
rexresearch
rexresearch1
Omar YAGHI, et al.
Metal-Organic Framework (MOF) Air Well
Metal-Organic Framework (MOF) Air Well
http://www.sciencealert.com/scientists-have-created-a-solar-powered-device-that-sucks-water-out-of-thin-air-even-in-the-desert?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+sciencealert-latestnews+%28ScienceAlert-Latest%29
Scientists Have Created a Device That Sucks Water
Out of Thin Air, Even in the Desert
FIONA MACDONALD
When it comes to future challenges, one of the biggest will be water scarcity - on a warming planet we're going to have plenty of seawater, but not enough fresh, clean water in the right places for everybody to drink.
And while a lot of research has focussed on desalination, a team of scientists have now come up with another possible solution - a device that pulls fresh water out of thin air, even in places with humidity as low as 20 percent. All it needs is sunlight.
It might sound too good to be true, but so far the research is solid. Called the 'solar-powered harvester', the device was created by teams from MIT and the University of California, Berkeley, using a special type of material known as a metal-organic framework (MOF).
To be clear, it's only in the prototype phase right now and has been tested in pretty limited situations, but the results so far have just been published in Science.
"This is a major breakthrough in the long-standing challenge of harvesting water from the air at low humidity," said one of the researchers, Omar Yaghi from UC Berkeley.
"There is no other way to do that right now, except by using extra energy. Your electric dehumidifier at home 'produces' very expensive water."
Two-thirds of the world's population is currently experiencing a shortage of clean water, but it's estimated that there's about 13,000 trillion litres of water worldwide present in the air all around us.
So far the prototype device has been tested under conditions of 20 to 30 percent humidity, and was able to pull 2.8 litres (3 quarts) of water from the air over a 12- hour period, using 1 kilogram (2.2 pounds) of MOF.
Successful tests have also been conducted on the rooftop at MIT, showing that it works in real-world conditions.
The team says that the device could easily be scaled up to provide a family with their freshwater needs for the day.
"One vision for the future is to have water off-grid, where you have a device at home running on ambient solar for delivering water that satisfies the needs of a household," said Yaghi.
"To me, that will be made possible because of this experiment. I call it personalised water."
Yaghi worked on the first MOF more than 20 years ago. Unlike regular sheets of metals, MOFs are structures where metals such as magnesium or aluminium are combined with organic molecules in an arrangement that creates rigid, porous structures ideal for storing gases or liquids.
Since then, more than 20,000 different kinds of MOFs have been created by researchers around the world, and are already being used to capture CO2, and efficiently store chemicals such as hydrogen or methane.
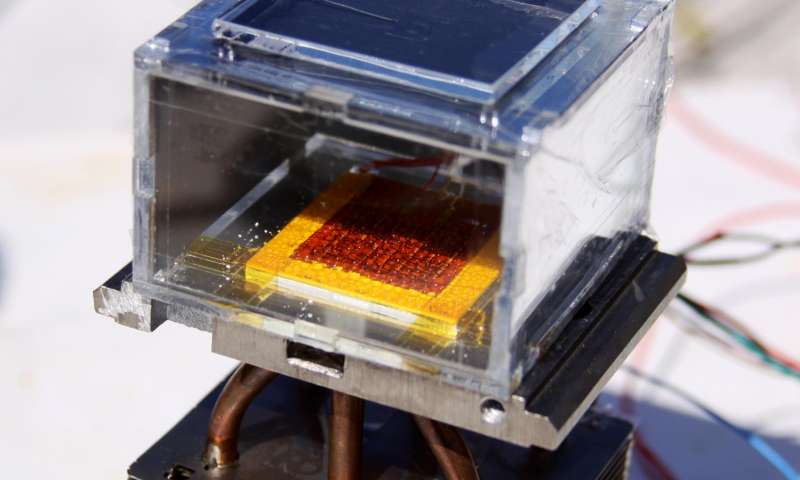
The MOF used in the solar-powered harvester, shown above, was synthesised in 2014, and contains a combination of zirconium metal and adipic acid, which binds water vapour.
The MIT team then took dust-sized crystals of this MOF and compressed them between a solar absorber and a condenser plate, and placed the whole thing inside a chamber that was exposed to the outside air.
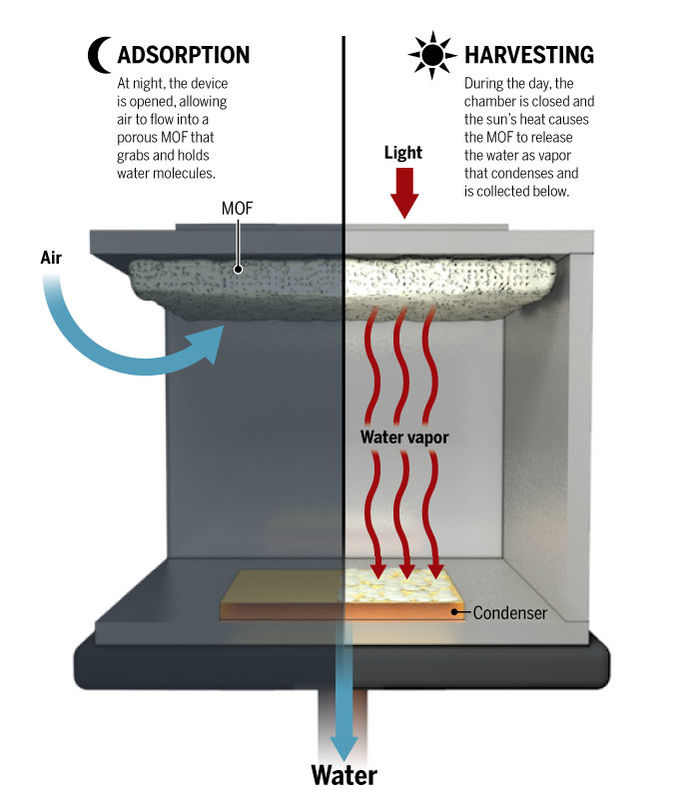
As ambient air diffuses through the MOF crystals, water molecules attach to the interior surfaces. X-ray diffraction studies of the system have shown that the water vapour molecules often gather in groups of eight, forming cubes.
Sunlight then heats the MOF up and pushes the bound water towards the condenser, which is the same temperature as the outside air. This vapour condenses as liquid water, and drips into a collector to provide clean drinking water.
"This work offers a new way to harvest water from air that does not require high relative humidity conditions and is much more energy efficient than other existing technologies," said MIT team leader, Evelyn Wang.
While it sounds pretty awesome already, the team admits there's a lot of room for improvement and fine-tuning, to make the device even more efficient.
Right now, the MOF can only absorb 20 percent of its weight in water, but other MOF materials could potentially absorb more than 40 percent.
The material can also be tweaked to be more effective at higher or lower humidity levels.
"It's not just that we made a passive device that sits there collecting water; we have now laid both the experimental and theoretical foundations so that we can screen other MOFs, thousands of which could be made, to find even better materials," said Yaghi.
"There is a lot of potential for scaling up the amount of water that is being harvested. It is just a matter of further engineering now."
"To have water running all the time, you could design a system that absorbs the humidity during the night and evolves it during the day," he added.
"Or design the solar collector to allow for this at a much faster rate, where more air is pushed in. We wanted to demonstrate that if you are cut off somewhere in the desert, you could survive because of this device."
"A person needs about a Coke can of water per day. That is something one could collect in less than an hour with this system."
http://science.sciencemag.org/content/356/6336/430
Science 28 Apr 2017: Vol. 356, Issue 6336, pp. 430-434
Water
harvesting from air with metal-organic frameworks powered
by natural sunlight
Hyunho
Kim,et al.
Solar heat helps harvest humidity
Atmospheric humidity and droplets constitute a huge freshwater resource, especially at the low relative humidity (RH) levels typical of arid environments. Water can be adsorbed by microporous materials such as zeolites, but often, making these materials release the water requires too much energy to be practical. Kim et al. used a metal-organic framework (MOF) material that has a steep increase in water uptake over a narrow RH range to harvest water, using only ambient sunlight to heat the material. They obtained 2.8 liters of water per kilogram of MOF daily at 20% RH.
Abstract
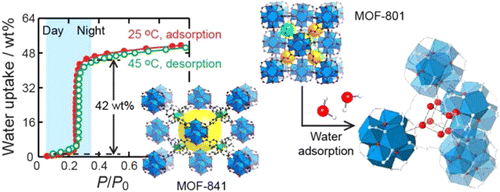
Atmospheric water is a resource equivalent to ~10% of all fresh water in lakes on Earth. However, an efficient process for capturing and delivering water from air, especially at low humidity levels (down to 20%), has not been developed. We report the design and demonstration of a device based on a porous metal-organic framework {MOF-801, [Zr6O4(OH)4(fumarate)6]} that captures water from the atmosphere at ambient conditions by using low-grade heat from natural sunlight at a flux of less than 1 sun (1 kilowatt per square meter). This device is capable of harvesting 2.8 liters of water per kilogram of MOF daily at relative humidity levels as low as 20% and requires no additional input of energy.
https://phys.org/news/2017-04-device-air-powered-sun.html
Device
pulls water from dry air, powered only by the sun
Imagine a future in which every home has an appliance that pulls all the water the household needs out of the air, even in dry or desert climates, using only the power of the sun.
That future may be around the corner, with the demonstration this week of a water harvester that uses only ambient sunlight to pull liters of water out of the air each day in conditions as low as 20 percent humidity, a level common in arid areas.
The solar-powered harvester, reported in the journal Science, was constructed at the Massachusetts Institute of Technology using a special material - a metal-organic framework, or MOF - produced at the University of California, Berkeley.
"This is a major breakthrough in the long-standing challenge of harvesting water from the air at low humidity," said Omar Yaghi, one of two senior authors of the paper, who holds the James and Neeltje Tretter chair in chemistry at UC Berkeley and is a faculty scientist at Lawrence Berkeley National Laboratory. "There is no other way to do that right now, except by using extra energy. Your electric dehumidifier at home 'produces' very expensive water."
The prototype, under conditions of 20-30 percent humidity, was able to pull 2.8 liters (3 quarts) of water from the air over a 12-hour period, using one kilogram (2.2 pounds) of MOF. Rooftop tests at MIT confirmed that the device works in real-world conditions.
"One vision for the future is to have water off-grid, where you have a device at home running on ambient solar for delivering water that satisfies the needs of a household," said Yaghi, who is the founding director of the Berkeley Global Science Institute, a co-director of the Kavli Energy NanoSciences Institute and the California Research Alliance by BASF. "To me, that will be made possible because of this experiment. I call it personalized water."
Yaghi invented metal-organic frameworks more than 20 years ago, combining metals like magnesium or aluminum with organic molecules in a tinker-toy arrangement to create rigid, porous structures ideal for storing gases and liquids. Since then, more than 20,000 different MOFs have been created by researchers worldwide. Some hold chemicals such as hydrogen or methane: the chemical company BASF is testing one of Yaghi's MOFs in natural gas-fueled trucks, since MOF-filled tanks hold three times the methane that can be pumped under pressure into an empty tank.
Other MOFs are able to capture carbon dioxide from flue gases, catalyze the reaction of adsorbed chemicals or separate petrochemicals in processing plants.
In 2014, Yaghi and his UC Berkeley team synthesized a MOF - a combination of zirconium metal and adipic acid -- that binds water vapor, and he suggested to Evelyn Wang, a mechanical engineer at MIT, that they join forces to turn the MOF into a water-collecting system.
The system Wang and her students designed consisted of more than two pounds of dust-sized MOF crystals compressed between a solar absorber and a condenser plate, placed inside a chamber open to the air. As ambient air diffuses through the porous MOF, water molecules preferentially attach to the interior surfaces. X-ray diffraction studies have shown that the water vapor molecules often gather in groups of eight to form cubes.
Sunlight entering through a window heats up the MOF and drives the bound water toward the condenser, which is at the temperature of the outside air. The vapor condenses as liquid water and drips into a collector.
"This work offers a new way to harvest water from air that does not require high relative humidity conditions and is much more energy efficient than other existing technologies," Wang said.
This proof of concept harvester leaves much room for improvement, Yaghi said. The current MOF can absorb only 20 percent of its weight in water, but other MOF materials could possibly absorb 40 percent or more. The material can also be tweaked to be more effective at higher or lower humidity levels.
"It's not just that we made a passive device that sits there collecting water; we have now laid both the experimental and theoretical foundations so that we can screen other MOFs, thousands of which could be made, to find even better materials," he said. "There is a lot of potential for scaling up the amount of water that is being harvested. It is just a matter of further engineering now."
Yaghi and his team are at work improving their MOFs, while Wang continues to improve the harvesting system to produce more water.
"To have water running all the time, you could design a system that absorbs the humidity during the night and evolves it during the day," he said. "Or design the solar collector to allow for this at a much faster rate, where more air is pushed in. We wanted to demonstrate that if you are cut off somewhere in the desert, you could survive because of this device. A person needs about a Coke can of water per day. That is something one could collect in less than an hour with this system."
http://pubs.acs.org/doi/abs/10.1021/ja500330a?src=recsys
Water Adsorption in Porous Metal–Organic
Frameworks and Related Materials
O.
Yaghi, et al.
Water adsorption in porous materials is important for many applications such as dehumidification, thermal batteries, and delivery of drinking water in remote areas. In this study, we have identified three criteria for achieving high performing porous materials for water adsorption. These criteria deal with condensation pressure of water in the pores, uptake capacity, and recyclability and water stability of the material. In search of an excellently performing porous material, we have studied and compared the water adsorption properties of 23 materials, 20 of which are metal–organic frameworks (MOFs). Among the MOFs are 10 zirconium(IV) MOFs with a subset of these, MOF-801-SC (single crystal form), −802, −805, −806, −808, −812, and −841 reported for the first time. MOF-801-P (microcrystalline powder form) was reported earlier and studied here for its water adsorption properties. MOF-812 was only made and structurally characterized but not examined for water adsorption because it is a byproduct of MOF-841 synthesis. All the new zirconium MOFs are made from the Zr6O4(OH)4(−CO2)n secondary building units (n = 6, 8, 10, or 12) and variously shaped carboxyl organic linkers to make extended porous frameworks. The permanent porosity of all 23 materials was confirmed and their water adsorption measured to reveal that MOF-801-P and MOF-841 are the highest performers based on the three criteria stated above; they are water stable, do not lose capacity after five adsorption/desorption cycles, and are easily regenerated at room temperature. An X-ray single-crystal study and a powder neutron diffraction study reveal the position of the water adsorption sites in MOF-801 and highlight the importance of the intermolecular interaction between adsorbed water molecules within the pores.
Rapid
Cycling and Exceptional Yield in a Metal-Organic
Framework Water Harvester
N.
Hanikel, et al.
[
PDF ]
https://news.berkeley.edu/2018/06/08/in-desert-trials-next-generation-water-harvester-delivers-fresh-water-from-air/
In desert trials, next-generation water harvester delivers fresh water from air
UC Berkeley scientists demonstrated that their device can collect drinkable water from the air each day/night cycle
US8852320 -- Preparation of Metal-Triazolate Frameworks
The disclosure provides for novel metal-triazolate frameworks, methods of use thereof, and devices comprising the frameworks thereof.
US2017081346 -- MESOSCOPIC MATERIALS COMPRISED OF ORDERED SUPERLATTICES OF MICROPOROUS METAL-ORGANIC FRAMEWORKS
US2017081345 -- METAL-ORGANIC FRAMEWORKS CHARACTERIZED BY HAVING A LARGE NUMBER OF ADSORPTION SITES PER UNIT VOLUME
US2017008915 -- ACID, SOLVENT, AND THERMAL RESISTANT METAL-ORGANIC FRAMEWORKS
ES2594616 -- Shaped bodies containing metal-organic frameworks
BR112013001263 -- FUNCTIONALIZATION OF ORGANIC MOLECULES USING METAL-ORGANIC FRAMEWORKS (MOFS) AS CATALYSTS
US2016008472 -- METAL-ORGANIC FRAMEWORKS WITH EXCEPTIONALLY LARGE PORE APERATURES
IN6714DEN2012 -- ORGANO-METALLIC FRAMEWORKS DERIVED FROM CARBENOPHILIC METALS AND METHOD OF MAKING SAME
WO2015195179 -- METAL ORGANIC FRAMEWORKS COMPRISING A PLURALITY OF SBUS WITH DIFFERENT METAL IONS AND/OR A PLURALITY OF ORGANIC LINKING LIGANDS WITH DIFFERENT FUNCTIONAL GROUPS.