The Technology of Low Temperature Carbonization
by
Frank M. Gentry
[ Chapter 1: Fundamentals ]
[ Note: The quality of the scanned graphics (tables & figures) are "uneven" at best despite repeated efforts to scan and tweak the images. -- R.N. ]
Chapter I
Fundamentals
Historical
Solid Fuels
Origin of Coal
Constitution of Coal
Destructive Distillation
Low Temperature Carbonization
Chemistry
Coal Assay
Thermochemistry
Heat Balance
Catalysis
Plastic Layer
Internal & External Heating
High & Low Temperature Carbonization
The low temperature carbonization of coal can lay claim to four distinct lines of descent from closely related fields of industry and research. A thorough understanding of its technology, therefore, requires a knowledge of the manufacture of coke and coal gas, of by-product recovery, and of complete gasification. Consequently, a brief review of the history and circumstances surrounding the origin and development of the various purposes for the processing of coal will be both interesting and helpful.
Even though the perpetual fires sacred among the superstitions of paganism were doubtlessly natural gases issuing from fissures in the earth in the vicinity of petroleum deposits and asphalt beds, there seems to be no published account of an investigation of the properties of combustible gases before 1658. In that year Shirley (1) communicated to the Royal Society an account of his investigation of a combustible gas issuing from a marsh in the vicinity of a coal mine near Wigan, in Lancashire. This same site was revisited by Clayton (2), Dean of Kildare, in 1739. Upon damming the water and digging beneath the creek bed, he discovered a coal deposit and, according to his account, he distilled some of the coal in a retort over an open fire, obtaining a black oil and non-condensible "spirit which catched fire at the flame of a candle." Although Van Helmont of Brussels had proposed the name of GAS in 1662, it is interesting to note that it had not come into general usage half a century later. Van Helmont writes in Latin, according to Richards (3), of his experiments in retorting charcoal and, referring to the gaseous distillate, he says, "This spirit. ...I call by the name of Gas."
Hales, in his "Vegetable Staticks," published in 1726, records the first destructive distillation of coal. He states that upon distilling 158 grains of Newcastle coal, he obtained 180 cubic inches of air (gas), weighing 51 grains. Six years prior to Clayton's visit to Wigan, Lowther (4) communicated to the Royal Society that the damp air issuing from a coal mine near Whitchaven could be ignited and would burn. Bladders were filled with the gas and carried away, afterwards being burned through a small pipe inserted in the bladder, according to his report.
Even though it is said by Molinari (5) that the Chinese employed petroleum vapor, distributed through wooden pipes for lighting purposes, as early as 900 AD, it is remarkable that over half a century elapsed from 1733 until 1792 before any actual application was made of combustible gases evolved through the destructive distillation of coal. The credit for practical application of coal gas to artificial illumination is due Murdock, according to Clegg (6). The date of his initial experiments is unknown, but in 1792 we find him manufacturing coal gas with which he illuminated his premises and offices at Redruth, in Cornwall. The first use of gas lighting as a commercial and economic substitute for lamps and candles was six years later when Murdock installed an apparatus in a factory at Soho.

An old wood-cut, taken from "Parl{es' Chemical Catechism"
published in 1810 and reproduced in Figure 1, illustrates the
state of the art at that time. It shows the approved
experimental apparatus for the destructive distillation of coal
as used in lectures to arouse popular interest. In 1807 a few
street lamps were illuminated with coal gas in Pall Mall,
London, but the public in general and even men of science were
decidedly prejudiced against gas lighting. In fact, it is said
that Davy considered the project ridiculous and inquired of one
of its advocates if he proposed to take the dome of St. Paul's
for a gasometer, to which the gentlemen, Clegg, replied that he
hoped to see them no smaller. A century later his expectations
were
realized many times over.
While iron is known to have been extracted as early as 4000 BC and coke was an article of commerce among the Chinese before 100 BC, the latter was not used in the metallurgy of iron until 1619, when it was introduced by Dudley. The following year in England, St. John was granted a patent for the first beehive oven. Aside from the small quantities consumed in ancient oriental trade and among certain European arts during the middle ages, the use of coke as a fuel may be said to date from the introduction of the blast furnace in the smelting of iron.
The memoirs of Becher in the end of the 17th century contain the earliest reference to coal as a source of by-products, according to Thorpe (7), and it is recorded by Wagner (8) that he received a patent in 1700 for an oven which permitted the recovery of tar in the coking of coal. He reported having found a method of treating coal, "so that it no longer smoakes nor stinks" and at the same time obtaining a -tar equal to the Swedish. Stahl is said to have been the inventor of a process used at Sulzbach, near Saarbrucken, prior to 1768, where coal was coked for iron smelting with a crude by-product recovery.
Seventeen years before the introduction of illuminating gas by Murdock in 1798, the Earl of Dundonald obtained a patent for distilling coal under the heat of its own combustion, thereby obtaining the tar and oils which it contains. From the patent it is plain that Dundonald was aware of carbonization in closed retorts at that date. In 1825 Faraday discovered benzene and twenty-two years later Mansfield (9) isolated it from coal tar. Runge (10) had isolated aniline from coal tar by 1834, but the real stimulus to the by-product coke industry did not come until 1856 when Perkin (11), a lad of eighteen years, discovered the first aniline dye, mauve or aniline purple, while attempting the synthetic preparation of quinine. On August 26 of the same year Perkin (12) received a patent and within a year the youthful chemist had built and placed in successful commercial operation a factory for producing the new dye from coal tar, without ever having seen inside of a chemical plant of any kind.
The great chemist Lavoisier discovered in 1793 that, when steam is passed over incandescent carbon, the reaction produces carbon monoxide and hydrogen, two combustible gases. This furnished the principle by which a cheap industrial gas could be made from coke or coal. The first gas producer, constructed as such, seems to have been made by Bischof in Germany in 1839, according to Rambush (13). He was followed closely by Ebelmen in France the following year. From an industrial standpoint, the development of gas producers dates from the patent granted the brothers Siemens in 1861 for a combined producer and regenerative furnace. Mond showed 28 years later that the by-products of producer plants could be recovered and made to render valuable service to the chemical and agricultural industries. This gave a decided stimulus to the producer gas industry.
Goethe in the episodes of his life "Dichtung und Wahrheit" describes his visit in 1741 to a burning hillside near Dutweiler, a village in the Palatinate, where he met Stauf, a coal philosopher, engaged in collecting the tar and oils obtained in the distillation of coal in crude ovens. How little did Goethe suspect that the black evil-smelling liquors of Stauf would some day be made to yield the richest and most vivid of colors, the sweetest of flavors, and the most fragrant of perfumes!
Solid fuels can, in general, be classified into three distinct classes, the cellulosic fuels, the sedimentary fuels, and the residuary fuels. The cellulosic fuels, of which wood is the most important member, have little value in modern industry because of their comparatively low heating quality and their cost. The sedimentary fuels consist of peat, lignite, and the two general grades of coal, bituminous and anthracite. They were, of course, originally cellulose-rich vegetation which was transformed during the geologic ages into the black, brittle, compact crust we find in the earth's today. They form the most important source of power which civilization has been able to master, either in their mineral or retorted state. The residuary fuels are obtained by heating the cellulosic and sedimentary fuels out of contact with air, forming charcoal and coke, respectively. They are distinguished by their high carbon and low volatile contents.
Outside of this classification, there remain those solid materials which contribute indirectly to our fuel resources, such as the shales which are not directly combustible, and synthetic compounds, representing the stored energy obtained from other sources of power, such as carbides. While the oil derived from shale and acetylene obtained from carbide have local must be stated that as a great source of energy they reached a position of primary importance. However, the vast deposits of shale constitute a great potential source of oil whose economic value will increase with the exhaustion of the petroleum resources.
The utilization of solid fuels follows three different methods: first, direct combustion; second, destructive distillation with simultaneous coking; and, third, complete gasification. Direct combustion is by far the simplest, oldest, and most extensively used. It consists in the burning of the raw carbonaceous material in the furnace in lump, powdered, or briquetted form. The residuum consists wholly of ash and clinker, devoid of all combustibles.
When fuel, rich in hydrocarbons, is distilled in closed retorts without the introduction of air, it is separated into the solid, liquid, and gaseous phases; in other words, into coke, tar, and gas. The coking of coal resolves itself into various methods, depending upon the disposition to be made of the products. The primary object in central or municipal gasworks is to produce a good grade of gas for domestic illumination and heating. Coke is a by-product in this process. Industrial coke ovens strive for a high quality of metallurgical coke. Tar and gas are by-products of this method. The low temperature distillation of coal aims at a compromise between these two systems and seeks to obtain a coke suitable as a smokeless fuel for industrial and domestic consumption with the simultaneous production and recovery of tar, its derivatives, and of high thermal value gas. Which of the products is of paramount importance depends on the coal, the process, and the economics of the case.
As distinguished from carbonization, complete gasification intends to convert the coal entirely into gaseous and liquid combustible matter, leaving only ash as a residuum. To this end, three means are resorted to: first, gasification by air; second, gasification by water; and, third, gasification by both air and water. The air producer method is based upon the incomplete combustion of carbon to carbon monoxide, only sufficient air being admitted to maintain a temperature at which the reaction can take place. Gasification by water is an intermittent process. Air is first introduced to raise the carbonaceous material to incandescence through the heat liberated in the complete combustion of carbon. After the air is shut off, steam is introduced to disintegrate and form a mixture of carbon monoxide and hydrogen. The production of semi-water gas combines these two principles and simultaneously introduces air and steam to the combustion chamber. Recent progress has been made in the use of pure oxygen mixed with steam to effect the continuous generation of semi-water gas.
At this point a brief review of the origin and constitution of coal is pertinent as preliminary to a study of the chemical process underlying carbonization. Today it is well established that the carbonaceous deposits, ranging from peat to anthracite, represent progressive changes which have taken place in the structure of decayed vegetable matter. Unquestionable evidence is furnished by fossil remains that among the early geologic ages, millions of years ago, the dead vegetation, which was so abundant at that time, accumulated in damp lowlands and bogs. There, with the exclusion of air by the water, a slow process of disintegration took place. The vegetable tissue, consisting mainly of cellulose, decomposed with the liberation of the oxides of carbon, marsh gas, and water, to form the material known as peat. As the bottom lands sank below the surface of the water and sedimentary deposits built up, a pressure developed which rendered the peat deposits more compact and consolidated, thus forming the lignites and sub-bituminous coals. The internal forces of the earth became active at that stage and the sedimentary rocks above and below the lignite deposits were violently thrown into folds, developing additional pressure with the generation of heat. The result of this upheaval and folding was the further consolidation of the mass and its transformation into bituminous coal. The remaining metamorphosis into anthracite is said to have been brought about by the cracking of the rock folds to permit the escape of entrapped hydrocarbons, evolved from the carbonaceous material.
The chemical representation of the follows, according to Heinrich and Ries (14):
[1] Cellulose 5 C6H10O5 > 6 CO2 + CO + 3 CH4 + 8 H2O + C20H22O4 Lignite
[2] Cellulose 6 C6H10O5 > 8 CO2 + CO + 5 CH4 + 10 H2O + C22H20O Bituminous
[3] Cellulose 7 C6H10O5 > 8 CO2 + 4 CH4 + 19 H2O + C30H16O Semi-Bituminous
Whatever the physical circumstances bringing about the vegetable transformation, or whatever the intermediate stages of the metamorphism, these equations give the initial and final stages in the transition.
The age of the coals in the United States ranges from the Carboniferous to the tertiary epochs. In general, the Carboniferous coals occur east of the 100th, the Cretaceous coals from the 100th to the 115th meridian, and the tertiary coals between the 120th meridian and the Pacific Ocean. An exception to this geologic distribution is a large area of Tertiary lignites in the Gulf States and a small area of Trianic coals in Virginia and North Carolina.
The constitution of coals found in the United States, determined by the proximate analyses as given by Parr (15), are shown in Table 1. These data are compiled from chemical determinations on a number of different samples and represent the average distribution of the constituents. The changes in the constitution of coal which take place with its geologic aging can be seen clearly. The moisture content of the fuel reaches its maximum in the peats and decreases to a low percentage in semi-anthracite. The volatile matter rises to a maximum in sub-bituminous coals and then varies inversely with the aging. On the other hand, the fixed carbon is almost directly proportional to the density of the coal. The ash remains about constant, but the heating value of the coal increases with the consolidation of the fuel, except for a slight decrease among the hard coals.
Table 2 gives the ultimate analyses of these fuels, showing their elementary constituents, as compiled by the author from numerous samples. The deoxygenation, occurring in the aging process, is quite apparent, but the dehydrogenation is not as rapid, although present. The percentage of nitrogen and sulfur seems to have no general relation to the antiquity of the fuel.


The ash referred to in the proximate analyses consists of a number of fused oxides, principally silica and lime. Representative analyses of the ash of various coals, as compiled by the author, are given in Table 3. Examination of this table discloses that the silica content increases quite regularly from peat to anthracite. The alumina increases also, but in a less orderly manner. The oxides of iron and calcium progressively decrease toward the harder coals. No generalizations can be drawn concerning the other oxides found in the ash, as they are largely dependent on the general geological and mineralogic structure in the immediate locality of the mines. The oxides of magnesium, and of anhydrides of phosphorous and of sulfur part of the ash. The melting point of the ash is well. The usual practice of washing the coal before carbonization can, of course, be resorted to for the purpose of reducing the ash content of the coke.
When coke is used as a fuel for domestic and industrial purposes, the formation of clinker on the grates should not be more of a nuisance than is ordinarily experienced with coal. If the coke is completely gasified and sold to the community for lighting and heating purposes, care must be taken to reduce the phosphorous and sulfur contents of the gas to a minimum, in order to prevent injury both to the consumers and to their property. It is not unlikely that the oxides present in the coal ash exert a catalytic effect in the decomposition of the hydrocarbons, as pointed out by Lessing (16) and as discussed later in this book.
Four general constituents of coal can be distinguished; the carbon residuum, the humous bodies, the resinous bodies, and the hydrocarbons. The last three undergo thermal decomposition with the formation of solid, liquid, and gaseous products, according to a table prepared by Lewes (17), while the first contains principally carbon and ash.
Constituents of Coal > Decomposition Products (Solid ~ Liquid ~ Gaseous)
Humous > Carbon ~ Water, Thin Tar ~ Carbon Oxides, Methane
Resins > Carbon, Pitch ~ Water, Rich Tar ~ Carbon Oxides, Ethylene, Unsaturated Hydrocarbons
Hydrocarbons > Carbon, Pitch ~ Heavy Tar ~ Methane, Ethane, Homologues
Carbon Residuum > Unaffecetd by heat
It appears that Dundoroff (18) first extracted resin from coal by the use of chloroform and other solvents. He found that these resins have a low melting point in the vicinity of 40° C to 80° C, and that they begin to decompose at 100° C to 140° C. The resinous bodies, which are soft in structure and dark brown in color, appear in the coal as slender rods. Chemical analysis shows then to contain 75% to 85% carbon; 8% to 10% hydrogen; 5% to 12 per cent oxygen; about 3% nitrogen; and about 1.5% sulfur.
Many of the volatile hydrocarbons of bituminous coal are decomposition products of the resinous and humous constituents and the characteristic cell structure of coke owes its existence to the melting and disintegration of these bodies. It will be noted from Table 4 that the humus differs from the resin mainly in the fixed carbon content.


In their researches on the low temperature carbonization of coal, Burgess and Wheeler (19) observed three significant facts. First, that for all coals there is a well defined point, between 700° C and 800° C, where the evolution of hydrogen increases rapidly, thereby indicating decomposition. Second, that the evolution of paraffin hydrocarbons takes place below 700° C and ceases above that temperature. And, third, that ethane, propane, butane, and other members of the series form a large part of the gas below 450° C. They concluded from these facts that coal was composed mainly of two substances, the first or more unstable of which yields paraffin hydrocarbons and no hydrogen, and the second of which becomes unstable at 700° C to 800° C, yielding hydrogen as its chief decomposition product. The first of these substances has been called by Clark and Wheeler (20) the resinic and the second the cellulosic constituent of the coal.
According to many authorities, low temperature tars are easy to distill, and, therefore, require less fuel than the tars from high temperature processes. Care must be taken in the distillation procedure, however, because of the large amount of light oil present. For this reason refrigerating equipment is sometimes provided in conjunction with the cooling apparatus to insure complete condensation. Distillation must be slow on account of the large quantities of tar acids present; otherwise, frothing will be severe and subsequent separation of by-products will be rendered difficult. The cresols emulsify with the water and cause frequent trouble. Agitators have been found to decrease the time of distillation and the use of superheated steam toward the end of the process is generally recommended to secure a good yield of heavy oil. In the distillation of coal, dissociation is much more pronounced at higher temperatures. Furthermore, carbon deposited from the decomposition exerts a reducing effect on the saturated hydrocarbons of the paraffin series that are present and results in the production of higher-boiling hydrocarbons.
When highly oxygenated coals, having in the neighborhood of 15% oxygen, are used, the condensed water, driven off in distillation up to 450° C, contains much more acid that that from coal of relatively lower oxygen content. The hydrocarbons do not appear in highly oxygenated coals until 290° C, instead of 240° C. The acidity of the distillate is due to acetic compounds. Liberated at low temperatures and originating from the ligneous or cellulosic content of high oxygen coals. Carbon dioxide is also evolved more voluminously than from the more resinous coals.
When carbonized at low temperatures, some fuels produce a char instead of a true coke. This char can be converted often into coke by subsequent treatment. It is often friable, lacking in the characteristic coke cell structure and is free burning. In some cases the char is briquetted for fuel, but the pitch, ordinarily used as a binder, causes a good deal of smoke and thus defeats one of the purposes of low temperature carbonization. Further treatment by heat eliminates this difficulty. On the other hand, some fuels, when carbonized at low temperature in certain retorts, yield a strong dense semi-coke. The character of the carbonaceous residuum, indeed, depends as much upon the process of carbonization as upon the raw coal.
Coal is such a heterogenous substance and its behavior so peculiar and complicated that it is almost impossible to make generalizations. Not only are the products in each instance so peculiar to the particular coal treated, but they depend upon the numerous physical and chemical circumstances surrounding the distillation. Such diverse factors as temperature gradient, thickness of the charger, pressure, and rate of carbonization, all have a direct bearing on the quantity of gas, tar, and coke that is obtained, as well as upon their quality and constitution.
Withdrawal of the products immediately after formation is desirable from the physical as well as the chemical standpoints. In the first case it means the saving of heat, because any excess heat absorbed by the gases is usually lost in cooling. A low temperature of evolution throws less work on the condensing plant, thereby increasing the overall efficiency, as well as decreasing the time of carbonization. From a chemical standpoint, it prevents cracking, which causes a deposition of carbon and an increase of gas yield at the expense of light oil and tar.
Low Temperature Carbonization ~
One of the most discussed factors in the destructive distillation of fuel has been the temperature of carbonization. When coal was first coked for illuminating gas over a century ago, this topic was as much in discussion as today. The early engineers recognized to some extent the value of low temperature processes, but as the production of gas was of primary importance to them, they resorted to the practice which gave the greatest yield and so adopted the high temperature method exclusively. The knowledge which has been gained concerning distillation products. Added to changing economic conditions, has given a new incentive to the carbonization of coal at low temperatures; and so again the discussion of thermal conditions has been brought forward, accompanied by its misunderstandings and its controversies.
The matter of thermal definition of the low temperature carbonization process is in considerable disagreement among the authorities. Parr and Layng (21) define it as below 750° C to 800° C, while Bone (22) considers it not beyond 550° C to 600° C, and Gluud (23) fixed the range as 500° C to 600° C. This disagreement may be understood by an examination of the coals considered and the particular type of product desired by each authority. Thus, Parr and his co-workers used Illinois coal and wished to secure a smokeless fuel for domestic use; Bone was interested in British coals; and Gluud was considering the production of primary tar, if primary tar, that is, tar with a small percentage of free carbon, is desired. It may be seen, therefore, that the temperature range depends upon the quality of products desired, as well as upon the method of processing. At most, it remains a balance between a good metallurgical coke and poor tar or a good tar and poor metallurgical coke.
By way of scientific definition, low temperature carbonization, as hereafter used, is taken to mean the destructive distillation of coal at or below the cracking temperature of the hydrocarbons in primary tar. This temperature is, of course, a function of the physical conditions of retorting; thus, for example, in vacuum distillation it may not exceed 450° C, and in case of pressure distillation may run beyond 1000° C under peculiar circumstances. It will vary in practice with the quality of the coal and with the economic balance in grade of products that is determined by local conditions. For the most part, however, under atmospheric pressure and for average coals, 750° C may be taken as the upper limit of low temperature carbonization. Gentry (24) has advocated this definition of low temperature carbonization as the only adequate and the most scientific one that has been proposed.
From an economic standpoint, the known losses in the present method of utilization of fuel fall under two categories; smoke, arising though incomplete combustion and forming not only a civic nuisance but a real fuel waste; and loss of valuable by-products through lack of proper recovery methods. It may be said that low temperature carbonization has for its purpose the abatement of the smoke nuisance on the one hand and the increase in overall efficiency of fuel utilization on the other hand. It does not mean conservation of natural resources, but the increase consumption of one economics good to obtain another.
The modus operandi is made clear when it is pointed out that certain hydrocarbons which are present in coal break down into their constituents, or crack, during the ordinary process of combustion. Elementary carbon is thus deposited in a finely divided state and carried through the stack before it has an opportunity to burn. It is plain that if these hydrocarbons are removed from the fuel by preliminary treatment, smoke can be eliminated almost entirely. Incidental to this operation is the question of what disposition is to be made of the hydrocarbons thus removed and which in themselves represent a pecuniary value. The answer of this is found in the fractional distillation of the tar to retrieve its valuable constituents for industrial use. Some of the products can be used as petroleum substitutes, commercial solvents, and as fertilizers. The coke obtained in this primary distillation will be characterized by the absence of smoke in its combustion, and it may be handles in the raw, briquetted, pulverized or gaseous form.
Quite naturally the history of low temperature carbonization is closely associated with that of coal gas. One of the first to recognize that a maximum yield of oil is obtained at low temperatures was Perkins (15), who secured a patent in 1853 for extracting oil from shale and other carbonaceous materials by distillation at a low temperature. The following year, Sparr (26) suggested the coking of coal for oil, rather than for gas, under the conditions of a high vacuum. In 1880 Scott-Moncrieff (27) proposed to free the atmosphere from smoke by partially coking the coal in high temperature retorts before combustion. Investigation, however, proved that only the outer layers of coal had been partially coked when removed from the retorts and the core remained as raw coal. Ten years later arker 28), the inventor of the Coalite process, secured a patent for the production of a smokeless fuel by distillation with superheated inert gases, such as steam, water gas, or coal gas, at 600° C to 650° C. Later, Parker (29) obtained patents for heating coal in the presence of steam below 450° C. These formed the basis upon which he developed his Coalite process, which will be discussed in Chapter VI.
In the United States, experiments had meanwhile been carried on from as early as 1902 at the University of Illinois. An announcement of the results was made in 1908 and further results were reported in 1912 by Parr (30, 31) and his various co-workers. While some of the earliest research on this subject was made in the United States, it has been extended, until recently, mostly by countries with limited or no petroleum resources and which recognized that their coal deposits could be made to yield a liquid fuel which would be of national importance. The World War gave great impetus to research in this field, particularly in England and Germany. The British Board of Fuel Research was established in 1917 to promote fuel economy and to coordinate fuel research. To that end, it has contributed a great deal of work to the carbonization of coal. The Kaiser Wilhelm Institute has performed a similar service in Germany.
In addition to a relatively small proportion of pyridine bases, low temperature tar contains, in general, two types of compounds: chemically neutral hydrocarbons, forming a mixture resembling paraffin base crude petroleum; and acidic hydrocarbons, having one or more phenolic hydroxyl groups. The former consist principally of alipathics with a certain proportion of naphthalene derivatives and aromatic compounds with extensive side chains. The proportion of acidic compounds in the primary tar depends, among other things, upon the oxygen content of the raw coal. Research at the Kaiser Wilhelm Institute has disclosed that a coal containing 3% oxygen, yields a tar, containing neutral hydrocarbons 45 or 6 times in excess of the acidic hydrocarbons present, whereas a coal containing 7.5% oxygen produces a tar composed of approximately equal proportions of neutral and acidic compounds.
The benzol ordinarily extracted from high temperature tar consists of a mixture of compounds of the aromatic hydrocarbon series, whose general formula is CnH2n-6, and whose chief member is benzene, although toluene and xylene are always present in quantities. Benzene and the other members of the cyclic hydrocarbons are not present as such within the coal, but they are derived through decomposition of other hydrocarbons by cracking during distillation. This is demonstrated by the fact that the primary tar, obtained when coal is distilled at low temperatures, contains no benzol in the ordinary meaning of the term. The word benzol is frequently employed, however, when referring to that fraction of low temperature tar, or to the light oil extracted by scrubbing the low temperature gas, which is suitable as a motor fuel and should more properly be designated a light oil or motor spirit. On the other hand, it has been pretty well established that the alipathic hydrocarbons belonging to the general formula CnH2n+2n are present as such within the coal. The formation of aromatic compounds from those of the paraffin series can be explained (32) by the fact that the higher paraffin hydrocarbons within the coal are broken into the lower members of the series and into olefins of the general formula CnH2n. Further heating will split the olefins into methane and acetylene, which latter is a member of the series CnH2n-2. Benzene can be formed by polymerization of the acetylene molecule.
The specific mechanism of the transformation from methane to anthracene has been outlined by Berthelot as follows, according to Audibert and Raineau (33):
[4] 2CH4 => C2H2 + 3H2
[5] 2C2H6 => 2CH4 + C2H2+ H2
[6] 2C2H4 => C2H6 + C2H2
which are the pyrogenic reactions entering into the first stage of the transformation. The second stage of the transformation is represented by the equations:
[7] 2C2H2 => C6H6
[8] C6H6 + C2H2 => C8H8
[9] C6H6 + 2C2H2 => C10H8 + H2
[10] 2C6H6 + C2H2 => C14H10 + 2H2
which are all pure polymerizing reactions.
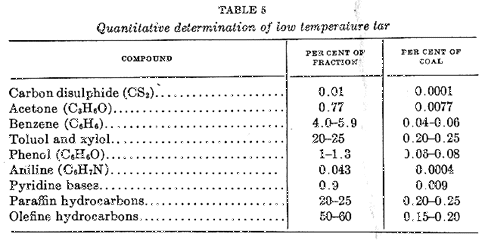
Schultz and Buschmann (34) in an examination of products, obtained from the Fellner-Ziegler process, found nearly a hundred different chemical compounds in the low temperature light oil, whose boiling point ranged from 30° C to 200° C; in the pressure condensed liquids below 30°C, and in the residual gas. Schultz and Buschmann (35) reported, in addition to water, hydrogen, and nitrogen, 20 hydrocarbons of the paraffin series; 9 hydrocarbons of the olefin series, representing each of the diolefins with chain and cyclic structures; 20 aromatic hydrocarbons, comprising 8 of the benzene series, 4 of the naphthalene series, 6 representatives of the indenes and hydrindenes, and 2 representatives of the more highly condensed aromatics. Only 3 compounds of the hydro-aromatic series derived from perhydornapthalene were identified. Four series of organic oxygenated compounds, consisting of aldehydes, ketones, phenols, and cumarones, were found. Phenols were represented by 8 simple aromatic phenols, one bivalent phenol and one napthol. Among the other oxygenated compounds, only 2 aldehydes, 4 ketones, and one cumarone were determined. Five sulfur compounds were identified, while the nitrogen derivatives were represented by 5 pyridine bases, 2 quinoline bases, one aromatic primary amine, and 2 alipathic nitrates, including hydrocyanic acid. From -250° C to 0° C there were about 12 substances which comprised some 6% by weight of the coal, whereas from 0° C to 200° C there were more than 50 chemical compounds whose total quantity was only about one-fifth of this. Table 5, after Schultz and Buschmann (35) gives a quantitative determination of certain constituents identified in the tar fraction from 0° C to 200° C.
Fieldner (36) has examined and compared the unpurified volatile products obtained from the McIntire externally heated primary retort at Fairmont WV with the products of low temperature distillation educed from a Utah non-smoking coal processes at two different temperatures in an externally heated retort and also in one heated internally with superheated steam. Table 6, after Fieldner (36) shows the makeup of the volatile products in the respective cases.



Gentry has compiled a list of chemical compounds which have been definitely identified in the volatile products of low temperature carbonization, as reported in the researches of a number of different authorities. This list in classified form is given in Table 7. Many additional compounds remain unidentified and a great deal of study will be required before their structure is definitely known. This is especially true of the high boiling constituents, which are difficult to isolate without decomposition. Many of the compounds listed in the following table do no occur in all low temperature volatile products, a great deal, of course, depending on the coal and on the process of carbonization. Some of the compounds listed, such as benzene and phenol, although occasionally present, are found only in very small quantities in true primary products.
A critical examination of Table 7 discloses the presence of a number of compounds involved in Berthelot's mechanism for the transformation from chain to cyclic hydrocarbons. That aromatic derivatives are decomposition products in coal carbonization becomes conclusive when a list of the volatile products of high and low temperature distillation are compared.
A number of attempts have been made to devise a satisfactory method of coal assay for purposes of carbonization, notably by Burgess and Wheeler (57) who experimented with a closed silica tube containing 2 gram samples which were heated at temperatures ranging from 400° C to 1000° C, and by Lessing (58) who advocated the insertion of a piston within the silica tube to compress the coal slightly. Gray and King (59) devised a standard apparatus for coal assay and conducted extensive experiments to determine its peculiarities and applicability in determining the yields to be expected from a given coal in full-scale operation. By correlating results obtained in the assay apparatus with those obtained in commercial retorts it has been possible to derive the necessary conversion factors for gas, tar, and coke. In cases where these factors have been determined by tests conducted by the Fuel Research Board, they will be reported hereafter under the discussion of each individual process. Other methods have been devised also by Fischer and Gludd (23), who used a small rotating cylinder, by Nielsen (61), by Layng and Hawthorne (62), and by Foxwell (63), all of whom used a method of internal heating.
In studying the thermochemistry of coal carbonization, it is necessary to bear in mind the distinction between gross and net heat values, that is, between the heat liberated or absorbed, referred to liquid tar and water and that referred to gaseous volatile products. The net value is the only one of importance in practice.
Coal is such a complex and homogenous substance that it is almost impossible to make a detailed analysis of the endothermic and exothermic reactions which occur during distillation. As a matte of fact, both exothermic and endothermic reactions take place simultaneously and only the net effect can be observed.
At high temperatures, Mahler (64) found that the heat of combustion of coal was about 460 BTU per pound less than that of all the products of carbonization, while Euchene (65) using a coal of the same analysis at lower temperatures, found an evolution of heat amounting to about 115 BTU per pound. Constam and Kolbe (66) studied a number of British coal and found an absorption of heat equal to as much as 6% of the thermal value with a Nottingham coal and as little as 2.1% of the calorific value with a Welsh coal.
Hollings and Cobb (67, 68) studied the thermal reactions of Mockton coal during low temperature carbonization. They observed that endothermic reactions predominated in the short region 410-470° C. A second endothermic period was found from 470-610° C. Above 610° C the reactions were decidedly exothermic in character, but between 750-800° C the reactions again became endothermic. This variation between the predominance of reactions involving the liberation and absorption of heat within various temperature limits explains sufficiently the discrepancies of earlier observations.
Strache and Fromm (69) studied the heat required for carbonizing various grades of fuel, ranging from wood to bituminous coal, at 750° C and found that the heat liberated was a function of the percentage of oxygen present in the fuel. With oxygen contents below 15%, the fuel is usually endothermic during its decomposition, while the fuels containing more than this amount of oxygen are exothermic in character. Of course, when very wet fuels are treated, the amount of heat required to evaporate the moisture may be so great as to entirely obscure any heat which might be liberated. In some cases, particularly in low temperature carbonization, where close temperature control is desirable, the exothermic reactions may be of importance. Thus, Parr and Layng (70) base their process upon this phenomenon and Gentry (71) has pointed out its importance in the carbonization of pulverized coal.
More recently, Davis and Place (72, 73) have investigated the thermal reactions of carbonization at the US Bureau of mines. They found that the exothermic heat varies not only with the oxygen content of the fuel but also with the conditions of distillation. Under conditions of low temperature carbonization, they found that most American coals have positive heat reactions. They confirmed the research of Klason (74) on wood cellulose, obtaining a liberation of 318 BTU/lb of pine sawdust.
Davis, Place and Edeburn (75) have made a very careful study of the heat of carbonization of various fuels by the calorimetric method devised by Davis (76). They showed that the primary decomposition of coal was exothermic in character, while the tendency of secondary reactions was endothermic. In other words, that the reactions involved in low temperature carbonization are principally those which liberate heat, whereas the reactions involved in the transition from low to high temperature carbonization products are those which absorb heat.
The difficulties of making such thermometric measurements are enhanced by the numerous variables affecting the results. Hulett and Capps (77) have shown that the pressure has considerable effect on the character and quantity of the products, and hence upon the heat of reaction, according to the principle laid down by Thompson (78) that the heat of formation of a compound is equal to the difference between its heat of combustion and the heat of formation of its products of combustion. The rate of heating determines the proportion of secondary reactions taking place simultaneously with the primary reactions and, therefore, causes a variation in the net heat evolved. Even then, the character of the inert gaseous atmosphere is not without its influence, as might be expected from its bearing on the reaction equilibrium. Thus, Davis and his coworkers found that at 930° F the heat evolved was about 85% greater in an atmosphere of carbon dioxide than in an atmosphere of hydrogen, when using Illinois and Ohio coals. Pre-oxidation renders the coal less exothermic, while preheating in hydrogen up to 390° F has no effect.
Analyses of the fuels, whose heat of carbonization was determined by Davis, Place, and Edeburn (75) are given in Table 8. These analyses are given on an air-dried basis. The heats of carbonization were determined in an atmosphere of nitrogen. The results are shown graphically for the coals in Figure 2 and for pine sawdust and peat in Figure 3. An examination of these illustrations discloses that all the fuels are slightly endothermic below the decomposition temperature, which is about 600° F for coals and 500° F for peat and sawdust. There is a rapid evolution of heat when primary decomposition sets in between 600° F and 800° F. The maximum heat evolution is reached at bout 900° F with coals and at about 1000°F with peat and sawdust. After attaining the maximum, the heat evolution curves drop off fairly rapidly and actually become endothermic again at 1050° F for the older and more consolidated coals.



Euchene (79) was one of the first to recognize the value of a heat a balance in coke ovens, but since his early experiments a great deal of valuable research has been done. The heat applied to decompose a fuel into its final products of coke, tar, and gas can be attributed to 8 factors, viz.: heat abstracted by moisture; heat abstracted by non-condensable gas as it leaves the retort; heat abstracted by tar oils; heat abstracted by liquor; sensible heat of the coke; sensible heat of the ash; internal heat of decomposition; and heat losses from the retort. Rambush (80) has proposed a method of estimating the heat balance of coal distillation by the use of tables, prepared to show the heat abstracted by each of the above factors as a function of the temperature of the gas outlet and yields of the various products.
The catalytic action of mineral matter in the coal, particularly in the ash, has long been overlooked, although attention was called to it in 1914 by Lessing (81). Experiments conducted by Lessing and banks (82) showed wide variations in the amount of coke produced when carbonizing sugar and cellulose in the presence of different inorganic compounds. The results were confirmed by Marson and Cobb (83). In view of the vital importance of catalysis in the synthesis of liquid hydrocarbons by hydrogenation of coal, it would not be at all surprising to find that the mineral matter in coal has a far more marked effect upon the volatile products than upon the solid residuum.
The mechanism of carbonization in a retort can be described as follows: the raw cola introduced into the chamber rapidly absorbs heat in its surface layer, thereby driving off free moisture, which moves inward and condenses in the interior regions of the cold charge; the temperature of the surface layer rises to 575° F or 750° F, within which temperature range it becomes fused or plastic; a rapid evolution of volatile products follows the fusion stage and the coke assumes a porous structure. From each wall of the retort the plastic layers migrate slowly inward until they meet at the center of the chamber, producing a line of demarcation always found as a central crack in the charge. The plastic layer is estimated to be from 0.5 inch to 1.5 inches in width, and it is characterized by a larger temperature drop across the fusion zone and a great resistance to the passage of gas through the layer. In fact, Evans (84) found that the resistance to passage of gas offered by the plastic layer was 3000 times that offered by screened lump coal, 75 times that offered by coal dust, and 300 times that offered by 1400° F coke.
Foxwell (63) investigated the temperature at which the plastic layer if formed and found that it lay between 752° F and 842° F, which is slightly above what is usually considered the fusion range. A pressure as high as 480 mm of water was required to force the passage of gas across the plastic layer, as compared to about 20 mm before and after fusion. Layng and Hawthorne (86) also investigated this problem for over 40 American coals and found pressures as high as 1500 mm of water in the case of Pocahontas coal. It is very apparent, therefore, that in coke ovens the gas evolved must develop quite a pressure to penetrate the plastic layer. The resistance offered by the fusion zone depends greatly upon the coal, particularly upon the degree to which it has been pre-oxidized. Figure 4 shows the result of some experiments by Parr (87) to determine the effect of pre-oxidation on the phenomena occurring during fusion. The reduction in resistance to gas passage, which accompanies pre-oxidation, evidently must be attributed to reduction in the coking power of the coal.
Ryan (88) has determined the progress of the fusion zone across a 16.5 inch Rothsburg coke oven and found that 16.3 hours were required for the two plastic layers to reach the center from each side when the flues were maintained at a temperature of about 2100° F. The progress of the plastic layer is shown clearly in Figure 5. The result of such a differentiation, using 0.5 inch increments to calculate the slope, is shown in Figure 6. With reference to the last illustration, it is seen that, when the raw coal is introduced into the hot retort, the surface layers in contact with the flue have a high heat transfer. Consequently, in the initial stages of coking, the plastic zone travels at the relatively high velocity of about 1.5 inches per hour. However, as the plastic layer recedes from the oven wall, it becomes more and more difficult to transfer the heat necessary to raise the charge to plasticity, and therefore the rate of travel rapidly falls to a minimum of about 0.3 inch/hour. During the progress of the plastic layer, additional heat has been expended immediately in front of the fusion zone to drive off the moisture which condensed on the interior of the charge, and to raise the temperature of the center of the mass. Finally, the chilling action of the center of the charge is checked and the rate of heat transfer to the plastic zone becomes greater, thereby raising again its rate of migration.



Because of the poor heat conductivity of coal, the time of carbonization is greatly influenced by the size of the particles, porosity of the charge, method of heating, shape of the retort, and other peculiarities of the particular process under examination. In addition to the time consumed in heat transfer, there is a time interval required. While the coal is maintained at the maximum temperature, until coking is complete. Both the time of carbonization and the rate of heating depend, among other factors, upon the ration of the mass of the charge to the surface of the charge.
The thermal conductivity of coal is so low that even with a high temperature gradient of 1100° C in the flues of the retort and 100° C in the center of the carbonization chamber, the rate of heat transfer rarely exceeds 0.8 inch/hour in practice. Thickness of the charge, therefore, becomes of primary importance when carbonizing between 400° C and 800° C. Measurements under commercial conditions have been made by Wellington and Cooper (89) with retorts of different thickness to study the rate of heat transfer and are given in Table 9. In accordance with the laws of heat transfer, we find that for a high thermal gradient there is a high velocity of heat transfer and a low time interval for the center of the charge to attain its maximum temperature. For a reduction of 50% in the thermal gradient, however, there is a reduction of but 35.5% in the velocity of heat transfer and an increase of 33.4% in the time of retorting.
The slow rate of heating of the charge is seen clearly by reference to Figure 7, where some data on the experimental determination of temperature inside of the carbonization chamber during coking are given. The measurements on the low temperature process are reported by Wellington and Cooper (89), who used a vertical fireclay retort, 11.5 feet high and 5 inches wide. It was heated externally with producer gas and the flues were maintained at 765° C. About 8 hours were required for the center of the charge to reach the initial temperature of the retort. The results presented for the high temperature process are from measurements made by McBride and Selvig (90) on standard Koppers ovens. These ovens were of the horizontal variety, 39 feet long and 18 inches wide. Over 20 hours elapsed before the interior of the charge reached its maximum, while the flues were kept at 1200° C. In the first case, the rate of heat transfer was about 0.625 inch/hour and in the second case about 0.850 inch/hour.

In Figure 8 are shown some isochronic temperatures across a coke oven for various times after charging, as measured by Ryan (88). The measurements begin with the 14th hour after charging, or just about 2 hours before the plastic layer reached the center of the retort, after which it is apparent that, once the fusion stage is over, the rate of temperature increase becomes rapidly greater until, after the 20th hour, the charge attains almost a uniform temperature throughout.

According to Newton's law, the flow of heat through a body by conduction, after the steady state has been attained, is expressed by the differential equation:
[11] dQ / dt = kA (sT / dx)
where Q is the unit of heat; T, the temperature; x, the distance in the direction of heat flow; t, the time; A, the area perpendicular to heat flow; and k the coefficient of heat transfer. The complexities of carbonization, however, render this equation inapplicable in its simple form for two reasons. In the first place, the coefficient of heat transfer is not constant, but is a function of both the time and the distance from the retort wall and, secondly, because the rate of change of temperature with respect to distance is also a variable with respect to the time as well as the distance. The coefficient of heat transmission for coke is considerably greater than for coal, hence, the manner in which the mean coefficient might vary can be gathered from consideration of Figure 5, recalling that coke exists in the center of the chamber. The manner in which the temperature gradient varies as a function of time and distance can be gathered by graphical differentiation of the family of curves in Figure 8 to determine their slope.
It will be seen from Figure 8 that for several hours after charging n account of the low thermal conductivity of the coal, the temperature range within the oven is very great. Different zones of the charge, therefore, undergo different carbonization transformations at the same period. Nielsen (91) has nicely illustrated this point under conditions of low temperature distillation by determining the variation of the percentage volatile matter remaining in the coke with the distance of the distillation residuum from the retort wall for a retort 6.5 inches thick. The carbonization was carried on for 6 hours at about 600° C before the coke was examined. It was found that even after this period of retorting, there is a range of from 10.4 % to 13.4% volatile matter in the coke, depending on the distance of the residuum from the chamber wall. The maximum volatile content occurs in the center of the charge where the temperature is a minimum.
The Fuel Research Board (92) has determined the longitudinal distribution of temperature in vertical retorts, both with and without the introduction of steam. The results are shown in Figure 9. The upper curve shows the change in temperature distribution, arising from the passage of 13.5% steam through the retort. The experiments were made on a Glover-West retort. Under ordinary conditions of distillation, only the lower 25% of the retort was above 300° C and 50% above 100° C. When steam distillation was used, 48% of the retort was above 300° C, and 98% above 100° C. In the zones of the retort, extending from 5 feet above the base to the top, constituting about 75% of the carbonization chamber, the use of 13.5% steam raised the temperature of the charge over 100%. It will be pointed out later that one of the functions of steam distillation is to assist in the uniform distribution of heat throughout the charge, heat being absorbed in the lower hot portions of the retort and distributed through the upper cooler zones.



The introduction of coke breeze has a material effect in the transfer of heat. Roberts (93) examined the time required for the interior of the charge to reach 360° C, and found that 58 minutes were required, when using raw Welsh coal; 46 minutes when 20% breeze was added; and 31 minutes when 30% breeze was mixed with the charge. Table 10 shows the result of his investigation. Apparently, the beneficial effects of coke breeze are derived from the fact that it increases the thermal conductivity of the charge and gives a certain amount of porosity to the mass, which allows the transfer of heat by internal convection currents.
It is easier to withdraw vapors soon after their formation in horizontal than in vertical retorts. In a static horizontal retort, where the material moves progressively, eduction pipes can be placed at intervals along the top of the retort to withdraw the vapors. Thus, the mechanical design of the retort is found to play an important part in determining the yield of the desired products.
Two methods of heating have been resorted to, external and internal. In external heating, the solid, liquid, or gaseous fuel is burned in an outer chamber and the coal is placed in a gas-tight retort. When carbonization is carried on by internal heating, the sensible heat of producer gas, generated externally or in the retort itself, superheated steam, molten lead, or some other agency, is employed to effect the distillation. One of the main differences between the two methods lies in the quantity of gas evolved. Thus, in the producer gas method of low temperature carbonization, the yield of gas is of the order 25,000 to 35,000 cubic feet per net ton of coal, a compared with 3,000 to 4,500 cubic feet obtained in external distillation. On the other hand, the great volume of gas yielded by the producer gas method is of low heating value, such as 250 BTU per cubic foot, as compared with the high calorific value of 800 to 1000 BTU per cubic foot obtained in the externally heated retorts.
The uniform distribution of heat in the retort by steaming suggests the advantage of internal heating over the external method. Nielsen (91) obtained the results in Table 11 by comparing the rate of evolution of gas from carbonization at low temperature in externally and internally heated retorts. The rate of evolution rises to a maximum after about three-quarters of an hour of carbonization, after which it gradually falls in value. Internally heated retorts attain an 18.5% higher maximum in the same period than those heated by external methods. The recession in the rate of evolution is also much more rapid when the heat is conducted through the charge by the internal heating process. By integrating these curves, that is, by measuring the area between them, the total quantity of gas evolved over any period of distillation can be found. Comparison of the two areas discloses that, over a period of 2.75 hours, 75% to 80% of all the gas evolved from the coal during low temperature carbonization in internally heated retorts has been yielded, whereas 70% to 75% has been given off in externally heated processes. For the same character and yield of products in the two cases, this is equivalent to a material reduction in the time of carbonization.

High & Low Temperature Carbonization ~
For the sake of concise presentation, it would be permissible, perhaps, to risk a generalized comparison. Considering a coal containing approximately 35% volatile matter, 7% ash, and 58% fixed carbon, it is reasonable to expect under ordinary circumstances the yields per net ton of raw fuel shown in Table 12.

The gaseous products of low temperature carbonization consist mainly of the lower saturated and unsaturated alipathics. Aromatic compounds are generally absent. The gases, while produced in lesser quantities than in high temperature ovens, are rich in their thermal value.
Low temperature tar contains more members of the paraffin series and fewer aromatic compounds than high temperature or coke oven tar. One of the chief characteristics of low temperature tar is its high tar acid content. The fraction up to 170° C, and the pitch usually contain about 40% tar acids, while the coke oven distillate runs as low as 15% or less. Very little phenol itself is produced, but this varies to a great extent with the coal. Of the tar acids, more cresol and homologues of phenol are present than in high temperature tar. Napthalene is absent, but other members of the series have been found in minor quantities. While coke oven tar has a specific gravity of about 1.17 at 15° C and contains about 6.5% free carbon, the low temperature product runs less than 1.10 specific gravity and usually contains less than 1% free carbon. The presence of free carbon in tar is partly indicative of cracking, which is reduced to a minimum at low temperatures. The presence of as much as 3% free carbon in some low temperature tars, however, is due to dust and not to cracking.
The evolution of ammonia gas from coal under destructive distillation begins below 300° C and ceases above 1200° C, so that it is to be expected that the ammonium sulfate yield of low temperature carbonization should be low. A great part of the nitrogen present remains in the coke and methods have been proposed for increasing the yield.
The two cokes are entirely different. The high temperature coke is coarser, denser, and harder to burn than low temperature coke. The latter is usually characterized by its friability, softness, fineness of texture, and high combustibility, but some semi-cokes are quite as hard and dense as the high temperature variety.
Since it is obvious that high temperature carbonization removes the hydrocarbons and resin out compounds, which cause the formation of smoke, as well as does low temperature carbonization, the question arises as to why the coke from gas ovens is not itself a suitable smokeless fuel for domestic consumption. The difference is that the former is not generally free-burning. The physical condition accounting for this difference in the free-burning property is said by Brownlie (94) to be in the porosity of the coke. High temperature coke is very spongy, while low temperature coke is more like charcoal. Furthermore, in the gas coke a graphitic film, deposited from the cracking of hydrocarbons, covers the walls of the pores and adds to its incombustibility.
