
rexresearch.com
Eddie
Chang, et al.
Cobalt Hexammine vs Ebola
( Hexamminecobalt(III) chloride, &c )



Cobalt Hexammine vs Ebola
( Hexamminecobalt(III) chloride, &c )



D.A. Knight : Biological Applications of
Cobalt(III) Complexes: From Artificial Endonucleases to
Antimicrobial Drugs
US Navy : CoHex: Broad Spectrum Anti-Viral Compound
Wikipedia : Hexamminecobalt(III) chloride
A. Aspaas & L. Stanley : Synthesis of Hexammine Cobalt (III) Chloride
Rutgers University : Preparation of HexamineCobalt(III) Chloride
Sigma-Aldrich Chemistry Co : H7891 Sigma -- Hexammine cobalt(III) chloride
Williams University : Preparation of an Inorganic Cobalt Complex : Co(NH3)nCl3
Preparation & Analysis of a Coordination Compound of Cobalt
Delahanty, et al : US2008182835 -- METHOD OF USING A COBALT-AMINE BASED METAL COMPLEX AS AN ANTIVIRAL COMPOUND...
Eddie Chang, et al : US20110027388 -- Cobalt Hexammine as a Potential Therapeutic Against HIV and/or Ebola Virus
US Navy : CoHex: Broad Spectrum Anti-Viral Compound
Wikipedia : Hexamminecobalt(III) chloride
A. Aspaas & L. Stanley : Synthesis of Hexammine Cobalt (III) Chloride
Rutgers University : Preparation of HexamineCobalt(III) Chloride
Sigma-Aldrich Chemistry Co : H7891 Sigma -- Hexammine cobalt(III) chloride
Williams University : Preparation of an Inorganic Cobalt Complex : Co(NH3)nCl3
Preparation & Analysis of a Coordination Compound of Cobalt
Delahanty, et al : US2008182835 -- METHOD OF USING A COBALT-AMINE BASED METAL COMPLEX AS AN ANTIVIRAL COMPOUND...
Eddie Chang, et al : US20110027388 -- Cobalt Hexammine as a Potential Therapeutic Against HIV and/or Ebola Virus
http://www.soflacs.org/semknightmar15.pdf
Dr
D. Andrew Knight
We have been exploring
the potential of the simple Werner complex, cobalt hexammine
"Cohex" for use as a broad spectrum antiviral and antibacterial
therapeutic. Cohex is a coordinatively saturated complex of
Co(III) surrounded by six ammonia ligands, is air and water
stable and has low toxicity. We have have reported on its
antiviral activity against both Sindbis virus (SV) and
adenovirus. Due to its kinetic inertness, Cohex interacts
principally with its environment via outer-sphere coordination
and through simple electrostatic interaction. One consequence is
that, unlike previously studied Co(III) systems, Cohex does not
hydrolyze nucleotides, but does show potent inhibition of
protein synthesis and dose-dependent antiviral properties. Our
recent studies on the activity of Cohex against Ebola and HIV
will be discussed.http://chemistry.fiu.edu/seminars/2011/seminar-on-wednesday-april-6th-at-11am/abstract.pdf
Biological
Applications of Cobalt(III) Complexes: From Artificial
Endonucleases to Antimicrobial Drugs
D. Andrew Knight,
Department of Chemistry, Florida Institute of Technology
D. Andrew Knight,
Department of Chemistry, Florida Institute of Technology
Artificial endonucleases based on a parent cobalt(III) cyclen complex have been used as artificial endonucleases and have demonstrated activity for the hydrolysis of DNA and RNA.
Using a cell-free translation system, we propose that cobalt cyclen complexes inhibit protein synthesis via a steric blockade and additionally through a
hydrolytic mechanism.
We have shown that a related cobalt(III) complex, cobalt hexammine shows broad spectrum anti-viral activity against Sindbis, HIV-1 and Zaire Ebola virus.
http://www.nrl.navy.mil/techtransfer/available-technologies/biomolecular-engineering/CoHex
CoHex:
Broad Spectrum Anti-Viral Compound


The Naval Research Laboratory (NRL) is developing a hexamminecobalt(III) (CoHex) based anti-viral compound for both clinical and first responder use.
Initial results with a variety of viruses (± ssRNA, -dsRNA, dsDNA, enveloped, non-enveloped) indicate that this compound is a very broad spectrum anti-viral agent.
Cohex is a small, stable, water-soluble, and inexpensive compound that can potentially be used as a therapeutic when there is no known drug therapy available, such as the case with H1N1 or an Ebola outbreak.
It can also be used with existing anti-viral drugs to provide an additive effect, which can reduce cost, as can be the case of HIV treatment, where less of a more expensive drug, such as AZT, is advantageous.
CoHex can also be used with current drugs against drug resistant strains, and may reduce the probability of drug resistance development.
Initial small animal testing also shows that CoHex has a much lower cytotoxicity than FDA approved cis-Platin making it a good source as a therapeutic agent
http://en.wikipedia.org/wiki/Hexamminecobalt%28III%29_chloride
Hexamminecobalt(III)
chloride




Hexaamminecobalt(III) chloride
Hexamminecobalt(III) chloride
CAS number 10534-89-1 Yes
Molecular formula H18N6Cl3Co
Molar mass 267.48 g/mol
Appearance yellow or orange crystals
Density 1.71 g/cm3,
Melting point decomposes
Solubility in water 0.26M (20 °C)
tribromide: 0.04M (18 °C)
Solubility soluble in NH3
geometry octahedral
Dipole moment 0 D
Hazards
R-phrases 36/37/38
S-phrases none
Main hazards poison
Related compounds
Other anions [Co(NH3)6]Br3
[Co(NH3)6](OAc)3
Other cations [Cr(NH3)6]Cl3
[Ni(NH3)6]Cl2
Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa)
Hexaamminecobalt(III) chloride is the chemical compound with the formula [Co(NH3)6]Cl3. This coordination compound is considered an archetypal "Werner complex", named after the pioneer of coordination chemistry, Alfred Werner. This salt consists of [Co(NH3)6]3+ trications with three Cl- anions. The term "ammine" refers to ammonia in its metal complexes, and the prefix hex (Greek: six) indicates that there are six ammonias per cation.
Originally this compound was described as a "luteo" (Latin: yellow) complex, but this name has been discarded as modern chemistry considers color less important than molecular structure. Other similar complexes also had color names, such as purpureo (Latin: purple) for a pentammine complex, and praseo (Greek: green) and violeo (Latin: violet) for two isomeric tetrammine complexes. [1]
Properties and structure
[Co(NH3)6]3+ is diamagnetic, with a low-spin octahedral Co(III) center. The cation obeys the 18-electron rule and is considered to be a classic example of an exchange inert metal complex. As a manifestation of its inertness, [Co(NH3)6]Cl3 can be recrystallized unchanged from concentrated hydrochloric acid: the NH3 is so tightly bound to the Co(III) centers that it does not dissociate to allow its protonation. In contrast, labile metal ammine complexes, such as [Ni(NH3)6]Cl2, react rapidly with acids reflecting the lability of the Ni(II)-NH3 bonds. Upon heating, hexamminecobalt(III) begins to lose some of its ammine ligands, eventually producing a stronger oxidant.
The chlorides in [Co(NH3)6]Cl3 can be exchanged with a variety of other anions such as nitrate, bromide, and iodide to afford the corresponding [Co(NH3)6]X3 derivative. Such salts are bright yellow and display varying degrees of water solubility.
Preparation
Since CoCl3 is not available, [Co(NH3)6]Cl3 is prepared from cobalt(II) chloride. The latter is treated with ammonia and ammonium chloride followed by oxidation. Oxidants include hydrogen peroxide or oxygen in the presence of charcoal catalyst.[2] This salt appears to have been first reported by Fremy.[3]
The acetate salt can be prepared by aerobic oxidation of cobalt(II) acetate, ammonium acetate, and ammonia in methanol.[4] The acetate salt is highly water-soluble to the level of 1.9M (20 °C), vs. 0.26M for the trichloride.
Uses
[Co(NH3)6]3+ is a component of some structural biology methods (especially for DNA or RNA, where positive ions stabilize tertiary structure of the phosphate backbone), to help solve their structures by X-ray crystallography[5] or by nuclear magnetic resonance.[6] In the biological system, the counterions would more probably be Mg2+, but the heavy atoms of Cobalt (or sometimes Iridium, as in PDB file 2GIS) provide anomalous scattering to solve the phase problem and produce an electron-density map of the structure.[7]
References
Huheey James E., "Inorganic Chemistry" (3rd edition 1983), p.360
Bjerrum, J.; McReynolds, J. P. (1946). "Hexamminecobalt(III) Salts". Inorg. Synth. 2: 216–221. doi:10.1002/9780470132333.ch69.
M. E. Fremy (1852). "Recherches sur le cobalt". Annales de chimie et de physique 35: 257–312.
Lindholm, R. D.; Bause, Daniel E. (1978). "Hexamminecobalt(III) Salts". Inorg. Synth. 18: 67–69. doi:10.1002/9780470132494.ch14.
Ramakrishnan, B.; Sekharudu, C.; Pan, B.; Sundaralingam, M. (2003). "Near-atomic resolution crystal structure of an A-DNA decamer d(CCCGATCGGG): cobalt hexammine interaction with A-DNA". Acta Crystallogr. D59: 67–72. PMID 12499541.
Rudisser, S.; Tinoco Jr., I. (2000). "Solution structure of Cobalt(III)hexammine complexed to the GAAA tetraloop, and metal-ion binding to G.A mismatches.". J. Mol. Biol. 295: 1211–1232. doi:10.1006/jmbi.1999.3421. PMID 10653698.
McPherson, Alexander (2002). Introduction to Macromolecular Crystallography. John Wiley & Sons. ISBN 0-471-25122-4.
http://ed.augie.edu/~awaspaas/inorg/hexaammine.pdf
Synthesis
of Hexammine Cobalt (III) Chloride
A. Aspaas & L. Stanley
A. Aspaas & L. Stanley
[
PDF ]
http://genchem.rutgers.edu/Coam.html
Preparation
of HexamineCobalt(III) Chloride
... This is a synthesis of a Coordination Compound.
The synthesis is relatively easy, but the procedure also involves some very interesting science.
The orange brown crystals of Co(NH3)6Cl3 shown above were grown slowly over a period of several days. This slow crystal growth created the large crystals.
The material you will produce in this experiment will be an orange powder. Faster crystallization produces smaller crystals. Because the smaller crystals reflect more light, they appear to be lighter in color.
COORDINATION COMPOUNDS
CoAmmol1.jpg (6912 bytes) Cobalt is a transition metal and as such its d orbitals are "being filled". They are part of the valence shell and are used in bonding.
In Co(NH3)6Cl3, the six empty hybrid d2sp3 orbitals are used to bond with the nonbonding electron pair on ammonia. CoAmmol2.jpg (7122 bytes)
The Co(NH3)6+3 ion is octahedrally surrounded by the six ammonia molecules and gives rise to a compact, relatively spherical ion.




























http://www.sigmaaldrich.com/catalog/product/sigma/h7891?lang=en®ion=US
H7891
Sigma
Hexammine cobalt(III) chloride
Hexammine cobalt(III) chloride
for use in transformations
Synonym: Cobalt hexammine trichloride, Hexaamminecobalt trichloride
CAS Number 10534-89-1
Linear Formula [Co(NH3)6]Cl3
Molecular Weight 267.48
EC Number 234-103-9
MDL number MFCD00036304
PubChem Substance ID 24895812
Properties
Related Categories Biochemicals, Molecular Biology, Molecular Biology Reagents More...
grade for molecular biology
form powder
storage temp. room temp
Suitable for
DNA condensation studies
induction of transitions of DNA from B to A or Z forms
induction of nucleic acid crystal growth
stabilization of tertiary tRNA interactions
preparation of ligation buffer
General description
Hexammine cobalt(III) is considered an analog of fully solvated magnesium, capable of activating some enzymes that requires magnesium. It is useful in DNA condensation studies.1
http://web.williams.edu/wp-etc/chemistry/epeacock/EPL_CHEM_153/153-LABMAN_PDF_05/6-PrepCoboltCompl.pdf
http://www.chemtopics.com/aplab/cocomplex.pdf
Preparation
& Analysis of a Coordination Compound of Cobalt
[
PDF ]
US2008182835
METHOD OF USING A COBALT-AMINE BASED METAL COMPLEX AS AN ANTIVIRAL COMPOUND...
METHOD OF USING A COBALT-AMINE BASED METAL COMPLEX AS AN ANTIVIRAL COMPOUND...
Inventor: DELEHANTY JAMES, et al.
The present invention is generally directed to a method of prophylaxis against viral infection of a cell or subject or a method of treating a subject infected with a virus including administering an antiviral composition having the general Structure III, wherein each of R1, R2, R3, R4, R5 and R6 is the same or different and includes an N-based ligand donor atom selected from the group consisting of ammonia, primary amine or secondary amine, or salt thereof. The present invention is also generally directed to a method of preparing an antiviral agent including providing a cobalt pentammine salt having a non-amine coordination site and mono-substituting the non-amine coordination site with a functional group incorporating a strong coordinator atom to cobalt to form a CoHex structure of Structure III, in which R1 incorporates the functional group having the strong coordinator atom coordinated with the cobalt atom, or a salt thereof.
FIELD OF THE INVENTION
[0001] The present invention is generally directed to the use of a cobalt-amine based metal complex as an antiviral compound and a method for the preparation thereof.
BACKGROUND OF THE INVENTION
[0002] Unlike antibiotics, there are significantly fewer antiviral drugs available. For example, for influenza there are only four: amantadine, rimantadine, oseltamivir (Tamiflu), zanamivir. These four drugs can be divided into two categories, the adamantane derivatives (amantadine, rimantadine) and the neuraminidase inhibitors (oseltamivir, zanamivir), on the basis of their chemical properties and activities against influenza viruses. Adamantanes inhibit influenza propagation by blocking the viral M2 protein ion channel, which prevents fusion of the virus and host-cell membranes and release of viral RNA into the cytoplasm of infected cells. Neuraminidase inhibitors, on the other hand, block the process of release of influenza virus from infected cells and, thereby, inhibit virus transmission to the neighboring cells. Another example of an antiviral drug is cidofovir, which has been found to be effective for treatment against cytomegalovirus, a virus that puts babies and people with HIV at risk. As with antibiotics, these antiviral agents exhibit problems with either resistance or toxicity, thereby limiting options for treatment of viral infection. Thus, there is some urgency in the development of new classes of antiviral drugs.
[0003] Certain metal-ion based antiviral complexes, such as the CTC series of cobalt(III)-based compounds has been shown to possess anti-inflammatory and antiviral activity. Structure I is a general formula for a CTC complex.
[0000]
EMI2.0

[0000] As illustrated, CTC complexes are generally complex chelate structures, with interconnected cobalt coordination sites, leaving only axial coordination cites accessible for activity. In particular, these axial positions contain labile, or easily altered or broken down, axial ligands. For example, in CTC-96 (also known as DOXOVIR, commercially available from Redox Pharm. Corp.), the most effective of the series, the labile axial ligands are 2-methyl-imidazoles.
[0004] Several CTC complexes have moderate in vitro and in vivo activity against herpes simplex virus types 1 and 2, varicella-zoster virus, cytomegalovirus, and Epstein-Barr virus. While the therapeutic activity of the CTC series has been known for several years, the mechanisms and the stage of the virus life cycle at which many of these compounds are effective are only beginning to be understood. For example, several CTC compounds can bind strongly to, and inhibit, Sp1, a DNA binding Zn finger protein, implying that the locus of inhibition may be through the group of metalloproteins that depend on the Zn finger motif. More recently, alternative mechanisms of inhibition have been postulated based on the inhibition of virus-mediated cell fusion.
[0005] Another type of Cobalt(III)-based compounds, based on the macrocyclic cyclen chelator, have been shown to bind very tightly to DNA/RNA, to hydrolyze the phosphodiester bonds of the nucleotides, and to inhibit protein translation in cell-free translation lysates. These cyclen complexes were of particular interest because they have only four out of six possible coordination sites on the Co(III) ion bound, leaving two cis-equatorial positions open for hydrolysis by the complex. For example, structure II below is a CoCyclen (or 1,4,7,10 tetraazacyclododecane) molecule illustrating the two cis-equatorial positions open for hydrolysis by the complex.
[0000]
EMI3.0

[0000] These cyclen complexes have been used for mechanistic studies of phosphodiester cleavage for both its efficient hydrolysis rates and kinetic inertness. That is, the complexes promote fast hydrolysis of the phosphodiester bond but are kinetically "slow" in letting go of the hydrolyzed phosphate. The kinetic inertness of the Co(III) may be overcome (i.e., at elevated temperatures) but, for gene-silencing, this property has the added advantage of disruption of gene function, particularly disruption of protein translation. However, even potent hydrolytic catalysts take much longer to degrade nucleic acids than enzymes.
[0006] Other antiviral approaches include "antisense" technology which includes the synthesis of oligodeoxynucleotides (ODN's) that bind to their complementary sequences on the mRNA, thereby blocking translation and inhibiting the production of the target protein. However, binding to the RNA is often not stable. Ribosomes can effectively compete with the oligonucleotides to bind with the RNA and consequently ensure continuous production of the target protein. The competitive edge of ribosomes is facilitated by their intrinsic "unwindase" activity, which allows them to read tangled messages, thus overcoming the effect of the antisense ODN.
[0007] One solution to this "unwindase" activity relies on the ability of the antisense oligonucleotides to employ the enzyme RNase H. RNase H recognizes the DNA:RNA duplex and acts as a DNA dependent RNA hydrolysis catalyst. This degrades the RNA leaving the antisense ODN free to bind to other mRNA molecules, where the RNA hydrolysis cycle is repeated. However, RNase H itself poses a drawback because DNA:RNA duplexes as short as 5 base pairs may be cleaved by RNase H, leading to poor specificity of the antisense ODN's.
[0008] The lack of stability of the antisense ODN is also a drawback for anitsense technology. Conventional ODN's are prone to nuclease degradation inside the cell. Modified ODN's have been synthesized with different backbones to improve their stability but they either fail to recruit RNase H or exhibit non-sequence specificity. A classical example of synthesized stable antisense oligos is S-DNA (phosphorothioate) oligos that possess enhanced stability but exhibit low sequence specificity because of their weak binding. In addition, they promiscuously bind cellular protein molecules thus reducing their effectiveness as antisense agents.
[0009] Alternatively, peptide nucleic acids, in which the backbone consists of N-(2-aminoethyl)-glycine units linked by peptide bonds instead of a sugar and phosphate groups, have gained considerable importance by virtue of their being nuclease resistant and their ability to form stable complexes with nucleic acids. More recently, morpholino oligos have also been reported to afford high efficacy, specificity, and resistance to nucleases. However, they fail to recruit RNase H relying only on the binding specificity of the oligo.
[0010] Another approach includes RNA interference (RNAi) therapeutics. The major difficulties with RNAi technology lie with the lack of a reliable method of targeting and delivery of double-stranded RNA (dsRNA). Issues, such as activation of the cells' antiviral defense mechanisms by long strand dsRNA, identification of a viable target region, and uncontrolled global changes in gene expression of cells when dsRNA strands are introduced into the cells, complicate any potential RNAi applications using short, interfering RNA (siRNA). While activation of cellular antiviral mechanisms may be desirable for antiviral applications, many viruses can shut down the defenses once entry is achieved. This natural viral defense strategy can be an impediment for siRNA therapeutics.
[0011] Since only short RNA strands can be used for siRNA, specificity of the target also can be an issue. Additionally, determining which sequences will work for siRNA still remains a problem to be solved for each target gene. Therefore, multiple regions are generally screened for each target. The screening requires using either synthetic RNAs, which are expensive, or with cloned DNA sequences, which is time consuming. Furthermore, the activity of the siRNA is not well understood at present-not all sequences will work and it is still a hit-and-miss proposition to find an active sequence.
BRIEF SUMMARY OF THE INVENTION
[0012] The CoHex complex of the present invention exhibits a potent inhibition of virus replication without possessing free cobalt(III) coordination sites and without hydrolyzing oligonucleotides.
[0013] An embodiment of the present invention includes a method of prophylaxis against viral infection of a cell including administering to a cell an antiviral composition having the structure of Structure III,
[0000]
EMI4.0

[0000] wherein each of R1, R2, R3, R4, R5 and R6 is the same or different and includes a N-based ligand donor atom selected from the group consisting of ammonia, primary amine or secondary amine, or salt thereof, so as to thereby provide prophylaxis against infection of the cell by a virus.
[0014] An alternative embodiment of the present invention includes a method of treating a subject infected with a virus including administering to the subject an antiviral composition comprising an antiviral effective amount of a compound having the structure of Structure III, above, wherein each of R1, R2, R3, R4, R5 and R6 is the same or different and includes a N-based ligand donor atom selected from the group consisting of ammonia, primary amine or secondary amine, or salt thereof, so as to thereby treat the subject infected by the virus.
[0015] An alternative embodiment of the present invention includes a method of prophylaxis against viral infection of a subject including administering to a cell an antiviral composition having the structure of Structure III, above, wherein each of R1, R2, R3, R4, R5 and R6 is the same or different and includes a N-based ligand donor atom selected from the group consisting of ammonia, primary amine or secondary amine, or salt thereof, so as to thereby provide prophylaxis against infection of the subject by the virus.
[0016] An alternative embodiment of the present invention includes a method of preparing an antiviral agent including providing a cobalt pentammine salt having a non-ammine coordination site and mono-substituting the non-ammine coordination site with a functional group incorporating a strong coordinator atom to cobalt to form a CoHex structure of Structure III, above, where each of R2, R3, R4, R5 and R6 is the same or different and includes a N-based ligand donor atom selected from the group consisting of ammonia, primary amine or secondary amine and R1 incorporates the functional group having the strong coordinator atom coordinated with the cobalt atom, or a salt thereof.
[0017] The foregoing and other features and advantages of the present invention will be apparent from the following, more particular description of a preferred embodiment of the invention, as illustrated in the accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
[0018] FIG. 1 is a graph plotting percentage of cell viability as a function of the concentration of a CoHex complex of the present invention as compared to a conventional antiviral compound.
[0019] FIG. 2 is a western blot exhibiting inhibition of luciferase protein translation in vitro by a CoHex complex of the present invention.
[0020] FIG. 3A is a graph plotting virus plaque counts in log plaque formation units as a function of the concentration of a CoHex complex of the present invention. FIG. 3B is a graph plotting a degree of inhibition of plaque formation as a function of concentration of the CoHex complex of the present invention based on the results illustrated in FIG. 3A. FIG. 3C is a graph plotting the fraction of cell survival of BHK cells during viral infection as a function of the concentration of a CoHex complex of the present invention relative to virus infected cells that received no CoHex complex treatment.
[0021] FIG. 4 is a graph plotting virus plaque counts in log plaque formation units per percent cell viability as a function of the concentration of a CoHex complex of the present invention.
[0022] FIG. 5 is a graph plotting percent cell viability as a function of the concentration of a CoHex complex of the present invention.
[0023] FIG. 6A is a micrograph illustrating healthy BHK cells in the absence of a virus and a CoHex complex of the present invention. FIG. 6B is a micrograph of BHK cells infected with a virus but no CoHex complex of the present invention. FIGS. 6C-6H are micrographs of BHK cells infected with a virus and concentrations of 0.15 mM, 0.3 mM, 0.6 mM, 1.2 mM 2.5 mM and 5 mM, respectively, of a CoHex complex of the present invention.
[0024] FIG. 7A is a raw data plot of simultaneous analysis of Sindbis virus protein synthesis during SV infection in the absence of a CoHex complex of the present invention. FIG. 7B is a raw data plot of simultaneous analysis of Sindbis virus protein synthesis during Sindbis virus infection in the presence of a CoHex complex of the present invention. FIG. 7C is representative flow cytometry data for the viability of Sindbis virus-infected BHK cells in the absence of a CoHex complex of the present invention. FIG. 7D is representative flow cytometry data for the viability of Sindbis virus-infected BHK cells in the presence of a CoHex complex of the present invention.
[0025] FIG. 8A is a chart illustrating the dose-dependent increase in cell viability in Sindbis virus-infected cells (solid square) as a function of the concentration of a CoHex complex of the present invention relative to uninfected cells (open circle). FIG. 8B is a chart illustrating the dose-dependent inhibition of EGFP expression in Sindbis virus-infected cells (solid square) as a function of the concentration of a CoHex complex of the present invention compared to uninfected cells (open circle).
[0026] FIG. 9 is a chart illustrating the dose-dependent increase in cell viability in Sindbis virus-infected cells as a function of the concentration of a CoHex complex of the present invention when the CoHex complex is administered at various times.
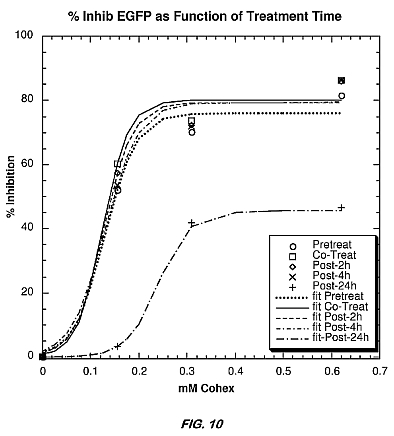
[0027] FIG. 10 is a chart illustrating the dose-dependent inhibition of EGFP expression in Sindbis virus-infected cells as a function of the concentration of a CoHex complex of the present invention when the CoHex complex is administered at various times.
[0028] FIG. 11 is a graph illustrating the dose-dependent increase in cell viability in Adenovirus-infected cells (right bar) as a function of the concentration of a CoHex complex of the present invention relative to uninfected cells (right bar).
[0029] FIG. 12 is graph comparing the increase in cell viability in Sindbis-infected cells (left bar) and Adenovirus-infected cells (right bar) normalized to respective infected control cells as a function of the concentration of a CoHex complex of the present invention.
[0030] FIG. 13 is graph comparing the increase in cell viability in Sindbis-infected cells (left bar) and Adenovirus-infected cells (right bar) normalized to respective uninfected control cells as a function of the concentration of a CoHex complex of the present invention.
[0031] FIG. 14 is a schematic illustrating a method for synthesizing an example of a functionalized CoHex complex of the present invention.










DETAILED DESCRIPTION OF THE INVENTION
[0032] Preferred embodiments of the present invention are now described with reference to the Figures. While specific details of the preferred embodiments are discussed, it should be understood that this is done for illustrative purposes only. A person skilled in the relevant art will recognize that other configurations and arrangements can be used without departing from the spirit and scope of the invention. It will also be apparent to a person skilled in the relevant art that this invention can also be employed in other applications.
[0033] The identification of new antiviral drugs is a challenging endeavor that is often built upon the balance between potent antiviral activity and minimal toxicity against host tissues. The present invention is generally directed to positively charged cobalt(III) complexes or salts thereof in octahedral ligand environments in which the ligands have strong coordinator atoms to Cobalt (i.e., CoHex complex), for example, where the ligand donor atoms are nitrogen (N)-based and/or interact either electrostatically or via hydrogen bonding, particularly ammonia, primary amines or secondary amines. The present invention may be demonstrated by the general formula of structure III, wherein R1 through R6 is the same or different and includes N-based ligand donor atoms of ammonia, primary amines or secondary amines.
[0000]
EMI5.0
[0034] A few examples of CoHex complexes of the present invention are found below. Structure IV has all six positions coordinated with a nitrogen atom of ammonia ligands, i.e., Co(NH3)6. Structure V is similar to the CoCyclen of Structure II, however, with all six positions coordinated with a N atom and with ammonia ligands at the cis-equatorial positions, rather than having these positions open for hydrolysis. In Structure V, some of the N-based ligand donors are chelating ligands. The CoHex complexes of the present invention are highly positively charged compounds. For example, the net charge for the complex of Structure IV of 3<+> at neutral pH. Preferably, they are prepared so as to be administered to a cell or subject as an acceptable salt.
[0000]
EMI6.0
[0035] Neither UV-VIS nor NMR shows evidence that Co(NH3)6 (i.e., Structure IV) has an ability to exchange its ammonia groups with free histidine, even at a ratio 100:1 histidine to Co(NH3)6 (results not shown). In other words, Co(NH3)6 does not appear to have labile ligands as does the conventional CTC complexes. Surprisingly though, the CoHex complexes of the present invention provide potent antiviral activity and relatively minimal toxicity against host tissues.
Preparation of Cobalt Complexes
EXAMPLE 1
[0036] While Co(NH3)6 is available commercially, the synthesis of its chlorine salt is fairly straight forward and using easily available reagents, for example using air to oxidize Co(II) to Co(III) according to the following formula.
[0000]
CoCl2+4NH4Cl+20NH3+O2->4[Co(NH3)6]Cl3+2H2O
[0037] The example discussed below is for Co(NH3)6, but one skilled in the art can appreciate that other CoHex complexes of the present invention may be commercially available or synthesized using similar methods. To prepare this Co(NH3)6 example, or more specifically the chlorine salt thereof, 9.6 g of CoCl2.6H2O (0.06 mol) and 6.4 g of NH4Cl (0.12 mol) were added to 40 mL of water in a 250 mL Erlenmeyer flask with a side arm. The mixture was shaken until most of the salts are dissolved. Then, 1 g of fresh activated decolorizing charcoal and 20 mL concentrated ammonia were added. The flask was next connected to the aspirator or vacuum line and air drawn through the mixture until the red solution became yellowish brown (about 2-3 hours). The air inlet tube was of fairly large bore (about 10 mm) to prevent clogging with the precipitated Co(NH3)6<3+> salt.
[0038] The crystals and charcoal were filtered on a Büichner funnel and then a solution of 6 mL of concentrated hydrochloric acid (HCl) in 75 mL of water was added, the mixture heated on a hot plate to effect complete solution and filtered while hot. Crystallization of the CoHex chloride was done by cooling to 0[deg.] C. and then slowly adding 15 mL of concentrated HCl. The crystals were washed with 60% and then with 95% ethanol and dried at 80-100[deg.] C.
Cytotoxicity of CoHex
EXAMPLE 2
[0039] The cytotoxicity of Co(NH3)6 (Structure IV) was assessed by monitoring its ability to inhibit proliferation of baby hamster kidney (BHK) cells. BHK cells were cultured as exponentially growing subconfluent monolayers in complete growth medium, particularly Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 1% (v/v) antibiotic/antimycotic agent and 10% (v/v) heat inactivated fetal bovine serum (FBS). Cells were grown in either T25 or T75 flasks (Acton, Mass.) and incubated at 37[deg.] C. under 5% CO2 atmosphere. A subculture was performed every 3-4 days.
[0040] The in vitro toxicity of Co(NH3)6 was determined using the CellTiter96(R) Proliferation Assay (Promega, Madison, Wis.). This quantitative calorimetric assay is based upon the enzymatic conversion of a tetrazolium salt substrate into a blue formazan product with a maximum absorbance at 570 nm. When incubated with this substrate, only viable cells convert the substrate into a blue product while nonviable cells do not. At the assay endpoint, the absorbance at 570 nm is directly proportional to the number of viable cells. To assess compound toxicity, BHK cells were seeded into the wells of a 96-well tissue culture microtiter plate (2*10<4 > cells/well) and cultured overnight at 37[deg.] C. in a humidified atmosphere containing 5% CO2. The next day, Co(NH3)6 was diluted into tissue culture media (at final concentrations ranging from 0 to 5 mM) and incubated with the cells for 72 hrs. Triplicate wells were included for each concentration. To control for any contaminating absorbance due to the compounds, control wells containing no cells were included at each compound concentration ("no cell" wells). At the end of the 72 hour culture period, 15 [mu]L of tetrazolium substrate was added to each well and the plate was returned to the incubator for 4 hours to allow viable cells to convert the substrate into the formazan product. At the end of the 4 hour incubation period, 100 [mu]L of solubilization solution was added to each well and the plate was subsequently incubated overnight to completely solubilize the contents of each well to a homogeneous color. The absorbance within each of the "no cell" wells was subtracted from the absorbance measured in the corresponding wells containing cells for each concentration of Co(NH3)6.
[0041] FIG. 1 illustrates cell viability with increasing concentrations of Co(NH3)6 as compared with a conventional cis-Platin complex. cis-Platin is an FDA approved and commercially available medicament used as an anti-cancer agent. As shown in FIG. 1, Co(NH3)6 elicited a dose-dependent inhibition of cellular proliferation in which concentrations below 2.5 mM were only mildly toxic, with no significant toxicity at concentrations below 0.3 mM. At concentrations above 2.5 mM, however, Co(NH3)6 increasingly inhibited cellular proliferation. At 2.5 mM Co(NH3)6 mediated about 80% cell viability, while achieving a maximum toxicity at 5 mM, the highest concentration tested. Also as demonstrated by FIG. 1, Co(NH3)6 is much less toxic than that of the FDA approved anti-cancer agent, cis-Platin. Lower toxicity makes a CoHex complex of the present invention a good source as a therapeutic.
[0042] From FIG. 1, a 50% cytotoxicity concentration (CC50) for CoHex, i.e., the concentration at which approximately 50% cell death occurs due to the administration of CoHex, was determined to be about 3.2 mM.
Activity of the CoHex Complex in Preventing Translation
[0043] The highly positively charged CoHex complex has built-in "stickiness" for highly-dense, negatively charged polyelectrolytes, such as DNA/RNA, such that it is tightly bound and does not easily release the nucleotides. This tight binding encourages blocking of translation and is similar in concept to "antisense" and RNAi technologies, but does not include some of the limitations inherent to the other approaches. In particular, because the CoHex complex itself binds strongly to nucleotides via static charges and hydrogen bonding, the tight binding overcomes any attempts by the cellular machinery to "unwind" the hybridization site.
[0044] CoHex complexes have been previously studied for its ability to "condense" double stranded DNA into toroidal-like superstructures under low salt conditions. Nonetheless, without the hydrolytic open binding sites, the CoHex complex of the present invention is not a potent gene-silencing agent. Surprisingly, however, a hydrolytic function is not necessary for suppressing protein translation. The CoHex complex has the characteristic of binding very tightly to nucleotides and has demonstrated that such a tight ionic-binding property is sufficient for the inhibition of protein expression and thus antiviral activities.
[0045] CoHex complexes, with their high positively charged density, are ideal for binding nucleotides and other polyelectrolytes. Although Cobalt(III) is not stable, by itself, in aqueous solutions, it can be stabilized by coordinating with donor atoms (preferably N-based ligands and chelators) that make strong contributions to the ligand field. While it is not clear why CoHex complexes are particularly useful for antiviral activity, the kinetic inertness, i.e., the inability of the close and tightly bound N atoms to easily disassociate from the Cobalt atom, may play a role in the particularly good antiviral activity of CoHex complexes. The role of close and tightly bound N atoms in CoHex's antiviral activity is particularly surprising, since it is the labile axial ligands, as in Structure II, and/or the availability of coordinating positions, as in Structure I, that is the apparent mode of antiviral activity of the conventional antiviral compounds.
[0046] Suppressing protein translation is to inhibit the ability of either DNA or RNA to act as templates for transcription and translation, respectively. This is done by blocking the site of transcription/translation or by hydrolyzing the DNA/RNA template. The CoHex complex of the present invention does not hydrolyze nucleotides, but nonetheless demonstrates potent protein-inhibition and dose-dependent antiviral properties by blocking the site of transcription/translation. For purposes of gene knock-out or antiviral applications, it is not necessary to fully digest a DNA or RNA strand to deactivate an organism or a gene, as long as the critical sites remain permanently blocked. The strong affinity of the CoHex complexes of the present invention for the bases and the phosphate groups of the nucleotides, therefore, is a critical advantage to the general goal of deactivating genetic materials. As such, the CoHex complex of the present invention can function as non-specific gene silencing compounds or general antiviral applications.
EXAMPLE 3
[0047] CoHex is capable of preventing translation of a messenger RNA (mRNA) in vitro. The ability Co(NH3)6 to inhibit translation of an mRNA luciferase template was assessed using the Rabbit Reticulocyte Lysate Translation System (Promega) according to the manufacturer's instructions. In the example discussed below, Co(NH3)6 in various concentrations was incubated with the mRNA for 10 minutes before the addition of the mRNA template to the translation lysate. Translated luciferase protein was run on a 10% SDSPAGE gel for 45 min at 130 V and detected by Western blot on a PVDF membrane. Proteins were detected using a streptavidinalkaline phosphatase conjugate and Western Blue substrate according to the instructions in the Transcend Non-Radioactive Translation Detection System (Promega). The results are provided in FIG. 2. In FIG. 2, Lane 1 represents the incubation of 0.01 mM Co(NH3)6. Lane 2 represents the incubation of 0.02 mM Co(NH3)6. Lane 3 represents the incubation of 0.05 mM Co(NH3)6. Lane 4 represents the incubation of 0.1 mM Co(NH3)6. Lane 5 represents the incubation of 0.2 mM Co(NH3)6. Lane 6 is a positive control in which no Co(NH3)6 is added. Lane 7 is a negative control in which no translation occurs. The lanes marked M are molecular weight marker lanes. As shown in FIG. 2, Co(NH3)6 blocks the RNA translation of luciferase protein at around concentrations of about 0.1 to 0.2 mM Co(NH3)6. Further, when compared to the same test using a conventional CoCyclen structure similar to Structure II rather than Co(NH3)6 (data not provided), Co(NH3)6 inhibits protein translation about an order of magnitude (or about 10 times) more than the conventional CoCyclen structure.
Antiviral Activity of the CoHex Complexes-Sindbis Virus
EXAMPLE 4
[0048] In order to provide an example of antiviral activity, an in vitro model of a positive single stranded RNA (+ssRNA) virus, recombinant Sindbis virus (633-EGFP strain), infection was used. In this construct, the gene encoding enhanced green fluorescent protein (EGFP) is placed under the control of the identical promoter sequence found upstream of the viral structural proteins. Thus, upon replication, virally-infected cells produce soluble EGFP at levels that are proportional to the amount of virus. The virus seed stock was expanded on BHK cells under serum free conditions.
[0049] BHK cells were grown in T-150 tissue culture flasks (Acton, Mass.) until 90% confluent. Subsequently, the cells were washed twice with Dulbecco's Phosphate Buffered Saline (PBS) and infected with the recombinant Sindbis virus at a multiplicity of infection of five plaque forming units (pfu) per cell in 2 ml of virus production serum free medium (VP-SFM). After 1 hour of incubation at 37[deg.] C., with rocking of flasks every ten minutes, an additional 13 ml of VP-SFM media was added to bring the volume to 15 ml. The next day, the cells were observed under light microscopy for signs of cytopathic effects and the presence of EGFP expression in infected cells was confirmed via fluorescence microscopy. Cellular debris was centrifuged and the supernatant was collected, aliquoted, and stored at -80[deg.] C. The majority of cells (greater than 70%) were positive for EGFP fluorescence.
Plaque Formation Assay
EXAMPLE 5
[0050] BHK cells (1*10<5 > cells) were seeded to the wells of a 24-well plate and grown overnight to a confluent monolayer. The next day, the cells were infected with the Sindbis virus at a ratio of about five plaque forming units (pfu) per cell in DMEM containing 2% FBS in the absence or the presence of increasing concentrations of Co(NH3)6. Infection was allowed to proceed for 48 hours, at which time the supernatants were collected and stored at -80[deg.] C. until further use.
[0051] For quantization of viral infection and replication, the sampled supernatants were thawed and serially diluted in 1% FBS DMEM. The diluted virus was used to infect monolayers of BHK cells grown in 6-well clustered plates. An aliquot (200 [mu]L) of virus from each selected dilution was incubated with BHK monolayers for 1 hr at 37[deg.] C. with rocking of plates every 10 min to prevent drying of the monolayers. After 1 hr, the cells were overlayed with warm melted 1.2% Bacto agar in water mixed with an equal volume of 2* Minimum Essential Medium (MEM). The overlay agar was allowed to solidify at room temperature. Then the plates were incubated at 37[deg.] C. for 48 hr to allow plaque formation. Two days later, the cells were stained with neutral red. The exact staining solution per well was 0.5 ml of 2*MEM, 0.5 ml distilled water, 0.11 ml of 0.33% neutral red solution (i.e., 3.3 g/L in PBS). After 1-2 hrs of staining, the remaining staining solution was aspirated off and plaques were observed as clear foci within the cell monolayer. Via this method the amount of virus in the cells (i.e., plaque counts) can be ascertained by visual counting the number of plaque formation units (pfu) per well and multiplying by the dilution factor to determine the concentration of pfu in the original supernatant.
[0052] FIG. 3A is a graph illustrating a dose dependent decrease in the plaque counts, in log pfu, for each increasing concentration of Co(NH3)6 used at 48 hours. A maximal inhibition of virus replication (i.e., about a 2.5 log decrease in pfu over the control with no Co(NH3)6) was seen at about 2.5 mM Co(NH3)6. FIG. 3A shows that Co(NH3)6 decreases plaque formation by almost 2 to 3 log units. These same general trends were observed in at 24-hours post infection (data not provided).
[0053] In FIG. 3B, the degree of inhibition of plaque formation units measured in FIG. 3A is plotted as a function of Co(NH3)6 concentration. The 50% inhibitory concentration (IC50) for Co(NH3)6 inhibition of Sindbis virus plaque formation was determined to be about 0.10+-0.04 mM.
[0054] The reductions in viral pfu of these magnitudes in vitro suggest in vivo significance. Previous studies have shown that a one log unit decrease in viral load in murine brains correlates with survival of infected animals. However, FIG. 3A does not take into consideration a significant decrease in cell proliferation due to increased cytotoxicity of Co(NH3)6 at concentrations over 2.5 mM or even a slight decrease in cell proliferation at concentrations less than 2.5 mM, as illustrated in FIG. 1. Nor does FIG. 3A account for the decrease in cell proliferation due to the virus replication itself.
EXAMPLE 6
[0055] Since the use of a CoHex complex of the present invention reduces but does not eliminate cell death due to the viral activity, the viability of BHK cells during Sindbis virus infection must also be determined as a function of the concentration of the CoHex complex. Because live cell counts will decrease with increasing CoHex complex due to cytotoxicity of the cells and virus replication, but increase due to the anti-viral protection of CoHex, the plaque counts may have decreased due to the fact that there were less live cells available to count. FIG. 3C is a graph depicting the fraction of cell survival of BHK cells during Sindbis viral infection as a function of Co(NH3)6 concentration at 48 hours after infection, in which cell survival is expressed as a "fraction cell survival" relative to Sindbis-infected cells that received no Co(NH3)6 treatments. The data of FIG. 3C was obtained using the CellTiter96 cell proliferation assay described above for the CoHex cytotoxicity data (i.e., with respect to FIG. 1) in which the cells were further infected with 5 plaque forming units per cell of Sindbis virus in DMEM containing 2% FBS in the absence or presence of various concentrations of Co(NH3)6, then assayed periodically.
[0056] Therefore, the fraction cell survival data of FIG. 3C was combined with the log pfu data of FIG. 3A to generate a ratio, Log(PFU) per percent cell survival, which although not a physical measure, is a general model of viruses in surviving cells. FIG. 4 shows this ratio for the various concentrations of Co(NH3)6 used during the infection of the BHK cells. As illustrated in FIG. 4, there has been a significant decrease in viral infection for the surviving cells with an increase in concentration of Co(NH3)6.
Cell Viability Study
EXAMPLE 7
[0057] Additional cell viability studies were done using a combined assessment of cellular morphology via light microscopy, or visual inspection of cell viability, and plasma membrane integrity measurements using a Trypan blue dye-exclusion assay. Specifically, BHK cells were seeded to the wells of a 96-well plate (2*10<4 > cells/well) and cultured overnight in complete growth medium. The cells were then incubated with increasing concentrations of Co(NH3)6 for 6 hours prior to the addition of Sindbis virus (1*10<5 > pfu/well). After a 48 hour infection period, the cells were resuspended and an aliquot of cells (10 [mu]L) was mixed with 90 [mu]L 0.2% trypan blue. The number of viable cells was determined by counting with a hemocytometer.
[0058] FIG. 5 illustrates the results of this viable cell count as a function of Co(NH3)6 concentration. FIG. 5 reflects that after 48 hours, cell membrane viability was only about 65% for the uninfected control and was down to 30% with virus but no Co(NH3)6 added. The initial addition of 0.15 mM Co(NH3)6 further decreased viability, but higher concentrations revived cell viability up to 80% at 1.2 mM, beyond which point, survivability dropped drastically. Due to the large scatter in the range of data values, there is not a significant difference between the 65% uninfected and the 80% 1.2 mM Co(NH3)6 values.
[0059] Also, a corresponding set of wells was identically prepared exclusively for observation using Trypan blue staining and light microscopy. FIGS. 6A-6H are light microscopy images showing cell morphology at various concentrations of Co(NH3)6 at 48 hours post infection following 6 hours of pretreatment with Co(NH3)6. Specifically, FIG. 6A shows healthy BHK cells in the absence of both Sindbis virus and Co(NH3)6. FIG. 6B shows BHK cells infected with the Sindbis virus but no Co(NH3)6. FIG. 6C shows BHK cells infected with the Sindbis virus and 0.15 mM Co(NH3)6. FIG. 6D shows BHK cells infected with the Sindbis virus and 0.3 mM Co(NH3)6. FIG. 6E shows BHK cells infected with the Sindbis virus and 0.6 mM Co(NH3)6. FIG. 6F shows BHK cells infected with the Sindbis virus and 1.2 mM Co(NH3)6. FIG. 6G shows BHK cells infected with the Sindbis virus and 2.5 mM Co(NH3)6. FIG. 6H shows BHK cells infected with the Sindbis virus and 5 mM Co(NH3)6. Viable cells remain attached to the tissue culture substrate and have an elongated, epithelial cell-like appearance. The arrows in FIGS. 6E-6G indicate examples of cells that have retained an elongated and adherent morphology. Non-viable cells are detached from the tissue culture substrate and are rounded. FIGS. 6B-6H illustrate that pronounced cell death is apparent in the presence of no Co(NH3)6 (FIG. 6B) and at lower concentrations of Co(NH3)6 (FIGS. 6C and 6D). However, viable cells appeared more pronounced at concentrations of 0.6 and 1.2 mM Co(NH3)6 (FIGS. 6E and 6F, respectively), again decreasing at 2.5 mM and 5.0 mM Co(NH3)6 (FIGS. 6G and 6H, respectively).
[0060] It is interesting to note that both the light microscopy results (FIGS. 6A-6H) and the cell viability count (FIG. 5) show similar results, and both suggest indicated that 1.2 mM concentration of Co(NH3)6 provides an optimal protection against Sindbis virus.
Flow Cytometry-EGFP Reporter Assay
EXAMPLE 8
[0061] Additional studies of antiviral activity were quantified by flow cytometry, a more sensitive fluorometric assay, to further validate the plaque assay and cell viability results. In these assays, Sindbis virus replication was assessed by monitoring viral structural protein synthesis using a recombinant Sindbis virus construct. In this construct, the gene encoding enhanced green fluorescent protein (EGFP) was placed under the control of the same promoter sequence that drives transcription of viral structural proteins. Thus, upon Sindbis virus replication, virally infected cells produce intracellular EGFP at levels that are proportional to the level of viral structural proteins.
[0062] BHK cells (1*10<5> ) were plated into the wells of 24-well plates in 1 mL of complete media and incubated with increasing concentrations of Co(NH3)6 for 6 hours prior to infection with Sindbis virus at a multiplicity of infection (moi) of 5 pfu per cell. The infection was allowed to proceed for 48 hours. In preparation for analysis by flow cytometry, cells from each well were removed and pelleted, washed with PBS, and resuspended in 500 [mu]L of PBS. One half of each sample was analyzed directly to determine the percentage of EGFP-positive cells (percent infected cells). The other half of each sample was incubated with 5 [mu]M propidium iodide for 1 minute prior to the determination of the percentage of non-viable cells. In all cases, analysis was performed on 1*10<4 > cells.
[0063] BHK cells were pretreated with Co(NH3)6 for 6 hours prior to infection with Sindbis virus. After 48 hours post infection, both the percentage of Sindbis-infected cells (as evidenced by EGFP fluorescence) and the percentage of viable cells (as determined by the exclusion of propidium iodide (PI)) were determined as a function of Co(NH3)6 concentrations.
[0064] FIGS. 7A and 7B are representative raw data plots of simultaneous analysis of SV protein synthesis during SV infection in the absence of Co(NH3)6 (FIG. 7A) and in the presence of 0.15 mM Co(NH3)6 (FIG. 7B). The area marked 'R1' corresponds to EGFP-positive (SV-infected) cells. In the presence of Co(NH3)6, a distinct decrease in the percentage of EGFP-positive (i.e., Sindbis-infected) cells was apparent. FIGS. 7C and 7D are representative flow cytometry data for the viability of SV-infected BHK cells in the absence of Co(NH3)6 (FIG. 7C) and in the presence of 0.15 mM Co(NH3)6 (FIG. 7D). The area marked 'R2' corresponds to propidium iodide (PI)-positive (non-viable) cells. In all cases, the x-axis corresponds to forward scatter (FSC). Quantification of cell viability revealed that during Sindbis infection, relative to infected cells that were not treated with Co(NH3)6 (FIG. 7C, region R2), cells treated with 0.15 mM Co(NH3)6 exhibited a significantly lower percentage of PI-positive (non-viable) cells (FIG. 7D, region R2).
[0065] Data demonstrating the dose-dependent nature of the Co(NH3)6-mediated increase in cell viability and inhibition of EGFP expression (i.e. inhibition of SV replication) as a function of Co(NH3)6 concentrations are presented in FIGS. 8A and 8B, respectively. In FIG. 8A, data are shown for the dose-dependent increase in cell viability in SV-infected cells (solid square) as a function of Co(NH3)6 concentrations relative to control, uninfected cells (open circle). In FIG. 8B, data are shown for the dose-dependent inhibition of EGFP expression in SV-infected cells (solid square) as a function of Co(NH3)6 concentration compared to control, uninfected cells (open circle). In FIGS. 8A and 8B, asterisks on the plots for the SV-infected cells correspond to levels of significance relative to the untreated infected control (as determined by a two-tailed Student's t-test): *(p<0.05); **(p<0.005); ***(p<0.001). When the data in FIG. 8B were fitted to a standard dose-response curve using a one-site dose response logistic curve fit function, an IC50 of 0.13+-0.04 mM was determined. This value agrees well with the IC50 value determined by the plaque assay above, (0.10+-0.04 mM).
[0066] A selectivity ratio may be calculated by comparing CC50 to IC50: CC50/IC50=about 25. This selectivity ratio measures the selectivity of Co(NH3)6 for Sindbis virus over BHK cells. Thus, Co(NH3)6 is about 25 times more selective for the virus than for the host cell.
Timing of CoHex Administration on Antiviral Activity
[0067] The flow cytometry assay discussed above was repeated with various concentrations of Co(NH3)6 administered as a pretreatment to Sindbis viral infection, as co-infection and at 2, 4 and 24 hours post-infection. FIG. 9 is a chart illustrating survival rates for BHK cells for the various concentrations of Co(NH3)6 for each administration timing. As seen in FIG. 9, timing of the Co(NH3)6 treatment has very little effect on the survivability of the cells. As such, Co(NH3)6 is an effective antiviral agent whether administered prior to viral infection, contemporaneously with viral infection, or after viral infection. However, it appears that early treatment of Co(NH3)6 generally increases the percentage of cell survival.
[0068] Similarly, FIG. 10 is a chart illustrating the effects of timing of Co(NH3)6 administration on EGFP expression by Sindbis virus in BHK cells. The chart measures percent inhibition of EGFP expression as a function of Co(NH3)6 concentration. As illustrated, Co(NH3)6 is equally effective when administered as a pretreatment, co-infection or early post-treatment. Even at 24 hours post-treatment, inhibition of EGFP expression is apparent, but not apparently as significantly as with earlier treatment.
Antiviral Activity of the CoHex Complexes-Adenovirus
EXAMPLE 10
[0069] Another example of antiviral activity is an in vitro model of a double stranded DNA (dsDNA) virus infection, Adenovirus. As with Sindbis virus, effective antiviral activity was demonstrated with Adenovirus in A549 cells. The same flow cytometry procedure discussed above for Sindbis viral infection in BHK cells was used for Adenovirus viral infection in A549 cells. In other words, the cells were incubated with 5 [mu]M propidium iodide for 1 minute prior to the determination of the percentage of non-viable cells. The percentage of viable cells (as determined by the exclusion of propidium iodide (PI)) were determined as a function of Co(NH3)6 concentration.
[0070] FIG. 11 illustrates survival rates for A549 cells uninfected and infected with Adenovirus assayed at 48 hours post infection with increasing concentration of Co(NH3)6 administered as a 6 hour pretreatment. As such, the presence of increasing concentration of Co(NH3)6, up to 0.0621 mM Co(NH3)6, demonstrates an increase in survival rate of A549 cells. A continued increase in survivability of A549 cells was found at a concentration of 1.25 mM Co(NH3)6, despite the fact that the toxicity began to take effect at this high concentration as evidenced by the reduction in cell survivability of the uninfected cells at 1.25 mM Co(NH3)6 (FIG. 1).
[0071] As illustrated in FIGS. 12 and 13, the normalized survivability rates of Co(NH3)6 treated cells is similar whether the viral infections are Adenovirus infections or Sindbis virus infections. In both cases, Co(NH3)6 increases cell survivability of virally infected cells.
Sequence Specific CoHex System
[0072] To be specific for a particular protein, the CoHex system of the present invention will need to possess an ability to recognize sequences of nucleotides that code for that protein. Thus, a full sequence-recognizing, artificial nuclease can be thought to consist of a very tight binding component and a sequence-recognition nucleotides component.
[0073] Thus, the present invention also includes the addition of nucleotide-binding groups, such as hybridization-capable oligonucleotides, or other nucleotide-binding groups (e.g., peptide nucleic acids, modified nucleic acids, triple-helix formers, or nucleotide-binding proteins and drugs), which are attached to functionalized CoHex complexes. The resulting system will be able to identify and bind to specific genes in order to silence transcription/translation of specific genes and/or attack specific viruses. Further, the attachment of a hybridizing oligonucleotide sequence to a CoHex complex may enable the CoHex complex to recognize nucleotide sequences that are longer than those typically used for RNAi applications. Therefore, CoHex complexes can potentially be used for both non-sequence-specific and sequence-specific inhibition of transcription/translation of viral proteins, as well as for other molecular biology tasks. The molecular recognition potential of the chemical nucleases, furthermore, means that these chemical nucleases can potentially be used in a novel approach to interdict whole classes of organisms (e.g., the filoviruses).
EXAMPLE 10
[0074] Although a variety of nucleotide-binding groups that identify and bind to a particular sequence may be added to the CoHex complexes of the present invention, one method for doing so includes starting with a copentammine salt, such as a chloropentammine cobalt(III) salt and mono-substituting the non-ammine coordination site with a functional group, which can bind to the CoHex complex. The functional group can subsequently be attached to an oligonucleotide or oligonucleotide sequence designed to identify and bind with specific viral DNA or RNA. For example, FIG. 14 illustrates a schematic method for synthesizing a CoHex complex having a mono-substituted functional group in the R1 position of Structure III, as shown in Structure VI below. In Structure VI and in FIG. 14, "OTf" refers to a triflate anion, CF3SO3<-> .
[0000]
EMI7.0
[0075] Structure VI was synthesized by mono-substitution of chloropentamminecobalt(III) chloride with trifluoromethanesulfonic acid. The brick red product was collected, then treated with (N-[[epsilon]-Maleimidocaproic acid]hydrazide) in dry acetone and stirred for 24 hrs. The pink solution was washed with dichloromethane to remove any organic impurities and then dried to give Structure VI as a pink powder. Structure VI, for example, may be particularly useful to bond with oligonucleotide or oligonucleotide sequences having a thiol functional group.
[0076] One skilled in the art can appreciate synthesis of CoHex complexes with a variety of functional groups as would be appropriate for a particular application either using the above described method or an appropriate alternative method. Such a CoHex complex would be capable of targeting and inhibiting translation of a particular sequence or virus.
US20110027388
Cobalt Hexammine as a Potential Therapeutic Against HIV and/or Ebola Virus
Cobalt Hexammine as a Potential Therapeutic Against HIV and/or Ebola Virus
Inventors: Eddie L. Chang (Silver Spring, MD, US) Lisa Hensley (Frederick, MD, US) Dzung C. Thach (Annandale, VA, US) Andy Knight (New Orleans, LA, US) Gene Olinger (Frederick, MD, US)
Assignees: The Government of the US, as represented by the Secretary of the Navy The Government of the United States, as represented by the Secretary of the Army
Hexaamminecobalt(III) chloride, also called Cohex, reduces the extent of viral infection, including difficult to treat infections caused by Ebola virus and HIV. Disclosed are methods for treating a viral infection, comprising administering to a patient a cobalt(III) hexammine compound in an amount effective to reduce an extent of a viral infection. Also disclosed are kits for delivery of a cobalt(III) hexammine compound by injection.
BACKGROUND
[0002]In this specification where a document, act or item of knowledge is referred to or discussed, this reference or discussion is not an admission that the document, act or item of knowledge or any combination thereof was at the priority date, publicly available, known to the public, part of common general knowledge, or otherwise constitutes prior art under the applicable statutory provisions; or is known to be relevant to an attempt to solve any problem with which this specification is concerned.
[0003]Hexaamminecobalt(III) chloride, also called Cohex, is notable for its ability to "condense" dsDNA into toroidal-like superstructures under low salt conditions. The metal ion itself, Co(III), with its high positive charge density, is an ideal candidate for binding nucleotides with their high negative charge density. Although Co(III) is not stable by itself in aqueous solutions, it is stabilized by coordinating with donor atoms (usually N) that make strong contributions to the ligand field. These coordinating donors could either be monodentate ligands, e.g., NH3, or polydentate chelators, such as cyclen, C8H20N4. The Co(III)-chelator complexes (e.g., cobalt cyclen complexes) have been used for mechanistic studies of phosphodiester cleavage for both its efficient hydrolysis rates and kinetic inertness, whereby the kinetic inertness of Co(III) ions results in the continued binding of the complex to the hydrolyzed phosphate.
[0004]Due to the kinetic inertness of Co(III) ions, the Cohex complex sequesters the "inner-sphere" ammonia ligands from most exchange-reactions in solution; therefore, the usual interactions with solution molecules are by "outer-sphere" coordination via water bridges to the ammonia ligands and via the high charge-density of the Co(III) ion. These two characteristics play an important role in the strong attachment of Cohex to either DNA or RNA and in enabling Cohex to often substitute for hydrated Mg2+(aq) as a cofactor in nucleic acid biochemistry.
[0005]For example, Cohex complexation with 5S RNA--where Cohex was used in place of Mg2+(aq)--was found to provide no significant shifts in the ?max of the absorption bands of Cohex, indicating that Cohex interaction with RNA was through outer-sphere complexation (and, of course, opposing charge attraction). It has also been reported that the number of binding sites on RNA was similar for Cohex and Mg2+(aq) and that the number was greater than expected for simple charge neutralization of the RNA backbone. These observations demonstrate that Cohex has a great propensity to bind to nucleotides at sites similar to Mg2+-binding sites and either inhibit or slow down the bio-functions of DNA and RNA.
[0006]While certain aspects of conventional technologies have been discussed to facilitate disclosure of the invention, Applicants in no way disclaim these technical aspects, and it is contemplated that the claimed invention may encompass one or more of the conventional technical aspects discussed herein.
BRIEF SUMMARY
[0007]Cohex can inhibit viral transcription/translation via interference with viral RNA. This interference can be either via general "blockade" of the nucleotide strands from transcription/translation or may be made more overt by attaching hybridizing oligonucleotide strands to the Cohex. It has been shown that Cohex does not hydrolyze nucleotides, but does show potent antiviral properties against the Sindbis virus and Adenovirus, which are positive single-stranded (ss) RNA, double-strand (ds) DNA, respectively, and furthermore can act as an antibiotic. See US Patent Application Publication Nos. 2008/0182835 and 2010/0004187, each of which is incorporated by reference in its entirety.
[0008]In one embodiment, a method for treating a viral infection comprises administering to a patient a hexaamminecobalt(III) compound (e.g., hexaamminecobalt(III) chloride) in an amount effective to reduce an extent of a viral infection.
[0009]In a further embodiment, a method for treating a viral infection comprises administering to a human patient a hexamminecobalt(III) compound in an amount effective to reduce an extent of an infection of the patient with Ebola virus or HIV.
[0010]In another embodiment, a kit for delivery of a hexamminecobalt(III) compound by injection comprises a hexamminecobalt(III) compound in a pharmaceutically acceptable carrier, and equipment for delivery thereof by injection, wherein the equipment comprises at least one of a container, injection tubing, or an injection needle.
BRIEF DESCRIPTION OF THE DRAWINGS
[0011]FIG. 1 is an illustration of hexacoordinated Co(III), hexamminecobalt(III) (chloride counterions not shown), and magnesium(II) hexahydrate, Mg(H2O)62+, both form octahedral coordination geometry with their respective ligands.
[0012]FIG. 2 is a double-Y semi-log plot is shown of the decrease in RT activity (left), as a measure of viral activity, or uninfected cell viability (right) for HIV-1 NL4-3 isolate. "% VC" means "% Virus Control" and "% CC" means "% Cell Control."
[0013]FIG. 3 is a double-Y semi-log plot is shown of the decrease in RT activity (left), as a measure of viral activity, or uninfected cell viability (right) for HIV-1 Ba-L isolate. "% VC" means "% Virus Control" and "% CC" means "% Cell Control."
[0014]FIG. 4 plots levels of GFP expression in cells infected with Zaire Ebola GFP, normalized against infected cells with no therapeutic (+/-control). Left plot: Relative GFP levels for A549 cells as a function of Cohex concentration, from 2.5 µM to 5 mM. Right plot: Relative GFP levels for HepG2 cells as a function of Cohex concentration
[0015]FIG. 5 plots of the levels of GFP expression in cells infected with Zaire Ebola GFP, normalized against infected cells with no therapeutic (+/-control). Left plot: Relative GFP levels for 293T cells as a function of Cohex concentration, from 2.5 µM to 5 mM. Right plot: Relative GFP levels for VeroE6 cells as a function of Cohex concentration.
[0016]FIG. 6 shows semi-log plots of the % viable (live) cells as a function of Cohex concentration. Left plot: A549 cells. Right plot: HepG2 cells.
[0017]FIG. 7 shows linear plots of the same data as FIG. 6, showing the region of greatest cytotoxic effect. Left plot: A549 cells. Right plot: HepG2 cells.
[0018]FIG. 8 shows linear plots of the % viable (live) cells as a function of Cohex concentration. Left plot: VeroE6 cells. Right plot: 293T cells.
[0019]FIG. 9 shows results for flow cytometric assay using PI as a marker for dead cells show almost no change between 0 to ˜1.2 mM Cohex.
[0020]FIG. 10 shows a curve fit of inhibition by Cohex. For purposes of fitting, the negative (-%) inhibitory % were turned into positive numbers; so 100%=100% inhibition. The IC50 for the fit was found to be 0.38 mM Cohex.





DETAILED DESCRIPTION
[0021]Hexaamminecobalt(III) (Cohex; FIG. 1), in particular the chloride salt thereof, is notable for its ability to "condense" dsDNA into toroidal-like superstructures under low salt conditions. The metal ion itself, Co(III), with its high (+)charge-density, is an ideal candidate for binding nucleotides with their high (-)charge density. Although Co(III) is not stable by itself in aqueous solutions, it is stabilized by coordinating with donor atoms (usually N) that make strong contributions to the ligand field. These coordinating donors could either be monodentate ligands, e.g., NH3, or polydentate chelators, such as cyclen, C8H20N4. The Co(III)-chelator complexes (e.g., cobalt cyclen complexes) have been used for mechanistic studies of phosphodiester cleavage for both its efficient hydrolysis rates and kinetic inertness, whereby the kinetic inertness of Co(III) ions results in the continued binding of the complex to the hydrolyzed phosphate.
[0022]Due to the kinetic inertness of Co(III) ions, the Cohex complex sequesters the "inner-sphere" ammonia ligands from most exchange-reactions in solution; therefore, the usual interactions with solution molecules are by "outer-sphere" coordination via water bridges to the ammonia ligands and via the high charge-density of the Co(III) ion. These two characteristics play an important role in the strong attachment of Cohex to either DNA or RNA5 and in enabling Cohex to often substitute for hydrated Mg2+(aq) as a cofactor in nucleic acid biochemistry. For example, Cohex complexation with 5S RNA--where Cohex was used in place of Mg2+(aq)--was examined and found to provide no significant shifts in the ?max of the absorption bands of Cohex, indicating that Cohex interaction with RNA was through outer-sphere complexation (and, of course, opposing charge attraction). It has also been reported that the number of binding sites on RNA was similar for Cohex and Mg2+(aq) and that the number was greater than expected for simple charge neutralization of the RNA backbone. These observations demonstrate that Cohex has a great propensity to bind to nucleotides at sites similar to Mg2+-binding sites and either inhibit or slow down the bio-functions of DNA and RNA.
[0023]Cohex may function as a new type of broad-spectrum antiviral compound. For example, Cohex can be effective in significantly enhancing cell viability and in depressing viral expression for Sindbis infected BHK cells, with similar significant effects of Cohex against adenovirus in A549. See US Patent Application Publication No. 2008/0182835. These observations point to the potential broad-spectrum nature of Cohex against viruses.
[0024]As disclosed herein, Cohex demonstrates antiviral properties against two additional viruses. Ebola virus is a negative-strand, filamentous, enveloped microorganism that belongs to the filoviridae family of viruses. Cohex can decrease the viral expression levels in a dose-dependent manner, in a variety of cells infected with the Ebola virus. Cohex also demonstrates antiviral properties against human immunodeficiency virus (HIV). HIV is a member of the genus lentivirus and belongs to the Retroviridae family. It has a single-strand (-)RNA genome, which is transcribed into a complementary DNA (cDNA) inside the host cell by an RNA-dependent DNA polymerase. The sense cDNA serves as a template for DNA-dependent DNA polymerase to make an antisense DNA copy, which forms a double-stranded viral DNA (dsDNA). The dsDNA is then transported into the cell nucleus where it gets integrated into the host cell's genome. Virus replication is initiated when the integrated DNA provirus is transcribed into mRNA.
DEFINITIONS
[0025]As used herein, the term "reduce an extent of the viral infection" with regard to a patient means that the ability of viruses to multiply within a patient is at least partially reduced.
[0026]As used herein, a "patient" can be a human or other mammal.
Antiviral Uses of Cohex
[0027]It is contemplated that Cohex could be used to treat a viral infection in a patient. In one embodiment, an effective amount of Cohex is administered to a patient suspected or known to have a viral infection. Optionally, a method of treatment includes identifying a patient who is or may be in need of such treatment. The patient can be a human or other mammal, including without limitation a primate, dog, cat, cow, pig, or horse.
[0028]In an embodiment, Cohex is administered to a patient known or suspected of being infected by a virus. In a further embodiment, Cohex is administered prior to exposure of the patient to a virus. In another embodiment, Cohex is administered subsequent to exposure of the patent to a virus.
[0029]The Cohex may be administered by any of various means including orally or nasally, or by suppository, or by injection including intravenous, intramuscular, or intraperitoneal injection, or combinations of any of these.
[0030]In an embodiment, equipment for injection of Cohex in a pharmaceutically acceptable comprises at least one of a container for the compound (such as a tube, bottle, or bag), injection tubing, or an injection needle.
[0031]The quantity of Cohex effective to treat an infection can be ascertained by one of ordinary skill in the art. Exemplary amounts of Cohex include 0.5, 1, 2, 4, 8, 10, 12, 14, 16, 18, or 20 mg/kg, or more.
[0032]Viral infections that can be treated include, but are not limited to, those associated with human immunodeficiency virus (HIV), human T cell leukemia virus (HTLV), Papillomavirus (e.g., human papilloma virus), Polyomavirus (e.g., SV40, BK virus, DAR virus), orthopoxvirus (e.g., variola major virus (smallpox virus)), EBV, herpes simplex virus (HSV), hepatitis virus, Rhabdovirus (e.g., Ebola virus), alphavirus (e.g., Sindbis virus), adenovirus, and/or cytomegalovirus (CMV). In preferred embodiments, the viral infection is by HIV or Ebola virus.
Preparation of Co(III) Hexammine
[0033]While Cohex is available commercially, its synthesis is fairly straight forward, using air to oxidize Co(II) to Co(III):
CoCl2+4NH4Cl+20NH3+O2?4[Co(NH3)6]Cl.s- ub.3+2H2O
[0034]9.6 g of CoCl2.6H2O (0.06 mol) and 6.4 g of NH4Cl (0.12 mol) were added to 40 ml of water in a 250 ml Erlenmeyer flask with a side arm and shaken until most of the salts are dissolved. Then 1 g of fresh activated decolorizing charcoal and 20 ml concentrated ammonia were added. Next the flask was connected to the aspirator or vacuum line and air drawn through the mixture until the red solution becomes yellowish brown (usually 2-3 hours). The air inlet tube if preferably of fairly large bore (˜10 mm) to prevent clogging with the precipitated Co(NH3)63+ salt.
[0035]The crystals and charcoal were filtered on a Buchner funnel and then a solution of 6 ml of concentrated HCl in 75 ml of water was added. The mixture was heated on a hot plate to effect complete solution and filtered while hot. The hexamminecobalt (III) chloride was crystallized by cooling to 0° C. and by slowly adding 15 ml of concentrated HCl. The crystals were filtered, washed with 60% and then with 95% ethanol, and dried at 80-100° C.
Cohex Activity Against HIV
[0036]There are two known strains of HIV: HIV-1 and HIV-2, of which HIV-1 is the more virulent virus and is the major cause of HIV infections. The first clinically useful drugs developed for HIV-1 were the nucleoside reverse transcriptase (RT) inhibitors. AZT, or 3-azido-3-deoxythymidine, is a synthetic pyrimidine analog of thymidine was actually initially developed as an anticancer drug before it became known as a popular anti-HIV compound. The active form of AZT is its phosphorylated triphosphate (TP) form, which is a competitive inhibitor of RT because AZT-TP binds to the HIV-1 RT better than to the natural substrate deoxythymidine triphosphate (dTTP).
[0037]Cohex was tested in a standard PBMC cell-based microtiter anti-HIV assay against one CXCR4-tropic HIV-1 isolate and one CCR5-tropic HIV-1 isolate. For this study peripheral blood mononuclear cells (PBMCs) were pre-treated with the compound for two hours prior to infection.
[0038]Cohex was stored at 4° C. as a powder and solubilized for tests. The solubilized stock was stored at -20° C. until the day of the assay. Stocks were thawed at room temperature on each day of assay setup and were used to generate working drug dilutions used in the assays. Working dilutions were made fresh for each experiment and were not stored for re-use in subsequent experiments performed on different days. Cohex was evaluated using a 3 mM (3,000 µM) high-test concentration with 8 additional serial half-log dilutions in the PBMC assays.
PBMC Assay
[0039]Freshly prepared PBMCs were centrifuged and suspended in RPMI 1640 with 15% FBS, L-glutamine, penicillin, streptomycin, non-essential amino acids (MEM/NEAA; Hyclone; catalog #SH30238.01), and 20 U/ml recombinant human IL-2. PBMCs were maintained in this medium at a concentration of 1-2×106 cells/ml, with twice-weekly medium changes until they were used in the assay protocol. Monocyte-derived-macrophages were depleted from the culture as the result of adherence to the tissue culture flask.
[0040]For the standard PBMC assay, the cells were plated in the interior wells of a 96 well round bottom microplate at 50 µL/well (5×104 cells/well) in a standard format developed by the Infectious Disease Research department of Southern Research Institute. Each plate contains virus control wells (cells plus virus) and experimental wells (drug plus cells plus virus). Test drug dilutions were prepared at a 2× concentration in microtiter tubes and 100 µL of each concentration was placed in appropriate wells using the standard format. 50 µL of a predetermined dilution of virus stock was placed in each test well (final MOI ˜0.1). Separate plates were prepared identically without virus for drug cytotoxicity studies using an MTS assay system (described below; cytotoxicity plates also include compound control wells containing drug plus media without cells to control for colored compounds that affect the MTS assay). The PBMC cultures were maintained for seven days following infection at 37° C., 5% CO2. After this period, cell-free supernatant samples were collected for analysis of reverse transcriptase activity and compound cytotoxicity was measured by addition of MTS to the separate cytotoxicity plates for determination of cell viability. Wells were also examined microscopically and any abnormalities were noted.
Reverse Transcriptase Activity Assay
[0041]A microtiter plate-based reverse transcriptase (RT) reaction was utilized (detailed in Buckheit et al., AIDS Research and Human Retroviruses 7:295-302, 1991). Tritiated thymidine triphosphate (3H-TTP, 80 Ci/mmol, NEN) was received in 1:1 dH2O:Ethanol at 1 mCi/ml. Poly rA:oligo dT template:primer (Pharmacia) was prepared as a stock solution by combining 150 poly rA (20 mg/ml) with 0.5 ml oligo dT (20 units/ml) and 5.35 ml sterile dH2O followed by aliquoting (1.0 ml) and storage at -20° C. The RT reaction buffer was prepared fresh on a daily basis and consisted of 125 µl 1.0 M EGTA, 125 µl dH2O, 125 µl 20% Triton X100, 50 µl 1.0 M Tris (pH 7.4), 50 µl 1.0 M DTT, and 40 µl 1.0 M MgCl2. The final reaction mixture was prepared by combining 1 part 3H-TTP, 4 parts dH2O, 2.5 parts poly rA:oligo dT stock and 2.5 parts reaction buffer. Ten microliters of this reaction mixture was placed in a round bottom microtiter plate and 15 µl of virus-containing supernatant was added and mixed. The plate was incubated at 37° C. for 60 minutes. Following incubation, the reaction volume was spotted onto DE81 filter-mats (Wallac), washed 5 times for 5 minutes each in a 5% sodium phosphate buffer or 2×SSC (Life Technologies), 2 times for 1 minute each in distilled water, 2 times for 1 minute each in 70% ethanol, and then dried. Incorporated radioactivity (counts per minute, CPM) was quantified using standard liquid scintillation techniques.
MTS Staining for PBMC Viability to Measure Cytotoxicity
[0042]At assay termination, the uninfected assay plates were stained with the soluble tetrazolium-based dye MTS (CellTiter 96 Reagent, Promega) to determine cell viability and quantify compound toxicity. MTS is metabolized by the mitochondria enzymes of metabolically active cells to yield a soluble formazan product, allowing the rapid quantitative analysis of cell viability and compound cytotoxicity. This reagent is a stable, single solution that does not require preparation before use. At termination of the assay, 20-25 µL of MTS reagent is added per well and the microtiter plates are then incubated for 4-6 hrs at 37° C., 5% CO2 to assess cell viability. Adhesive plate sealers were used in place of the lids, the sealed plate was inverted several times to mix the soluble formazan product and the plate was read spectrophotometrically at 490/650 nm with a Molecular Devices SPECTRAmax plate reader.
Assay Results
[0043]The PBMC data were normalized by dividing by either the average control, infected, untreated value for the infection measurements (% Viral Control) or by the control, uninfected, untreated value for the cytotoxicity measurements (% Cell Control). The normalized values were then analyzed for IC50 (50% inhibition of virus replication), CC50 (50% cytotoxicity), and therapeutic index values (TI=CC/IC; also referred to as Antiviral Index or AI).
[0044]Cohex was tested for antiviral efficacy against one CXCR4-tropic HIV-1 isolate and one CCR5-tropic HIV-1 isolate in PBMCs. For this study PBMCs were pre-treated with the compound for two hours prior to infection. FIG. 2 illustrates the decrease in RT activity (left), as a measure of viral activity, or uninfected cell viability (right) for HIV-1 NL4-3 isolate. FIG. 3 illustrates of the decrease in RT activity (left), as a measure of viral activity, or uninfected cell viability (right) for HIV-1 Ba-L isolate. In these Figures, "% VC" means "% Virus Control" and "% CC" means "% Cell Control." The results of the testing are summarized in Table 1.
[0045]Cohex displayed definite antiviral activity against the virus isolates evaluated in this study, with an average IC50 value of 31.2 µM. There did not appear to be any difference in the activity of the compound based on co-receptor tropism, as the compound had approximately equal activity against both virus isolates tested. Cytotoxicity was observed with the compound at concentrations above 100 µM (TC50=833 µM), resulting in an average Therapeutic Index value of 26.7. These results can be summarized with IC50, CC50, and TI values given in Table 1.
TABLE-US-00001 TABLE 1 Summary of Cohex Activity Against HIV-1 in PBMCs Therapeutic Compound HIV-1 Isolate IC50 CC50 Index Cohex Ba-L 33.8 µM 833 µM 24.7 NL4-3 28.6 µM 29.1
[0046]The results show that Cohex displays very similar activity against HIV as against other types of viruses, attesting to the very broad-spectrum nature of the compound. The antiviral activity is not as high as specific antiviral drugs, like AZT, but there are situations where the use of Cohex can be an advantage.
Cohex Activity Against Ebola Virus
[0047]Ebola was first discovered simultaneously in 1976 in Sudan and in the Democratic Republic of the Congo (formerly Zaire). While its origins are still not firmly established, Ebola likely came from the rain forests of Africa. The primary reservoir is likely not nonhuman primates, but rather that the virus is zoonotic, transmitted to humans from ongoing life cycles in animals or arthropods.
[0048]Ebola viruses belong to the filoviridae family and has five known strains (subtypes): Bundibugyo, Cote d'Ivoire, Sudan, Zaire, and Reston. The Bundibugyo, Sudan, and Zaire strains have caused outbreaks of Ebola hemorrhagic fever among humans in Africa, killing up to 90% of those infected. Of the Ebola viruses, the Zaire strain is the most virulent and the Reston strain is the least virulent.
[0049]The Ebola virus is transmitted via contact with bodily fluids of infected persons and can take from two days to three weeks for symptoms to appear. Disease symptoms start with fever, muscle aches and a cough before progressing to severe vomiting, diarrhea and rashes, along with kidney and liver problems. Death generally occurs as the result of either one or a combination of dehydration and/or massive bleeding from leaky blood vessels, kidney, and liver failure. The World Health Organization has documented 1,850 cases of Ebola (mostly in sub-Saharan Africa) since its discovery; only 600 (32 percent) of the victims survived. (32 percent) of the victims survived.
[0050]As with all viruses of the order Mononegavirales, filoviruses, such as Ebola, contain a single-stranded, negative-sense RNA molecule as their genome. The genomes of filoviruses are quite large at approximately 19,000 bases in length and contain seven sequentially arranged genes. Filovirus proteins can be subdivided into two categories, those that form the ribonucleoprotein (RNP) complex and those that are associated with the envelope. The proteins associated with the nucleocapsid are involved in the transcription and replication of the genome, whereas the envelope-associated proteins primarily have a role either in assembly of the virion or in receptor binding and virus entry.
[0051]There is no known cure for Ebola disease. Existing antiviral drugs do not work well against this virus and the best doctors can do is attempt to maintain the patient's body fluids and electrolytes levels under intensive care; while bleeding problems may require transfusions of platelets and/or fresh blood.
Activity of Cohex Against Ebola Virus in Cell Culture
[0052]For EC50 assays, cells were plated onto 96-well plates and incubated at 37° C. for 24 hours before adding compound followed by cell infection with Zaire Ebola GFP virus, a virus strain that contains a GFP gene. The infected cells were allowed to grow for an additional 48 hours before reading on a Molecular Devices spectrofluorometer (X=485 nm, M=515 nm). Controls were done for +virus/-compound and -virus/-compound. The -virus/+compound controls were part of the CC50 tests. Dosage of Cohex ranged from 2.5 µM to 5 mM and were done in triplicates. Error bars for the figures are for standard error (SE) of the mean.
[0053]The results for A549 cells and HepG2 cells are shown in the left and right panels of FIG. 4, respectively. It is seen that there appears to be a general flat response from 2.5 µM until around 0.1 mM Cohex, at which point, GFP expression drops until there is nearly 100% suppression (-100%) of viral expression at concentrations above 1 mM Cohex.
[0054]The results for 293T and VeroE6 cells are shown in the left and right panels of FIG. 5, respectively. For 293T cells, there is a monotonic decrease in GFP expression with increasing Cohex, even starting as low as 2.5 µM Cohex. For VeroE6 cells, there is also a decrease in GFP expression with increasing Cohex, but the slope of the decrease is much less pronounced than for the other cells. There is another difference in the cells of FIG. 4 from FIG. 5. The values for concentrations below 0.1 mM in FIG. 1 fluctuate between 0 and +50 enhancement of GFP with large error bars, whereas the values in FIG. 2, for the same region of concentration, all show (except for 1 point) negative GFP enhancement (i.e., in the suppression of expression region). Thus, the behavior of Cohex for the different cell types exhibit differential amounts of viral expression decrease, but they all show decreasing levels of GFP fluorescence with increasing Cohex concentrations, especially above 0.1 mM.
[0055]In order to check whether the decreasing GFP levels were simply due to decreasing numbers of viable cells, in vitro cytotoxicity studies were performed for the same cell lines. That is, the same concentration ranges as used above were used in a CellTiter-Glo Luminescent Cell Viability Assay by Promega. This assay is based on quantitation of the ATP present in cells, which signals the presence of metabolically active cells, that is, a decrease in luminescence correlates with a decrease in the number of viable cells. The cells were plated out on 96-well plates, as above, and incubated at 37° C. for 24 hours before adding compound. The treated cells were then allowed to grow for an additional 48 hours before reading on the BMG Lumistar set on the ATP protocol.
[0056]In addition to the luminescence assay, a flow cytometry assay was performed using propidium iodide as a "dead" stain for A549 cells. The flow cytometry assay protocol for A549 cell line is similar to protocols known in the art, and is as follows. The cells were grown until confluent and reseeded at 100,000 cells/well in 1 ml in 24-well plates. The monolayers were allowed to form overnight at 37° C. under 5% CO2. The Cohex dilution series was added to appropriate wells and the plate incubated for 48 hours at 37° C. under 5% CO2. The cells were then washed, pelleted, resuspended in buffer, and transferred to BD falcon tubes for flow analysis. A BD FACSort cytometer and BD CellQuest software was used to quantify cell viability. Prior to flow analysis, 10 µL of propidium iodide (PI) at 0.05 mg/ml was added to each tube to stain dead cells. Analysis was performed on 1×104 events/well.
[0057]FIG. 6 shows the result of the cytotoxicity assay for A549 and HepG2 cells plotted on semi-log scale. There appears to be no toxic effect until about 0.1 mM, after which there is a decreasing % of viable cells. To better show the region from 2.5 µM to 0.1 mM, FIG. 7 provides linear-scale plots to emphasize the concentration region that does affect cytotoxicity.
[0058]Both 293T and VerE6 cells lines show much less cytotoxic susceptibility to Cohex, leveling off between 70 to 80% viability, even at 5 mM Cohex. There is a variety of reactions to Cohex by different cell lines, but none of the cells were 100% killed, whereas suppression of GFP expression tends to bottom out close to -100% (except for VeroE6).
[0059]It is further notable that, in addition to variability between cell lines, different markers can also differ in their assessment of viability. As an example, the results of a flow cytometry measurement using propidium iodide (PI) as a marker for dead cells shown in FIG. 9. it can be seen that PI appears to measure a cell property (cell permeability) that is much less affected by Cohex than the luminescence study (ATP levels).
[0060]The IC50 for Cohex for the different cell lines can be estimated from FIGS. 1 and 2. By using a log concentration scale, the data can be fitted to the classic sigmoidal shape using a non-linear least-squares fitting program, seen in FIG. 10. The IC50 for the fit was found to be 0.38 mM Cohex.
[0061]The results with various cell types are shown in Table 2.
TABLE-US-00002 TABLE 2 Summary of Cohex IC50 for Various Cell Types A549 HepG2 VeroE6 293T IC50 (mM) 0.48 0.24 1.66 1.28
Cohex Animal Study Against Ebola
[0062]An efficacy study was conducted in mice to test whether Cohex would have a therapeutic affect against Ebola virus exposure. Initially, to determine whether the mice would tolerate the Cohex, they received intraperitoneal (IP) injections of Cohex once a day for 10 days at levels of 0.5, 1, 2, 4, and 8 mg/kg in this study. The mice tolerated the compound very well, with no adverse reactions reported.
[0063]To examine the efficacy of Cohex, mice were treated by IP injection with either phosphate buffered saline (PBS) or Cohex in PBS one hour before virus exposure, and further treated once a day for 9 more days. In comparing the results of the mice treated with PBS versus those treated with 8 mg/kg of Cohex, it was found to be statistically very likely (p=0.01 in a chi-squared test) that the 8 mg/kg treatment improved survival rates over the PBS treatment in mice infected with Ebola virus.
[0064]The general advantages of a broad-spectrum drug, such as Cohex, are its low-cost, stability, and, of course, ability to attack multiple microorganisms. When there is no treatment available, as in the case of Ebola virus, Cohex could be the only source of treatment. For viruses, such as HIV, where drugs with very high TI already exist, Cohex can be used in a combination drug therapy regime. There are several advantages to doing this: (1) as a broad-spectrum compound, Cohex can fight against opportunistic infections by other microorganisms; (2) Cohex may have a synergistic effect on existing anti-HIV drugs; (3) Cohex can significantly decrease the cost of anti-HIV treatment; (4) Cohex can slow the development of viral drug-resistance by presenting a very different mechanism that must be overcome.
[0065]All numbers expressing quantities of ingredients, constituents, reaction conditions, and so forth used in the specification are to be understood as being modified in all instances by the term "about." Notwithstanding that the numerical ranges and parameters set forth, the broad scope of the subject matter presented herein are approximations, the numerical values set forth are indicated as precisely as possible. Any numerical value, however, may inherently contain certain errors resulting, for example, from their respective measurement techniques, as evidenced by standard deviations associated therewith.
[0066]Although the present invention has been described in connection with preferred embodiments thereof, it will be appreciated by those skilled in the art that additions, deletions, modifications, and substitutions not specifically described may be made without departing from the spirit and scope of the invention. Terminology used herein should not be construed in accordance with 35 U.S.C. §112, 6 unless the term "means" is expressly used in association therewith.
Interim Guidance for Emergency Medical Services (EMS) Systems and 9-1-1 Public Safety Answering Points (PSAPs) for Management of Patients with Known or Suspected Ebola Virus Disease in the United States
October 1, 2014
Who this is for: Managers of 9-1-1 Public Safety Answering Points (PSAPs), EMS Agencies, EMS systems, law enforcement agencies and fire service agencies as well as individual emergency medical services providers (including emergency medical technicians (EMTs), paramedics, and medical first responders, such as law enforcement and fire service personnel).
What this is for: Guidance for handling inquiries and responding to patients with suspected Ebola symptoms, and for keeping workers safe.
How to use: Managers should use this information to understand and explain to staff how to respond and stay safe. Individual providers can use this information to respond to suspected Ebola patients and to stay safe.
Key Points:
The likelihood of contracting Ebola is extremely low unless a person has direct unprotected contact with the blood or body fluids (like urine, saliva, feces, vomit, sweat, and semen) of a person who is sick with Ebola or direct handling of bats or nonhuman primates from areas with Ebola outbreaks.
When risk of Ebola is elevated in their community, it is important for PSAPs to question callers about:
Residence in, or travel to, a country where an Ebola outbreak is occurring;
Signs and symptoms of Ebola (such as fever, vomiting, diarrhea); and
Other risk factors, like having touched someone who is sick with Ebola.
PSAPS should tell EMS personnel this information before they get to the location so they can put on the correct personal protective equipment (PPE) (described below).
EMS staff should check for symptoms and risk factors for Ebola. Staff should notify the receiving healthcare facility in advance when they are bringing a patient with suspected Ebola, so that proper infection control precautions can be taken.
The guidance provided in this document is based on current knowledge of Ebola. Updates will be posted as needed on the CDC Ebola webpage. The information contained in this document is intended to complement existing guidance for healthcare personnel, Infection Prevention and Control Recommendations for Hospitalized Patients with Known or Suspected Ebola Hemorrhagic Fever in U.S. Hospitals
Background
The current Ebola outbreak in West Africa has increased the possibility of patients with Ebola traveling from the affected countries to the United States.
1 The likelihood of contracting Ebola is extremely low unless a person has direct unprotected contact with the body fluids of a person (like urine, saliva, feces, vomit, sweat, and semen) of a person who is sick with Ebola or direct handling of bats or nonhuman primates from areas with Ebola outbreaks.
2 Initial signs and symptoms of Ebola include sudden fever, chills, and muscle aches, with diarrhea, nausea, vomiting, and abdominal pain occurring after about 5 days. Other symptoms such as chest pain, shortness of breath, headache, or confusion, may also develop. Symptoms may become increasingly severe and may include jaundice (yellow skin), severe weight loss, mental confusion, bleeding inside and outside the body, shock, and multi-organ failure.
3 Ebola is an often-fatal disease and care is needed when coming in direct contact with a recent traveler from a country with an Ebola outbreak who has symptoms of Ebola. The initial signs and symptoms of Ebola are similar to many other more common diseases found in West Africa (such as malaria and typhoid). Ebola should be considered in anyone with fever who has traveled to, or lived in, an area where Ebola is present. 2 The incubation period for Ebola, from exposure to when signs or symptoms appear, ranges from 2 to 21 days (most commonly 8-10 days). Any Ebola patient with signs or symptoms should be considered infectious. Ebola patients without symptoms are not contagious. The prevention of Ebola includes actions to avoid exposure to blood or body fluids of infected patients through contact with skin, mucous membranes of the eyes, nose, or mouth, or injuries with contaminated needles or other sharp objects.
Emergency medical services (EMS) personnel, along with other emergency services staff, have a vital role in responding to requests for help, triaging patients, and providing emergency treatment to patients. Unlike patient care in the controlled environment of a hospital or other fixed medical facility, EMS patient care before getting to a hospital is provided in an uncontrolled environment. This setting is often confined to a very small space and frequently requires rapid medical decision-making and interventions with limited information. EMS personnel are frequently unable to determine the patient history before having to administer emergency care.
Coordination among 9-1-1 Public Safety Answering Points (PSAPs), the EMS system, healthcare facilities, and the public health system is important when responding to patients with suspected Ebola. Each 9-1-1 and EMS system should include an EMS medical director to provide appropriate medical supervision.
Case Definition for Ebola Virus Disease (EVD)
The CDC’s most current case definition for EVD may be accessed here:http://www.cdc.gov/vhf/ebola/hcp/case-definition.html.
Recommendations for 9-1-1 Public Safety Answering Points (PSAPs)
State and local EMS authorities may authorize PSAPs and other emergency call centers to use modified caller queries about Ebola when they consider the risk of Ebola to be elevated in their community (e.g., in the event that patients with confirmed Ebola are identified in the area). This will be decided from information provided by local, state, and federal public health authorities, including the city or county health department(s), state health department(s), and CDC.
For modified caller queries:
It will be important for PSAPs to question callers and determine if anyone at the incident possibly has Ebola. This should be communicated immediately to EMS personnel before arrival and to assign the appropriate EMS resources. PSAPs should review existing medical dispatch procedures and coordinate any changes with their EMS medical director and with their local public health department.
PSAP call takers should consider screening callers for symptoms and risk factors of Ebola. Callers should be asked if they, or someone at the incident, have fever of greater than 38.6 degrees Celsius or 101.5 degrees Fahrenheit, and if they have additional symptoms such as severe headache, muscle pain, vomiting, diarrhea, abdominal pain, or unexplained bleeding.
If PSAP call takers suspect a caller is reporting symptoms of Ebola, they should screen callers for risk factors within the past 3 weeks before onset of symptoms. Risk factors include:
Contact with blood or body fluids of a patient known to have or suspected to have Ebola;
Residence in–or travel to–a country where an Ebola outbreak is occurring (a list of impacted countries can be accessed at the following link: http://www.cdc.gov/vhf/ebola/outbrea...a/index.html); or
Direct handling of bats or nonhuman primates from disease-endemic areas.
If PSAP call takers have information alerting them to a person with possible Ebola, they should make sure any first responders and EMS personnel are made confidentially aware of the potential for Ebola before the responders arrive on scene.
If responding at an airport or other port of entry to the United States, the PSAP should notify the CDC Quarantine Station for the port of entry. Contact information for CDC Quarantine Stations can be accessed at the following link: http://www.cdc.gov/quarantine/quaran...tlistfull.html
Recommendations for EMS and Medical First Responders, Including Firefighters and Law Enforcement Personnel
For the purposes of this section, “EMS personnel” means pre-hospital EMS, law enforcement and fire service first responders. These EMS personnel practices should be based on the most up-to-date Ebola clinical recommendations and information from appropriate public health authorities and EMS medical direction.
When state and local EMS authorities consider the threat to be elevated (based on information provided by local, state, and federal public health authorities, including the city or county health department(s), state health department(s), and the CDC), they may direct EMS personnel to modify their practices as described below.
Patient assessment
Interim recommendations:
Address scene safety:
If PSAP call takers advise that the patient is suspected of having Ebola, EMS personnel should put on the PPE appropriate for suspected cases of Ebola (described below) before entering the scene.
Keep the patient separated from other persons as much as possible.
Use caution when approaching a patient with Ebola. Illness can cause delirium, with erratic behavior that can place EMS personnel at risk of infection, e.g., flailing or staggering.
During patient assessment and management, EMS personnel should consider the symptoms and risk factors of Ebola:
All patients should be assessed for symptoms of Ebola (fever of greater than 38.6 degrees Celsius or 101.5 degrees Fahrenheit, and additional symptoms such as severe headache, muscle pain, vomiting, diarrhea, abdominal pain, or unexplained hemorrhage). If the patient has symptoms of Ebola, then ask the patient about risk factors within the past 3 weeks before the onset of symptoms, including:
Contact with blood or body fluids of a patient known to have or suspected to have Ebola;
Residence in—or travel to— a country where an Ebola outbreak is occurring (a list of impacted countries can be accessed at the following link: http://www.cdc.gov/vhf/ebola/outbrea...a/index.html); or
Direct handling of bats or nonhuman primates from disease-endemic areas.
Based on the presence of symptoms and risk factors, put on or continue to wear appropriate PPE and follow the scene safety guidelines for suspected case of Ebola.
If there are no risk factors, proceed with normal EMS care.
EMS Transfer of Patient Care to a Healthcare Facility
EMS personnel should notify the receiving healthcare facility when transporting a suspected Ebola patient, so that appropriate infection control precautions may be prepared prior to patient arrival. Any U.S. hospital that is following CDC's infection control recommendations and can isolate a patient in a private room is capable of safely managing a patient with Ebola.
Interfacility Transport
EMS personnel involved in the air or ground interfacility transfer of patients with suspected or confirmed Ebola should wear recommended PPE (described below).
Infection Control
EMS personnel can safely manage a patient with suspected or confirmed Ebola by following recommended isolation and infection control procedures, including standard, contact, and droplet precautions. Particular attention should be paid to protecting mucous membranes of the eyes, nose, and mouth from splashes of infectious material, or self-inoculation from soiled gloves. Early recognition and identification of patients with potential Ebola is critical. An EMS agency managing a suspected Ebola patient should follow these CDC recommendations:
Limit activities, especially during transport, that can increase the risk of exposure to infectious material (e.g., airway management, cardiopulmonary resuscitation, use of needles).
Limit the use of needles and other sharps as much as possible. All needles and sharps should be handled with extreme care and disposed in puncture-proof, sealed containers.
Phlebotomy, procedures, and laboratory testing should be limited to the minimum necessary for essential diagnostic evaluation and medical care.
Use of Personal protective equipment (PPE)
Use of standard, contact, and droplet precautions is sufficient for most situations when treating a patient with a suspected case of Ebola as defined above. EMS personnel should wear:
Gloves
Gown (fluid resistant or impermeable)
Eye protection (goggles or face shield that fully covers the front and sides of the face)
Facemask
Additional PPE might be required in certain situations (e.g., large amounts of blood and body fluids present in the environment), including but not limited to double gloving, disposable shoe covers, and leg coverings.
Pre-hospital resuscitation procedures such as endotracheal intubation, open suctioning of airways, and cardiopulmonary resuscitation frequently result in a large amount of body fluids, such as saliva and vomit. Performing these procedures in a less controlled environment (e.g., moving vehicle) increases risk of exposure for EMS personnel. If conducted, perform these procedures under safer circumstances (e.g., stopped vehicle, hospital destination).
During pre-hospital resuscitation procedures (intubation, open suctioning of airways, cardiopulmonary resuscitation):
In addition to recommended PPE, respiratory protection that is at least as protective as a NIOSH-certified fit-tested N95 filtering facepiece respirator or higher should be worn (instead of a facemask).
Additional PPE must be considered for these situations due to the potential increased risk for contact with blood and body fluids including, but not limited to, double gloving, disposable shoe covers, and leg coverings.
If blood, body fluids, secretions, or excretions from a patient with suspected Ebola come into direct contact with the EMS provider’s skin or mucous membranes, then the EMS provider should immediately stop working. They should wash the affected skin surfaces with soap and water and report exposure to an occupational health provider or supervisor for follow-up.
Recommended PPE should be used by EMS personnel as follows:
PPE should be worn upon entry into the scene and continued to be worn until personnel are no longer in contact with the patient.
PPE should be carefully removed without contaminating one’s eyes, mucous membranes, or clothing with potentially infectious materials.
PPE should be placed into a medical waste container at the hospital or double bagged and held in a secure location.
Re-useable PPE should be cleaned and disinfected according to the manufacturer's reprocessing instructions and EMS agency policies.
Instructions for putting on and removing PPE have been published online at http://www.cdc.gov/HAI/prevent/ppe.html and http://www.cdc.gov/vhf/ebola/pdf/ppe-poster.pdf[PDF - 2 pages].
Hand hygiene should be performed immediately after removal of PPE.
Environmental infection control
Environmental cleaning and disinfection, and safe handling of potentially contaminated materials is essential to reduce the risk of contact with blood, saliva, feces, and other body fluids that can soil the patient care environment. EMS personnel should always practice standard environmental infection control procedures, including vehicle/equipment decontamination, hand hygiene, cough and respiratory hygiene, and proper use of U.S. Food and Drug Administration (FDA) cleared or authorized medical PPE. For additional information, see CDC’s Interim Guidance for Environmental Infection Control in Hospitals for Ebola Virus.
EMS personnel performing environmental cleaning and disinfection should:
Wear recommended PPE (described above) and consider use of additional barriers (e.g., shoe and leg coverings) if needed.
Wear face protection (facemask with goggles or face shield) when performing tasks such as liquid waste disposal that can generate splashes.
Use an EPA-registered hospital disinfectant with a label claim for one of the non-enveloped viruses (e.g., norovirus, rotavirus, adenovirus, poliovirus) to disinfect environmental surfaces. Disinfectant should be available in spray bottles or as commercially prepared wipes for use during transport.
Spray and wipe clean any surface that becomes potentially contaminated during transport. These surfaces should be immediately sprayed and wiped clean (if using a commercially prepared disinfectant wipe) and the process repeated to limit environmental contamination.
Cleaning EMS Transport Vehicles after Transporting a Patient with Suspected or Confirmed Ebola
The following are general guidelines for cleaning or maintaining EMS transport vehicles and equipment after transporting a patient with suspected or confirmed Ebola:
EMS personnel performing cleaning and disinfection should wear recommended PPE (described above) and consider use of additional barriers (e.g., rubber boots or shoe and leg coverings) if needed. Face protection (facemask with goggles or face shield) should be worn since tasks such as liquid waste disposal can generate splashes.
Patient-care surfaces (including stretchers, railings, medical equipment control panels, and adjacent flooring, walls and work surfaces) are likely to become contaminated and should be cleaned and disinfected after transport.
A blood spill or spill of other body fluid or substance (e.g., feces or vomit) should be managed through removal of bulk spill matter, cleaning the site, and then disinfecting the site. For large spills, a chemical disinfectant with sufficient potency is needed to overcome the tendency of proteins in blood and other body substances to neutralize the disinfectant’s active ingredient.
An EPA-registered hospital disinfectant with label claims for viruses that share some technical similarities to Ebola (such as, norovirus, rotavirus, adenovirus, poliovirus) and instructions for cleaning and decontaminating surfaces or objects soiled with blood or body fluids should be used according to those instructions. After the bulk waste is wiped up, the surface should be disinfected as described in the bullet above.
Contaminated reusable patient care equipment should be placed in biohazard bags and labeled for cleaning and disinfection according to agency policies. Reusable equipment should be cleaned and disinfected according to manufacturer's instructions by trained personnel wearing correct PPE. Avoid contamination of reusable porous surfaces that cannot be made single use.
Use only a mattress and pillow with plastic or other covering that fluids cannot get through. To reduce exposure among staff to potentially contaminated textiles (cloth products) while laundering, discard all linens, non-fluid-impermeable pillows or mattresses as appropriate.
The Ebola virus is a Category A infectious substance regulated by the U.S. Department of Transportation’s (DOT) Hazardous Materials Regulations (HMR, 49 C.F.R., Parts 171-180). Any item transported for disposal that is contaminated or suspected of being contaminated with a Category A infectious substance must be packaged and transported in accordance with the HMR. This includes medical equipment, sharps, linens, and used health care products (such as soiled absorbent pads or dressings, kidney-shaped emesis pans, portable toilets, used Personal Protection Equipment [e.g., gowns, masks, gloves, goggles, face shields, respirators, booties] or byproducts of cleaning) contaminated or suspected of being contaminated with a Category A infectious substance.
4. Follow-up and/or reporting measures by EMS personnel after caring for a suspected or confirmed Ebola patient
EMS personnel should be aware of the follow-up and/or reporting measures they should take after caring for a suspected or confirmed Ebola patient.
EMS agencies should develop policies for monitoring and management of EMS personnel potentially exposed to Ebola.
EMS agencies should develop sick leave policies for EMS personnel that are non-punitive, flexible and consistent with public health guidance
Ensure that all EMS personnel, including staff who are not directly employed by the healthcare facility but provide essential daily services, are aware of the sick leave policies.
EMS personnel with exposure to blood, bodily fluids, secretions, or excretions from a patient with suspected or confirmed Ebola should immediately:
Stop working and wash the affected skin surfaces with soap and water. Mucous membranes (e.g., conjunctiva) should be irrigated with a large amount of water or eyewash solution;
Contact occupational health/supervisor for assessment and access to post-exposure management services; and Receive medical evaluation and follow-up care, including fever monitoring twice daily for 21 days, after the last known exposure. They may continue to work while receiving twice daily fever checks, based upon EMS agency policy and discussion with local, state, and federal public health authorities.
EMS personnel who develop sudden onset of fever, intense weakness or muscle pains, vomiting, diarrhea, or any signs of hemorrhage after an unprotected exposure (i.e., not wearing recommended PPE at the time of patient contact or through direct contact to blood or body fluids) to a patient with suspected or confirmed Ebola should:
Not report to work or immediately stop working and isolate themselves;
Notify their supervisor, who should notify local and state health departments;
Contact occupational health/supervisor for assessment and access to post-exposure management services; and
Comply with work exclusions until they are deemed no longer infectious to others.
1 http://www.cdc.gov/vhf/ebola/hcp/pat...hospitals.html
2 http://www.cdc.gov/vhf/ebola/hcp/case-definition.html
3 http://www.cdc.gov/vhf/ebola/hcp/cli...-settings.html
4 http://phmsa.dot.gov/portal/site/PHM...gnextfmt=print
http://www.cdc.gov/vhf/ebola/hcp/int...ed-states.html
Got Ebola ?
https://www.sciencedirect.com/science/article/abs/pii/S0968089607008887
Antiviral properties of cobalt(III)-complexes
Co(III)hexammine, significantly inhibited Sindbis virus replication in baby hamster kidney (BHK) cells in a dose- and time-dependent manner. In plaque assays, the incubation of Co(III)hexammine with Sindbis virus resulted in a dose-dependent decrease in virus replication when measured at both 24 and 48-h post-infection. Over the concentration range of 0–5 mM Co(III)hexammine, the IC50 for the inhibition of viral replication was determined to be 0.10 ± 0.04 mM at 48 h. Additionally, when BHK cell monolayers were pretreated with Co(III)hexammine for 6 h prior to Sindbis infection, optimal cellular morphology and plasma membrane integrity were observed at 0.6–1.2 mM Co(III)hexammine. Analysis by flow cytometry confirmed that Co(III)hexammine mediated a concomitant dose-dependent increase in BHK cell viability and a decrease in the percentage of Sindbis virus-infected cells (IC50 = 0.13 ± 0.04 mM). Our findings demonstrate for the first time that Co(III)hexammine possesses potent antiviral activity. We discuss our findings within the context of the ability to further functionalize Co(III)hexammine to render it a highly specific antiviral therapeutic reagent.
https://www.academia.edu/35596791/Synthesis_of_Hexaammine_Cobalt_III_Chloride_Characterization_and_study_of_its_Application
Synthesis of Hexaammine Cobalt (III) Chloride; Characterization and study of its Application
by Saim Khalid
Method for reducing impurities in hexammine cobalt halide compounds
US5102633 (A)
Selective precipitation of cobalt and nickel amine complexes
US3928530 (A)
METHOD FOR PRODUCING COBALTIC HEXAMMINE COMPOUNDS AND COBALT METAL POWDER
WO8002567 (A1)