
rexresearch.com
Solar-Powered Still
http://www.sciencedaily.com/releases/2019/08/190805112151.htm
ScienceDaily, 5 August 2019
Nature Sustainability, 2019
DOI: 10.1038/s41893-019-0348-5
A polydimethylsiloxane-coated metal structure for all-day radiative cooling.
Lyu Zhou, et al.
"In the future, this electricity-free tech could help cool buildings in metropolitan areas."
Engineers have designed a new system that can help cool buildings in crowded metropolitan areas without consuming electricity, an important innovation at a time when cities are working to adapt to climate change.
The system consists of a special material -- an inexpensive polymer/aluminum film -- that's installed inside a box at the bottom of a specially designed solar "shelter." The film helps to keep its surroundings cool by absorbing heat from the air inside the box and transmitting that energy through the Earth's atmosphere into outer space. The shelter serves a dual purpose, helping to block incoming sunlight, while also beaming thermal radiation emitted from the film into the sky.
"The polymer stays cool as it dissipates heat through thermal radiation, and can then cool down the environment," says co-first author Lyu Zhou, a PhD candidate in electrical engineering in the University at Buffalo School of Engineering and Applied Sciences. "This is called radiative or passive cooling, and it's very interesting because it does not consume electricity -- it won't need a battery or other electricity source to realize cooling."
"One of the innovations of our system is the ability to purposefully direct thermal emissions toward the sky," says lead researcher Qiaoqiang Gan, PhD, UB associate professor of electrical engineering. "Normally, thermal emissions travel in all directions. We have found a way to beam the emissions in a narrow direction. This enables the system to be more effective in urban environments, where there are tall buildings on all sides. We use low-cost, commercially available materials, and find that they perform very well."
Taken together, the shelter-and-box system the engineers designed measures about 18 inches tall (45.72 centimeters), 10 inches wide and 10 inches long (25.4 centimeters). To cool a building, numerous units of the system would need to be installed to cover a roof.
The research will be published on Aug. 5 in Nature Sustainability. The study was an international collaboration between Gan's group at UB, Boon Ooi's group at King Abdullah University of Science and Technology (KAUST) in Saudi Arabia, and Zongfu Yu's group at the University of Wisconsin-Madison. Along with Zhou, co-first authors are Haomin Song, PhD, UB assistant professor of research in electrical engineering, and Jianwei Liang at KAUST. The study was funded in part by the National Science Foundation.
A system that works during the day and in crowded environments
The new passive cooling system addresses an important problem in the field: How radiative cooling can work during the day and in crowded urban areas.
"During the night, radiative cooling is easy because we don't have solar input, so thermal emissions just go out and we realize radiative cooling easily," Song says. "But daytime cooling is a challenge because the sun is shining. In this situation, you need to find strategies to prevent rooftops from heating up. You also need to find emissive materials that don't absorb solar energy. Our system address these challenges."
When placed outside during the day, the heat-emanating film and solar shelter helped reduce the temperature of a small, enclosed space by a maximum of about 6 degrees Celsius (11 degrees Fahrenheit). At night, that figure rose to about 11 degrees Celsius (about 20 degrees Fahrenheit).
How innovative architecture can drive radiative cooling
The new radiative cooling system incorporates a number of optically interesting design features.
One of the central components is the polymer/metal film, which is made from a sheet of aluminum coated with a clear polymer called polydimethylsiloxane. The aluminum reflects sunlight, while the polymer absorbs and dissipates heat from the surrounding air. Engineers placed the material at the bottom of a foam box and erected a solar "shelter" atop the box, using a solar energy-absorbing material to construct four outward-slanting walls, along with an inverted square cone within those walls.
This architecture serves a dual purpose: First, it helps to sponge up sunlight. Second, the shape of the walls and cone direct heat emitted by the film toward the sky.
"If you look at the headlight of your car, it has a certain structure that allows it to direct the light in a certain direction," Gan says. "We follow this kind of a design. The structure of our beam-shaping system increases our access to the sky. The ability to direct the emissions improves the performance of the system in crowded areas."
WO2018102573Inventor(s): GAN QIAOQIANG; YU ZONGFU; LIU ZHEJUN; SONG HAOMIN; SINGER MATTHEW; LI CHENYU +
System and method for solar vapor evaporation and condensation
Applicant(s): UNIV NEW YORK STATE RES FOUN
A solar vapor generator system and method are provided. In some embodiments, the system has near perfect energy conversion efficiency in the process of solar vapor generation below room temperature. Remarkably, when the operation temperature of the system is below that of the surroundings, the total vapor generation will be higher than the upper limit that can be produced by the input solar energy.
Background of the Disclosure
[0002] The advent of the steam engine was one of the key developments that led to the first Industrial Revolution. Since then, the use of steam has influenced many aspects of modern life. For instance, thermal steam generation and condensation was one of the dominant technologies for seawater desalination before the introduction of reverse osmosis technologies. Although membrane-based technologies became the dominant solution to desalination, they are usually energetically demanding with serious environmental impacts arising from cleaning and maintenance. As a result, there is emerging global interest in developing alternative desalination technologies to address these issues. Solar vapor generation with no electrical input is proving to be a promising and environmentally benign solution, especially in resource limited areas.
However, conventional techniques for generating solar vapor typically rely on costly and cumbersome optical concentration systems to enable bulk heating of a liquid, resulting in relatively low efficiencies (e.g., 30%-40%) due to heat absorption throughout the entire liquid volume that is not directly translated into vapor production. Recently, various advanced and expensive metallic plasmonic and carbon-based nanomaterials have been explored for use in solar vapor/steam generation. However, the vaporization efficiencies of these reported structures are still relatively low under 1 sun illumination (e.g., 48% (10) ~ 83%). [0003] For practical outdoor solar still applications, stable and continuous solar illumination is not achievable in most areas of this planet due to varying weather conditions. Even with inexpensive moderate solar concentrators, a stable incident power higher than AM 1.5 solar light still cannot be guaranteed. Additionally, since most solar stills are covered by glass or other similar collection material, condensation can lead to optical scattering and a decrease in the incident solar power. Therefore, vapor generation under < 1 solar illumination condition is an important, long-felt need, despite being neglected in most previously reported work. Brief Summary of the Disclosure
[0004] The present disclosure provides an alternative approach to solar vapor generation using a supported substrate. In an extremely cost-efficient and effective embodiment, the substrate is a carbon black-dyed cellulose-polyester blend (CCP) and the support is expanded polystyrene foam (EPS). A system according to some embodiments of the disclosed technology achieved a record thermal conversion efficiency of -88% under non-concentrated solar illumination of 1 kW/m<2>. This corresponds to an optimized vapor generation rate that is ~3 times greater than that of natural evaporation. Stable and repeated seawater desalination tests were performed in a portable prototype both in the laboratory and an outdoor environment, and achieved a water generation rate that was 2.4 times that of a commercial product. Also, desalination systems according to some embodiments of the present disclosure largely avoid the costs for seawater intake and pretreatment that are generally required for conventional reverse osmosis processes. Compared with previously reported advanced nanostructures, this CP -EPS system is extremely low-cost in terms of both materials and fabrication, environmentally benign, and safe to handle during production. These attributes enable such a system to be easily expanded to a large scale system. Furthermore, embodiments of the present system may be used for simultaneous fresh water generation and treatment from heavily contaminated source water. Membrane filters and photocatalysts may also be incorporated to purify contaminated source water. Considering the challenges in contaminated/waste water treatment and reuse, the development of low cost, electricity-free, and multi-functional technologies represents a significant advance in the field.
[0005] In some embodiments, the approach further utilizes cold vapor below room temperature, and provides a near unity conversion efficiency of absorbed solar energy. Due to the energy contribution from the surroundings, the measured total vapor generation is higher than the upper limit that can be produced by a given incident solar energy. Importantly, this breakthrough technique was realized using the extremely low cost CCP-foam system under 1 sun illumination, with no need for advanced and expensive nanomaterials. In addition, features for optically absorbing and evaporative materials for solar still systems are shown: i.e., under a given environment, a stronger natural evaporation capability will result in a lower surface temperature. This provides applications in solar still technology, evaporative cooling and solar evaporated mining applications, evaporation-driven generators and recently reported water- evaporation-induced electricity. Description of the Drawings
[0006] For a fuller understanding of the nature and objects of the disclosure, reference should be made to the following detailed description taken in conjunction with the accompanying drawings, in which:
Figure 1 depicts the physical mechanism of vapor generation. (A) Energy balance and heat transfer diagram of the CCP-foam under strong solar illumination. The surface temperature, T2, is higher than the room (ambient) temperature, Ti. (B) A photograph of CCP-foam floating on top of water surface and its corresponding thermal image under dark environment— the surface temperature is below room temperature. (C) Energy balance and heat transfer diagram of the CCP-foam under dark environment or low intensity illumination. (D) A photograph of a CCP-air gap-foam structure floating on top of water and its corresponding thermal image under dark environment— the surface temperature is even lower than the CCP-foam structure.
Figure 2 shows vapor generation under low density light illumination. (A) Photographs of a CCP-foam (upper panel) and a CCP-air gap-foam (lower panel) under 0.6 sun illumination. (B) Thermal images of the CCP-foam (upper panel) and the CCP-air gap- foam (lower panel) under 0.6 sun illumination. (C) Comparison of measured water weight change versus time of CCP-foam and CCP-air gap-foam. The upper limit that can be produced by 0.6 sun input solar energy is plotted by the solid curve. (D) Thermal images of the CCP-foam (upper panel) and the CCP-air gap-foam (lower panel) under 0.2 sun illumination. (E) Comparison of measured water weight change versus time of CCP- foam and CCP-air gap-foam. The upper limit that can be produced by 0.2 sun input solar energy is plotted by the solid curve.
Figure 3 shows the physical interpretation of energy balance of solar vapor generation systems. (A) Energy flow diagram under dark conditions: the input energy from the environment is in balance with the evaporation energy. (B) Energy flow diagram of a below-room-temperature system with a weak light input: the output evaporation energy is the sum of the light input and the environment input. (C) Energy flow diagram of a room- temperature system: the output evaporation energy is in balance with the surrounding and light input. (D) Energy flow diagram of a hot system: the input solar energy is the sum of the evaporation energy and the loss to the environment. Figure 4A and 4B show the increased surface area under 1 sun illumination. (4A(A))
Exemplary schematic diagram to reduce the light density by introducing larger surface area structures. (4A(B), 4A(D)-4A(E)) Thermal distribution images and corresponding photographs of three exemplary samples (4A(B)) a flat CCP -foam, (4A(D)) a triangle structure with T of 37.8<°>, (4A(E)) a triangle structure with T of 22.9<°>. (4B(C)) Comparison of measured water weight change versus time of the three exemplary CCP-foam samples (spheres)— wherein the calculated upper limits that can be produced by 1 sun input solar energy are plotted by solid curves. (4A(F)-4A(G)) The thermal distribution images and corresponding photographs of CCP-air gap-foam structures with (4A(F)) T =37.4<°>and (4A(G)) T =22.4<°>. (4B(H)) Comparison of measured water weight change versus time of these two CCP-air gap-foam samples (spheres)— wherein the calculated upper limits that can be produced by 1 sun input solar energy are plotted by solid curves.
Figure 5 A shows the configuration of a water diffusion height experiment for three sample substrates: white substrate (left); CCP (center); sodium alginate treated CCP (right). Figure 5B is a thermal image of the three sample substrates of Figure 5 A showing the resulting water diffusion heights.
Figure 6 shows the optical absorption spectrum of the CCP and the transmission spectrum of the diffuser. The absorption is -96.9% by weighting absorption spectrum (topmost curve) with the AM 1.5 solar irradiance, which contributes to a high efficiency. The shaded area shows the solar irradiation spectrum as a reference. The transmission spectrum (middle curve) indicates that the transmitted light by the diffuser will basically keep the energy distribution of AM 1.5 at different wavelengths.
Figure 7 shows an experimental setup for solar vapor generation. CCP-foam is illuminated using the solar simulator.
Figure 8 shows an apparatus used to characterize dark evaporation in controlled environment (a commercial glove box is 61 cm x 46 cm x 38 cm with controlled relative humidity and temperature inside the box).
Figure 9 is an illustration of an embodiment of a solar evaporator module floating on top of water surface, wherein each module contains an electricity/solar-driven fan to accelerate the convection.
Figure 10 shows an embodiment of the presently-disclosed carbon substrate in a NaCl brine under 1 sun illumination with a picture being recorded every 30 minutes. One can see the salt crystal accumulated on top of the black substrate surface, which will decrease the vapor evaporation rate. Intriguingly, the salt crystals tended to accumulate on the substrate surface (up to image 10), which may simplify the collection of salt in practice.
Figure 11 shows the mass change over time of the sample under 1 sun illumination. Notice that as salt builds up on our material, only a slight decrease in performance is observed (up to image 10). Therefore, the performance of the salt collector should be very stable and can be replaced easily. Moreover, when the solar simulator is turned off after 8-hour illumination, the salt will be dissolved from the CCP surface back into the bulk water, demonstrating the minimum maintenance requirements.
Figures 12A and 12B show a preliminary experiment in an outdoor environment. Each container has 450 ml water with 40 gram salt. After 10 hour test (Figure 12B), obvious salt can be obtained from the carbon substrate surface (left container) while the control sample did not have any output (right container). Therefore, the presently-disclosed strategy can be used for a solar mining using low concentration solution. At least 8 grams of salt were obtained from the carbon substrate surface in the experiment.
Figure 13 depicts a system according to another embodiment of the present disclosure.
Figure 14 (A) Scanning Electron Microscope (SEM) image of uncoated fiber-rich paper.
(B) SEM image of CCP under low and high magnifications (inset). (C) Top line:
Absorption spectra of uncoated white paper; Bottom line: Absorption spectra of CCP. Absorption spectra were measured by an integration sphere; Inset: Photograph of these two pieces of paper. (D) Comparison of water weight change versus time under four different conditions: i) water in dark environment; ii) water under 1 kW/m<2>illumination; iii) floating white paper under 1 kW/m<2>illumination and iv) floating CCP under 1 kW/m<2>illumination. (E) The surface temperature distribution of the four samples measured in Figure 14(D) measured using a thermal imager: the upper left panel corresponds to i) of Figure 14(D); the upper right panel corresponds to ii) of Figure 14(D); the lower left panel corresponds to iii) of Figure 14(D) and the lower right panel corresponds to iv) of Figure 14(D).
Figure 15A Photographs of a CCP with (upper panel) and without the insulating EPS foam (lower panel) floating on top of water.
Figure 15B Photograph of the CCP-foam structure with cover foam to eliminate evaporation from the water surface surrounding the CCP-foam structure. Figure 15C Comparison of water mass change due to evaporation versus time under four different conditions: water under 1 kW/m<2>, exfoliated graphite on foam from previous work, CCP without insulating foam, and CCP with insulating foam.
Figure 15D Surface temperature distribution of an exemplary CCP with (upper panel) and without the insulating EPS foam (lower panel) floating on the water.
Figure 16 (A) The water mass change as a function of time under 1, 3, 5, 7 and 10 times concentrated solar illumination, respectively. (B) The temperature change as a function of time under 1, 3, 5, 7 and 10 times concentrated solar illumination, respectively. The solid lines represent vapor temperatures measured by a thermometer installed above the CCP- foam. The dashed lines represent bulk water temperatures measured under the foam, while the lines are as for Figure 16(A). (C) The solar thermal conversion efficiency (light gray dots) and corresponding evaporation rate (black dots) as a function of solar intensity. (D) Direct comparison of solar thermal conversion efficiencies obtained by previously reported structures and an exemplary CCP-foam according to an embodiment of the present disclosure.
Figure 17 (A) Energy balance and heat transfer diagram in an exemplary CCP-foam architecture during the vapor generation process. (B) Diagram of the detail near the surface of the CCP structure during the vapor generation process.
Figure 18 (A) Evaporation rate of exemplary CCP-foam samples on salt water and pure water as the function of cycle number. The two solid lines are reference lines to show the stable performance. (B) An SEM image of an exemplary CCP sample after 1 hour evaporation in salt water. (C) Evaporation rate of CCP sample in salt water over an 8- hour evaporation period as a function of illumination time. (D) Photographs and (E) thermal images of an exemplary CCP-foam on salt water at times corresponding to the evaporation rate of salt water in Figure 17(C).
Figure 19A (A) Schematic illustration of a conventional desalination solar still. (B)
Photograph of a 5x5 CCP array with a total area of 100 cm<2>according to an embodiment of the present disclosure. (C) and (D) are thermal images of the CCP array before (C) and after (D) solar illumination. (E-G) Photographs of experimental systems with (E) a CCP- foam array on salt water, (F) bare salt water with a layer of black aluminum foil placed at the bottom, and (G) bare salt water with no CCP-foam. (I) The photograph of a prototype system placed outdoors on a lake. (J) The photograph of a control experiment with a commercial product (left) and the exemplary system (right) during the experiment.
Condensation can be seen at the inner surfaces of the covers.
Figure 19B (H) Hourly water weight change with the exemplary CCP-foam array on the water surface (dots), black aluminum foil at the bottom (triangles), and salt water (squares) as a function of illumination time; the top dashed line is the hourly bulk water temperature under CCP foam; middle dashed line is the hourly bulk water temperature with the black aluminum foil at the bottom of the container; bottom dashed line is the hourly water weight change of salt water. (K) The solar intensity (upper panel) and outdoor temperature curves (lower panel) from 8:00 am to 6:00 pm on May 6, 2016. Figure 20 (A) Comparison of the water solution used to ultrasonically clean a CCP sample after different amounts of time. (B) Photographs of the CCP sample after different amounts of ultrasonic cleaning time. (C) Optical absorption spectra of the CCP sample after ultrasonic cleaning.
Figure 21 (A) Surface temperature distribution of a black Al foil (left) and a CCP sample (right) placed on top of a heat plate set at 40 °C. (B) Direct measurement of the temperature at three positions using a thermal couple sensor probe.
Figure 22 Photographs of an experimental setup to measure the temperature of (A) vapor and (B) bulk water.
Figure 23 Optical absorption spectrum of a black Al foil measured by an integration sphere.
Inset: the photograph of a black Al foil.
Figure 24 is a diagram depicting another embodiment of the present disclosure.
Figure 25 is a diagram depicting another embodiment of the present disclosure.
Figure 26A is a side view of an exemplary solar still according to an embodiment of the present disclosure.
Figure 26B is a top view diagram of the solar still of Figure 26A.
Figure 26C is a photograph of the exemplary solar still constructed according to Figures 26A and 26B.
Figure 27 is a diagram of an exemplary floating CCP-foam with air gap for thermal isolation (side view).
Figure 28 is a chart depicting another embodiment of the present disclosure. Detailed Description of the Disclosure
[0007] Unless defined otherwise herein, all technical and scientific terms used in this disclosure have the same meaning as commonly understood by one of ordinary skill in the art to which this disclosure pertains. The disclosure includes all combinations of all components and steps described herein. Throughout this application, the singular form includes the plural form and vice versa.
[0008] By utilizing extremely low-cost materials in this invention, economically viable large-area systems are now possible with no energy input required for operation. This prospect is particularly attractive for addressing global freshwater shortages, especially for individuals to purify water for personal needs (i.e., ~2 liter/day) in developing regions. Because embodiments of the present disclosure do require special micro/nanofabrication processes and do not require solar concentrators, the disclosed technology is extremely low-cost and amenable to scaling up over large or huge areas for real applications.
[0009] Without being bound by any theory, due to the superior absorption, heat conversion, and insulating properties of the presently-disclosed CCP-foam structure, most of the absorbed energy can be used to evaporate surface water with significantly reduced thermal dissipation compared with previously reported architectures. Without being bound by any theory, due to the thermal insulation between the surface liquid and the bulk volume of the water and the suppressed radiative and convective losses from the absorber surface to the adjacent heated vapor, a record solar thermal conversion efficiency of > 88% under illumination of 1 kW/m<2>(corresponding to the evaporation rate of 1.28 kg/(m<2>h)) was realized using an embodiment of the disclosure having no solar concentration. When scaled up to a 100 cm<2>array in a portable solar water still system, the outdoor fresh water generation rate was 2.4 times of that of a leading commercial product. Furthermore, seawater desalination was also demonstrated with reusable stable performance.
[0010] To enhance the vapor generation rate, typically the approach is to increase the operation temperature for a given solar illumination. However, this will inevitably increase the thermal loss to the surroundings mainly via conduction, convection and radiation losses.
Therefore, high temperature solar vapor generation (e.g., with solar concentration) inherently suffers from limits in energy conversion efficiencies. [0011] In some embodiments, present disclosure provides techniques which take an opposite approach, using solar energy to generate cold vapor below room temperature, to provide surprising results. This is a breakthrough pathway for efficient solar vapor generation since under illumination at low power densities, the absorbed-light-to-vapor energy conversion efficiency can reach -100% when the evaporation temperature is lower than the room temperature. Under this condition, the environment will provide additional energy for vapor generation, resulting in a total vaporization rate that is higher than the upper limit that can be produced using the input solar energy alone. This cold vapor generation technique was experimentally validated and demonstrated limit-breaking vaporization rates using an extremely low cost CCP-foam system.
[0012] With reference to Figure 13, in a first aspect, the present disclosure may be embodied as a solar vapor generation system 10 having an open-topped vessel 12 for holding a solution, for example, a water-based solution. A substrate 20 is configured to be placed in the open-topped vessel 20. The substrate 20 is configured to wick solution from the vessel 12. The substrate 20 may be supported near an exposed surface of the solution (i.e., near the top of the open-topped vessel 12) by a support 22. The support may have a density less than water. The support 22 may be thermally insulative and/or thermally stable. The support 22 may be a foam. The support 22 may be configured to not absorb water. The support 22 may comprise expanded polystyrene foam (EPS), polyurethane foam, polyvinyl chloride foam, polyethylene form, a phenol formaldehyde resin foam, or other foam materials or combinations of one or more materials. The support 22 may include an air gap, to separate at least a portion of the substrate 20 from the support 22 allowing air to pass between a portion of the support 22 and the substrate 20 (see, e.g., Figure 27).
[0013] The system 10 may further comprise a housing 14. The substrate 20 and the support 22 may be located within the housing 14. In some embodiments, at least a portion of the vessel 12 may be located within the housing 14. The housing 14 may be configured so as to admit solar energy. For example, the housing 14 may have a transparent top. For example, the housing 14, or a portion thereof, may be made from a transparent plastic, a transparent glass, a transparent polymer membrane (e.g., microwave membrane), etc. In some embodiments, an interior surface of the cover is coated with a non-toxic, anti-mist super-hydrophobic surface treatment. [0014] The system 10 may further comprise an air mover 30 configured to cause air (e.g., ambient air) to move adjacent to the substrate 20. The air mover 30 may be an electrically- powered fan 30, which may be powered by way of, for example, a solar cell 32.
[0015] In some embodiments, a temperature of the substrate 20 is maintained substantially at or below an ambient temperature. For example, in embodiments having a housing 14, the housing may be a temperature-controlled housing 14 for maintaining an ambient temperature above the temperature of the substrate 20. By maintaining a temperature substantially at an ambient temperature, it is intended that the temperature of the substrate be maintained to within 5 °C of the ambient temperature. In some embodiments, substantially at the ambient temperature means to maintain the temperature to within 1, 2, 3, or 4 °C or any other value therebetween to within a decimal position. In some embodiments, the substrate is maintained at a temperature below the ambient temperature.
[0016] In some embodiments, the system 10 is used as a solar still. For example, in such embodiments, the system 10 may be used to desalinate water for use as drinking water. In such embodiments, the system 10 may further comprise a condenser for condensing the generated vapor. For example, the housing 14 may be configured such that vapor condenses on the housing 14 (i.e., an inner surface of the housing) for recovery of the condensate. In other embodiments, a condenser, such as a condensation trap, may be located within the housing or outside of the housing. [0017] As will be further described below under the heading "Further Discussion," the substrate 20 may be configured as a planar sheet generally parallel to a top surface of the solution. In another embodiment, the substrate is tent-shaped, comprising two planar sheets connected to one another along an adjoining edge. The two planar sheets of a tent-shaped substrate may connect at any angle, for example, at an angle of between 1.0 and 180.0 degrees, all values and ranges therebetween to the first decimal place (tenths). In some embodiments, the two planar sheets connect at an angle of between 20.0 and 45.0 degrees, inclusive and all values and ranges therebetween to the first decimal place (tenths).
[0018] The substrate may be a porous material, such as, for example, a fabric. The substrate may comprise paper and/or plastic, for example, a porous fabric material comprising paper and/or plastic. In some embodiments, the substrate is a hydroentangled, non-woven 55% cellulose / 45% polyester blend, such as TechniCloth™ Wiper TX609, available from Texwipe. The word "paper" does not signify, expressly or implicitly, any equivalence between the "paper" used in some embodiments of the subject disclosure and alternative paper material including any prior substrate which may have been called "paper," but which may have a different or unknown composition or arrangement of fibers. The material may comprise material or material(s) suitable for the purposes of the present substrate as will be apparent in light of the present disclosure.
[0019] In some embodiments, the substrate comprises a cellulose/polyester blend. The blend may comprise about 35% to about 75% cellulose, including all integers and ranges therebetween, and about 45% to about 65% polyester, including all integers and ranges therebetween. In an embodiment, the blend may comprise about 55% cellulose and about 45% polyester. In another embodiment, the substrate may consist essentially of cellulose, while in a different embodiments, the substrate does not consist essentially of cellulose.
[0020] In some embodiments, the substrate is made from non-woven fibers. In other embodiments, the substrate is made from woven fibers (e.g., yarns). In other embodiments, the substrate is a composite material. For example, the substrate may be made from one or more non-woven layers and/or one or more woven layers. In another example of a composite, the substrate may be made from more than one layer, each layer made from the same or different materials. Plastic or paper filter (virgin kraft paper) may also be used as the substrate. In a further embodiment, the substrate does not consist essentially of any one of the following: coral fleece fabric, cotton, wool, nylon, jute cloth, coir mate or polystyrene sponge.
[0021] In some embodiments, the substrate has a dark hue au naturale. In some embodiments, the substrate is coated, dyed, or otherwise colored to attain a dark hue. In some embodiments, the substrate is black or substantially black. For example, the substrate may be coated, dyed, or otherwise colored with carbon black. In some embodiments, the carbon black comprises nanoporous carbon black, microporous carbon black, or a mixture thereof. In another embodiment, the carbon black consists essentially of nanoporous carbon black. Selecting carbon black of a particular sized porosity may be helpful in cleaning contaminated water. However, it is not necessary for the distillation of water, in which general purpose black carbon may be used. Other black or dark pigments may also be used to dye or coat the substrate.
[0022] In some embodiments, the substrate may have a length of about 8 cm to about 14 cm and all integers and ranges therebetween. The length was determined by the water transportation capability of the substrate. The exemplary length of about 10 cm to about 14 cm was used in an exemplary embodiment for a hydroentangled (non-woven) substrate consisting of about 55% cellulose and about 45% polyester. The width may be greater for more substrates with greater liquid transport potential. The length may be less than 10 cm or greater than 14 cm according to the application at hand. [0023] In some embodiments, the substrate may have a width of about 8 cm to about 14 cm and all integers and ranges therebetween. The width was determined by the water
transportation capability of the substrate. The exemplary width of about 8 cm to about 14 cm was used with a hydroentangled (non-woven) substrate consisting of about 55% cellulose and about 45% polyester. The width may be greater for more substrates with greater liquid transport potential. The width may be less than 8 cm or greater than 14 cm according to the application at hand.
[0024] In some embodiments, the substrate has the shape of a cross. In some embodiments, the substrate has the shape of a square or rectangle. The substrate may be any shape suitable to the application. [0025] In some embodiments, the substrate is corrugated, in whole or in part (see, e.g.,
Figure 27). For the corrugation, smaller angles with straight and sharp angle tips may be advantageous. Considering the moving sun light, using corrugation having a smaller depth may be better because using a large depth may cause a shadow effect whereby some substrate will be shielded from light. An upper limit of the corrugation depth may be selected such that the solution can be transported to the entire surface of the substrate. Corrugation not only significantly increases the surface area, but also maintains the evaporated vapor at a relatively low temperature so that energy loss to heat the water and vapor can be suppressed, without being bound by any theory.
[0026] In some embodiments, the substrate and its support float at the surface of the solution. For example, the solution may be source water to be distilled. In such embodiments, where the substrate and its support float on the source water, the dimensions of the support and of the substrate may be selected so that the ends of the substrate overlap the edges of the support and contact the source water as shown in Figure 2 A.
[0027] In some embodiments, the support has a length of about 8 to about 10 cm. In some embodiments, the support has a width of about 8 to about 10 cm. The support has a height of about 8 to about 14 cm. The height can be greater for more absorbent substrates or substrates with enhanced liquid transport (wicking) capability. As before, these dimensions were optimized for a hydroentangled (non-woven) substrate consisting of about 55% cellulose and about 45% polyester. The dimensions of the support and of the substrate may be selected so that the ends of the substrate overlap the edges of the support as shown in Figure 2A. Other support sizes may be used and the above are merely exemplary dimensions used to illustrate the present disclosure.
[0028] Figure 24 depicts a solar vapor evaporation and condensation system 100 according to another embodiment of the present disclosure. A water source 104 is configured to provide a supply of water to an open-topped vessel 112. For example, the water source 104 may be higher than the vessel 112 such that water flows by gravity. In some embodiments, the water source 104 may be a dark in color— for example, black— so that the contained water may be heated via solar heating. The system 100 may include a valve 106 configured to regulate the flow of water from the water source 104. The valve 106 may be any suitable type of valve, such as a manually-controlled valve. In some embodiments, the valve 106 may be controlled automatically, for example, based on a water level in the vessel 112. The vessel 112 may be thermally isolative. For example, the vessel 112 may have a double-walled construction. Other thermally isolative configurations will be apparent to the skilled person in light of the present disclosure.
[0029] A support 122 is disposed within the vessel 112, and a substrate 120 is disposed on the support 122. As described above, the support 122 may be made from any suitable material, such as, for example, EPS foam. Also as described above, the substrate 120 may be made from a suitable wicking material, such as, for example, CCP. Other materials may be used for the support 122 and/or the substrate 120. The some embodiments, the support 122 is configured to float on water contained within the vessel 112. The substrate 120 may be configured to wick water contained within the vessel 112. The system 100 may include a solar concentrator 130— such as, for example, a Fresnel lens— for increasing the solar energy directed towards the substrate 120.
[0030] The system 100 further includes a housing 140, which may be in the shape of a cone, a dome, a pyramid, or any other shape suitable to the purpose as is described herein. The housing 140 is arranged to contain the vessel 112 within. In this way, water vapor evaporating from the water in the vessel 112 will condense on an inner surface of the housing 140 and run down the inner surface for collection in a collection container 150. The collection container 150 may be constructed so as to encourage condensation. For example, the collection container 150 may be constructed using a single-layer of material, such as a plastic or metal material. The system 100 may further include an outlet 152 whereby condensate (distillate) may be accessed for further use/storage.
[0031] In another embodiment, a system 200 is configured to be used in a body of water 290 (see, e.g., Figure 25). For example, the system 200 may be designed to float in a body of water 290, such as, for example, a lake, pond, river, man-made pools, etc. A substrate 220 is disposed on a support 222, and configured to wick water from the body of water 290 (e.g., the substrate 220 may overlap the support 222 and contact the water). The substrate 220 and support 222 may be CCP-EPS foam, or other suitable materials as further described in this disclosure. A housing 240 is configured to contain the substrate 220 and support 222. The housing 240 is arranged such that water vapor evaporated from the substrate 220 is contained within the housing 240 and caused to condense on an inner surface of the housing 240. The housing 240 includes a collection channel 242 arranged to collect condensate which forms on the inner surface of the housing 240. In this way, the condensate will run down the inner surface of the housing 240 into the collection channel 242 where it is collected for use/storage. In some embodiments, the collection channel 242 or a portion thereof is advantageously arranged to be disposed within the bulk water 290 such that the bulk water cools the collection channel 242. [0032] In some embodiments, the support includes an air gap 323 between a portion of the substrate 320 and a portion of the support 322 (see, e.g., Figure 27). Such an air gap may serve as a thermal isolator to minimize thermal dissipation into the bulk water.
[0033] In another aspect, the present disclosure may be embodied as a method 400 for solar vapor generation including placing a solution, such as a water-based solution in an open- topped vessel (see, e.g., Figure 28). A substrate may be disposed 403 in and/or on the solution. The substrate may be configured in any way described herein. The substrate may be disposed 403 on the solution using a support, such as a foam support, to float the substrate at or near a top surface of the solution. The substrate is exposed 406 to solar energy thereby causing evaporation of the solvent (e.g., water), or increasing the rate of evaporation of the solvent over the rate at which evaporation would occur without a substrate and/or exposure to solar energy. The method 400 includes maintaining 409 the substrate at a temperature which is below the ambient temperature. The method may include moving air adjacent to the substrate to further increase the rate of evaporation and/or cool the substrate.
[0034] Some embodiments include chemically treating the substrate and/or the carbon to be more hydrophilic. In some embodiments, the substrate and/or the carbon is treated with sodium alginate.
[0035] As previously mentioned, in some embodiments, the subject invention provides methods and systems for solar distillation of water comprising a substrate on a support. The substrate may be referred to herein as a wick.
[0036] The sides, base, distillate channel, and collection container may each independently comprise metal, plastic or wood. The plastic may be acrylic. For the base, plastic or metal are preferred.
[0037] Optionally, foam or other material less dense than water may be added to ensure that the system floats (see, e.g., Figure 19A(I)). For example, a foam ring or open square may be attached to the lower sides of the system. [0038] In an alternative embodiment, at least an interior surface of the base may angled so that the substrate and its support are angled to face the sun.
[0039] Some embodiments of the presently-disclosed techniques are particularly advantageous for use in mining applications, and more particularly, in salt mining applications. Solar salt mining is a common practice to obtain a plethora of different salts ranging from table salt, NaCl, to Lithium-based salts (e.g., Lithium Carbonate, Lithium Hydroxide, Lithium Chloride, etc.), and Sodium/Potassium/Iodine salts for battery, food, and medical applications. While salt processing plants have the ability to process large amounts of raw salt product every year, these plants rarely run at full capacity due to bottlenecks in the production of raw salts from solar evaporation of salt brine. Using embodiments of the present disclosure, the solar evaporation of salt brines can be increased by 3-5x times the natural rate. A low cost carbon nanomaterial based substrate was developed and shown to be >88% efficient at converting solar light into heat (see below under the heading "CCP Discussion and Experimental Details"). This carbon substrate can easily be applied using a roll-to-roll process for extremely feasible scalability and modular systems, allowing the continued use of the existing infrastructure for solar evaporation ponds while providing greatly improved solutions to enhance salt production. To further maintain current infrastructure, the material used may be mechanically stable, thereby allowing the continued use of current collection vehicles to drive over and scoop up the raw salts. In addition to being low cost and scalable, the present carbon-based substrate is chemically inert as to prevent contamination and preserve purity of salt products.
[0040] In another aspect suitable for use in mining applications, the present disclosure may be embodied as an apparatus for improved salt separation in an evaporation pond. The apparatus is similar to the above-described system where the open-topped vessel is a pre-existing evaporation pond. As such, the apparatus includes a substrate configured to wick solution from the evaporation pond. The apparatus may include a support, configured to support the substrate at a position near the surface of the solution. A temperature of the substrate is maintained below an ambient temperature. The substrate of such an apparatus may be of any type described herein and may be configured as a planar sheet or a tent-shaped configuration as described herein.
[0041] In some embodiments, the substrate is configured in a geometric shape— i.e., having a geometric circumferential shape. In a particular example (illustrated in Figure 8), the substrate is hexagonally shaped such that a plurality of substrates may be arrayed to cover a large area. Other shapes and array configurations will be apparent in light of the present disclosure and are within the scope of the disclosure.
[0042] The substrate may configured for mechanical separation of the salt. For example, the substrate may be a durable material capable of withstanding mechanical separation (scraping, beating, etc.) As such, the substrate may be reusable, such that once the salts have been removed (substantially removed), the substrate may be used to obtain salts again. In some embodiments, the substrate is washable. Here again, such ability to be washed allows for re-use of the substrate.
[0043] While solar salt mining focuses on the evaporation of brine water to collect the salts left behind, embodiments of the present system will also enable reclamation of the evaporated water in a condenser unit. In this way, miners and staff may be provided with a fresh supply of drinking water. This means for no additional energy input, other than the natural solar radiation, raw salt production can be enhanced 3-5x while saving time, money, and other resources associated with providing these often remote mining locations with clean drinking water. [0044] In addition, the CCP structure can also be applied to evaporation enhancement for water having only a low concentration of salt. In such applications, accumulated salt can re- dissolve into the water solution, providing a "self-cleaning" feature and reducing the maintenance required for operation. Additionally, Figure 10 shows a test embodiment wherein salt tended to accumulate on the surface of the substrate. This tendency may provide an advantage in collecting the accumulated salt. For example, mechanical separation of the salt from the substrate may be easier if the majority of accumulated salt is on a surface of the substrate.
[0045] Additionally, the presently-disclosed process includes the geometric assembly of the substrate. Based on geometry, the carbon substrate can be arranged to induce higher airflow speed which increases evaporation rates, prevents adsorption of salts onto the surface of the substrate and easily transfers salts to different collection containers, which aids in overall collection and ease of use/maintenance. As such, the apparatus for salt separation may include one or more air movers (for example, as shown in Figure 8).
[0046] In contrast to water purification applications, solar mining may utilize extra components/devices to accelerate the vapor generation rate. For instance, electricity driven or solar driven fans can be employed in the solar vapor generation for salt mining. According to preliminary experiment results, an air flow from 0.4 to 2 m/s can enhance the vapor generation rate by 1000% (dark environment) ~ 15% (under 3X sun illumination). In particular, solar driven fans can be included in each solar evaporator model (Figure 8). In addition, large scale fans can also be installed at the edge of the pond.
Further Discussion
Loss channels in solar vapor generation systems and the strategy to realize the perfect efficiency
[0047] As illustrated in Figure 1 A, major loss channels include net radiation, convection and conduction losses. Therefore, the power flux exchanged with the environment in the solar vapor generation process can be described as:
P = Coptqi - es(7/2<4>- 7\<4>) - h T2- - qwater(1)
[0048] Here, a is the optical absorption coefficient, Copt is the optical concentration, qi the normal direct solar irradiation (i.e., 1 kW/m<2>for 1 sun at AM 1.5), e the optical emission, s the Stefan-Boltzmann constant (i.e., 5.67x 10<"8>W/(m<2>-K<4>)), T2 the temperature at the surface of the evaporative material, Ti the temperature of the adjacent environment, h the convection heat transfer coefficient, and qwater the heat flux to the bulk water. This equation describes most major processes (if not all) involved in the evaporation process, i.e., the absorption of light, aCoptqi, the net radiative loss to the surroundings, es(?<4>2- ??), the convective loss to the ambient, h(T2 - Ti), and the radiative and conductive loss to the bulk water, qwater. By manipulating the energy distribution among these channels, unique solar vapor generation mechanisms can be realized. For instance, a selective absorber and a bubble wrap cover can be introduced to decrease the infrared thermal radiation (e) and the convective loss (h) to the surroundings, respectively, to produce 100 °C steam under one sun illumination. However, for high temperature solar vapor generation systems, these losses can only be reduced but not eliminated completely. An important question is what happens when T2= Ttl In this steady case (with a stable surface temperature), the system will actually take energy from the environment and the absorbed solar energy can only be consumed in the liquid-to-vapor phase transition, corresponding to near perfect solar energy conversion. Next, a thermally isolated CCP on foam was employed as a low- cost test bed to analyze the energy balance and heat transfers under both dark and illuminated conditions.
Experimental embodiments and results
Materials [0049] In an exemplary embodiment, a substrate of carbon-coated cellulose and polyester blend (CCP) was fabricated using commercially available materials: paper (Texwipe™ TX609) and carbon powder (Sid Richardson Carbon & Energy Company). In some embodiments, evaporation performance can be further manipulated by engineering features of carbon nanomaterial s. For example, the light-absorbing substrate can be enhanced with hydrophilic features. In particular, it may be advantageous to provide a substrate that comprises a black material able to absorb water and sunlight simultaneously and evaporate moisture at a higher rate. To improve these characteristics, the porosity of a carbon nanomaterial may be manipulated in some embodiments. In some embodiments, the substrate and/or the carbon may be chemically treated to increase hydrophilicity. In some embodiments, the substrate and/or the carbon may be treated with sodium alginate. [0050] In an experiment to demonstrate such features, water diffusion height was employed as the figure of merit to evaluate the absorptivity of materials under test (Figure 5A). In the experiment, water diffusion height was measured in substrates made from three sample materials: a first sample comprising a white substrate (left sample); a second sample comprising a substrate coated with a carbon nanomaterial (center sample); and a third sample comprising a carbon-coated substrate similar to the second sample and further treated with sodium alginate (right sample). As shown by the infrared imaging in Figure 5B, the water diffusion height of the first sample was approximately 23 cm. In the second sample, water diffusion height was approximately 37 cm, demonstrating improved water absorptivity in the CCP material. In the third sample, the hydrophilicity of the sample was improved by the sodium alginate, resulting in a water diffusion height of approximately 43 cm.
Methods
Sample fabrication
[0051] 2 g carbon powder was dispersed into 400 mL water. 8 mL acetic acid was added to make carbon powder easier to attach to fibers. The solution was mixed in a 1000 ml beaker and blended well using an ultrasonic cleaner (Branson Ultrasonics Bransonic® B200) for 5 minutes. Subsequently, the prepared white substrate was put into the mixed solution to vibrate and stir for 3 minutes so that carbon powders can dye the substrate uniformly. After that, the CCP was dried at 80 °C on a heating stage. This procedure was repeated three to four times to realize a desired dark color.
Sample characterization
[0052] The absorption spectrum using an integration sphere spectroscopy (Thorlabs IS200-4 integrated with Ocean Optics USB2000+, Ocean Optics Jaz, and Avantes AvaSpec- NIR256-1.7TEC for ultraviolet, visible and infrared wavelength range, respectively). By weighting optical absorption spectrum of CCP (the topmost curve in Figure 6) with the AM 1.5 solar irradiance, the optical absorption was -96.9%.
Solar vapor generation
[0053] To measure the water evaporation rate, a 150 mL beaker with an inner diameter of 5 cm filled with -140 g water was placed under an intensity -tunable solar simulator (Newport 69920), as shown in Figure 7. Three pieces of diffuser (10 inch x 8 inch x 0.050 inch polystyrene sheet, Plaskolite) were used to generate a uniform light distribution. As shown by the middle curve in Figure 6, the overall transmission spectrum was almost wavelength- independent. Therefore the diffuser will not change the spectral feature of the incident light. The solar light intensity was measured using a power meter (PM100D, Thorlabs Inc.) equipped with a thermal sensor (S305C, Thorlabs Inc.) at the same height of the CCP. The CCP was first illuminated for approximately 30 minutes for stabilization. Then the evaporation weight change was measured by an electronic scale (U.S. Solid, with the resolution of 1 mg) every 10 minutes. The surface temperature of CCP was characterized using a portable thermal imager (FLIR ONE®). To calibrate the temperature, a piece of white substrate without illumination was adopted as a reference for room temperature in the same thermal imaging. Its temperature shown in the thermal distribution image was calibrated by a thermometer (GoerTek). In this case, the error in the temperature characterization due to distance from the sample to the thermal imager can be minimized.
Dark evaporation
[0054] Water evaporation is a natural process which occurs under any conditions regardless of solar illumination. As shown in Figure IB, a 19.6 cm<2>CCP was attached to a foam substrate floating on top of water. Its surface thermal distribution was then characterized using a portable thermal imager (FLIR ONE®). The dark evaporation rate of bare water surface was characterized in a glove box with controlled relative humidity and temperature (ETS Model
5501-11, electro-tech system, Inc., Figure 8). In this experiment, two sets of measurements were performed by fixing the relative humidity and temperature inside the box, respectively. Each condition was stabilized for 1 hour before the characterization.
[0055] One can see that the surface temperature of the CCP is -14.3 ± 0.2 °C (T2), which is lower than that of the room temperature (i.e., Ti = 22.3~23.3°C). This was characterized in a laboratory environment (with the humidity of 16-25% in winter time at Buffalo, New York) showing that the average evaporation rate in the dark environment was 0.275 kg/(m<2>h). Due to natural evaporation, this process will consume 6.78>< 10<5>J/(m<2>h) energy from the environment (considering the enthalpy of vaporization at 14.3 °C). Therefore, the energy balance and heat transfer diagram under dark environment (or low intensity illumination condition) is different from that in a previously reported solar heating situation. As shown in Figure 1C, the heat transfer is actually from the environment to the CCP surface due to the lower temperature of the sample. According to equation (1), the convective input power, Peon = -h(T2 - Ti), is approximately 2.88x 10<5>J/(m<2>h) (h was assumed to be 10 W/(m<2>-K)) under dark conditions. This heat transfer direction is valid as long as the CCP surface temperature is lower than the surrounding temperature. In addition, the system has no net radiation loss when T2= Ti. Instead, according to the equation Prad = -es(?<4>2- T<4>,) (e is 0.969 for the CCP, Figure 6), the radiative input power can be calculated to be 1.56>< 10<5>J/(m<2>h). The remaining input is contributed by qwater from the substrate dipped in the water and the foam substrate (although it is suppressed significantly). Therefore, the CCP foam system actually takes energy from the environment rather than losing it. From this standpoint, an advantageous material/structure for solar vapor generation should have a higher evaporation rate under dark conditions in oder to achieve a lower equilibrium temperature. As a result of this insight, the foam under the CCP was removed so as to introduce an air gap (CCP-air-foam), the evaporation rate was then enhanced to 0.340 kg/(m<2>h), resulting in a lower temperature of -13.6 °C at the CCP surface as shown in Figure ID. To examine how this arrangement influences solar vapor generation, light illumination was used to accelerate the vapor generation.
Low intensity illumination
[0056] In this experiment, a solar simulator (Newport) was employed to illuminate the
CCP samples (Figures 2A and 7). The light beam was filtered by an optical diffuser (Figure 6) to get a more uniform beam spot with the power density of -0.6 kW/m<2>{i.e., equivalent to the power of 0.6 Sun at AM 1.5). However, the temperature distribution was not uniform even under uniform solar illumination. One can see that the surface temperature of the central part of the CCP-foam sample (upper panel in Figure 2B) increased up to 35.3 °C, while the CCP-air-foam (lower panel in Figure 2B) surface temperature increased up to 29.7 °C. They are both higher than the room temperature. Therefore, the loss channels highlighted in Figure 1 A will result in lower solar energy conversion efficiency in these areas. One can see from Figure 2C that these measured average evaporation rates {i.e., 0.68 kg/(m<2>h) and 0.80 kg/(m<2>h)) are both below the upper limit that can be produced by the input solar energy {i.e., 0.90 kg/(m<2>h), the solid curve). It should be noted that the CCP-air-foam sample realized a better vapor generation rate under the same illumination, confirmed by its lower surface temperature. [0057] To minimize these loss channels, the incident power was reduced to -0.2 kW/m<2>.
As shown by the upper panel in Figure 2D, the central area temperature of the CCP-foam structure was reduced to 22.9 °C. Other areas on this sample are all below room temperature. In addition, the highest temperature of the CCP-air-foam structure was 20.1 °C (lower panel in Figure 2D), all below room temperature. Under this situation (i.e., Figure 1C), a total vapor generation rate of 0.39 kg/(m<2>h) was obtained for the CCP-foam sample and 0.48 kg/(m<2>h) for the CCP-air-foam sample, respectively, as shown by spheres in Figure 2E. Remarkably, they are all beyond the theoretical upper limit of the vapor generation rate that can be produced by the input solar energy (i.e., -0.30 kg/(m<2>h), the solid curve in Figure 2E). It should be noted that the dark evaporation "background" was not subtracted for the reasons discussed below.
The background evaporation
[0058] In previously reported solar vapor generation literature, the dark evaporation was usually considered as a background which was subtracted from the total vapor generation to obtain the net solar-induced vapor generation. However, by simply comparing Figures 1 A and 1C, one can see that the energy balance and heat flow direction under dark conditions were different from those under illuminated conditions. To test this argument, one can simply turn off the solar light and characterize the remaining evaporation rate immediately. Since the surface temperature cannot return to the sub-room-temperature operation immediately, the dark evaporation is not the "background" of the solar vapor generation. Then the question is: What is the "background"? Or, is there any "background" for solar evaporation?
[0059] To interpret this intriguing problem, here the energy balance was analyzed using a "water container" model, as illustrated in Figure 3. Under dark conditions (Figure 3 A), the system took energy from the environment. The energy lost to natural evaporation, Pout, was in balance with the input energy (Pin) from convection, conduction, radiation and others (if any). The system temperature T2 was lower than the room temperature Ti, and was dependent on the intrinsic evaporation capability of the system under this environment (including temperature, humidity, pressure, system architecture, etc., Figure 8 and Table 1 below). When a solar energy input was introduced as shown in Figure 3B, the system temperature increased. During this unsteady process, the system held more energy from the solar input due to its thermal capacity. When the system temperature increased up to the room temperature (Figure 3C), the input energy channel from the environment closed. Ultimately, the output energy consumed by the evaporation was in balance with the input solar energy with 100% conversion efficiency under the new steady state. When the input solar energy was increased further (Figure 3D), the system temperature T2 was higher than Ti. Then the energy was lost through conduction, convection and radiation channels. In this case, the evaporation energy was always smaller than the input energy. Therefore, the absorbed solar energy conversion efficiency was definitely smaller than 100% and the obtained vapor generation rate could not surpass the theoretical upper limit. In particular, when the light was turned off, the evaporation rate did not change immediately due to the stored thermal energy in the system. One can see that in this process, no dark "background" should be considered since there was no energy flow from the environment to the system (as illustrated in Figure 3 A). Importantly, this physical picture pointed out a strategy to realize the vapor generation rate beyond the solar upper limit, as will be discussed in the next section.
TABLE 1 : Measured dark evaporation rates of a bare water
Image available on "Original document"
Surpassing the solar upper limit: Reducing the power density using larger surface areas
[0060] As illustrated in Figure 3B, below-room-temperature operation allows for obtaining total vapor generation rates that surpass the solar input limit (Figure 2E). However, due to the weak solar illumination, the total vapor generation rate was still relatively low. A first embodiment for realizing this below-room-temperature strategy under a practical 1 sun illumination is to increase the actual surface area within a given projection area, for example, as illustrated in Figure 4A(A). To demonstrate this strategy, a set of triangle structures was fabricated with different apex angles (T) and their surface temperature distributions was compared with a flat sample. As shown in Figure 4A(B), the highest temperature on the flat CCP sample was 42.6 °C. The measured mass change and the theoretical upper limit data were plotted in Figure 4B(C). Since the surface temperature of the flat CCP sample was higher than the room temperature, corresponding to the lossy system in Figure 3D, the measured vapor generation rate (-1.21 kg/(m<2>h), see top set of spheres) was lower than that of the theoretical limit (-1.58 kg/(m<2>h), the top curve).
[0061] When the same light was employed to illuminate the triangle samples with larger surface areas (Figures 4A(D)-4A(E)), the temperature decreased significantly compared with the flat sample shown in Figure 4A(B). Here four temperature points are indicated at different areas along the side walls. One can see that a major area of the sample in Figure 4A(D) (6>=39<°>) was still higher than the room temperature. As a result, a total evaporation rate of -1.50 kg/(m<2>h) was observed, which was -88.9% of the input solar energy (see middle set of spheres and the bottom curve in Figure 4B(C)). This efficiency was improved compared with the flat CCP sample in Figure 4A(B). More intriguingly, for the sample with larger surface areas (6>=23<°>) as shown in Figure 4A(E), the surface temperature was decreased further with major areas below- room-temperature. In this case, a total vapor generation rate of -2.02 kg/(m<2>h) was observed (bottom set of spheres in Figure 4B(C)), which was higher than the theoretical upper limit (-1.65 kg/(m<2>h), see the bottom curve in Figure 4B(C) and Table 2 below). Ultimately, the foam under these two triangle samples was removed to get CCP-air triangle samples to further enhance the convection contribution from the surroundings and accelerate the evaporation rate. As shown by Figures 4A(F)-4A(G), the surface temperatures can be reduced further under the same illumination conditions, indicating the improved vapor generation rates. As shown in Figure 4B(H), total vapor generation rates of 1.58 kg/(m<2>h) were obtained for the sample in
Figure 4A(F) and 2.20 kg/(m<2>h) for the sample in Figure 4A(G), respectively. In particular, the best result of 2.20 kg/(m<2>h) was even faster than those reported by other systems under 1-2 sun illumination (e.g., -1.09 kg/(m<2>h) under 1 sun and -1.93 kg/(m<2>h) under 2 sun reported by others, see dashed lines in Figure 4B(H)). This encouraging result indicates the potential to realize ultra-efficient and high performance solar stills based on extremely low cost materials.
TABLE 2: The values of solar intensity and the enthalpy of evaporation used in the calculation.
Solar intensity Enthalpy of (kW/m<2>) evaporation (J/g)
Upper panel of Fig. 2B 0.609 2419.5
Lower panel of Fig. 2B 0.600 2435.7
Upper panel of Fig. 2D 0.203 2448.2
Lower panel of Fig. 2D 0.203 2453.6
Left panels of Fig. 4A(B) 1.001 2399.9
Left panels of Fig. 4A(D) 1.136 2433.9 Left panels of Fig. 4A(E) 1.146 2439.1
Left panels of Fig. 4A(F) 1.127 2437.1
Left panels of Fig. 4A(G) 1.181 2444.2
Calculation of the solar vapor generation rate
[0062] In describing the present techniques for limit-breaking solar vapor generation rate beyond the input solar energy limit, the theoretical upper limit was estimated as described below. [0063] In this calculation, the solar energy was assumed to transfer solely to the liquid- vapor transition without any other losses. Therefore, the obtained solar vapor generation rate was equal to the solar intensity (J/(m<2>h)) divided by the enthalpy of evaporation (J/kg).
[0064] The solar intensity was measured by placing the aforementioned S305C thermal sensor perpendicular to the light beam. For triangle structures shown in Figures 4A and 4B, the solar intensity at different height was slightly different due to the diffraction of the beam. In this case, the highest value at the top position was employed to calculate the theoretical upper limit so that the limit-breaking experiment result is unambiguous. For instance, in the left panel of Figure 4A(G), the strongest illumination at the top of the triangle sample, 1.181 sun as the solar intensity (i.e., 1.181 kW/m<2>= 4.2516x l0<6>J/(m<2>h)) was employed. [0065] The enthalpy of evaporation is temperature dependent. Therefore, an analysis was performed of the temperature distribution on the CCP surface, which was non-uniform
(Figures 2 and 4). The energy flow condition varied on the same CCP sample due to the nonuniform temperature distribution. Since the enthalpy of evaporation is smaller at higher temperature, the enthalpy of evaporation corresponding to the highest temperature on the CCP surface was selected to calculate the theoretical upper limit. For example, in the left panel of Figure 4A(G), the enthalpy of evaporation of 2444.2 J/g (i.e., 2.4442 l0<6>J/kg) at 25.6 °C was adopted (i.e., the highest temperature on the CCP surface). Under the 1.181 sun solar illumination, the theoretical upper limit of the vapor generation rate was 1.739 kg/(m<2>h).
Considering the actual optical absorption of -96.9%, the theoretical upper limit was 1.685 kg/(m<2>h). All values used in the calculation are listed in Table 2 above.
CCP Discussion and Experimental Details
CCP for solar vapor generation
[0066] A hydrophilic porous material, a fiber-rich nonwoven 55% cellulose / 45% polyester blend (TechniCloth™ Wiper TX609, available from Texwipe™) was selected for use in a test embodiment. This substrate was chosen for its extremely low cost {i.e., retail price of ~$1.05/m<2>), chemical-binder-free make up, and has excellent water transport properties. Its microstructure is shown in Figure 14A, having 10-20^m-wide fiber bundles. The substrate was dyed using low cost carbon black powders {e.g., SidRichardson Carbon & Energy Co., retail price of $2.26/lb). [0067] Sample preparation: 0.8 g carbon powder (Sid Richardson Carbon & Energy Co.) was dispersed into a 160 mL water. 3 mL acetic acid was added to make carbon powder easier to attach to fibers. The mixed solution was blended well using an ultrasonic cleaner (Branson Ultrasonics Bransonic™ B200) for 5 minutes. Subsequently, the 2 cm x 2 cm white paper (TechniCloth™ Wiper TX609, available from Texwipe™) was put into the mixed solution to vibrate for 3 minutes so that carbon powders can dye the paper uniformly. After that, the CCP was dried at 80 °C on a heating stage. This procedure was repeated three to four times to realize a dark shade (see Figure 14C).
[0068] As a result of the dying process, the fibers were coated with carbon nanoparticles, as shown in Figure 14B. The direct comparison between the white paper and the carbon-coated paper is shown in the inset of Figure 14C. The optical absorption of the CCP was very strong with the average absorption of -98% throughout the visible to near IR domain (from 250 nm to 2.5 µp?, measured by a spectrophotometer equipped with an integration sphere, Shimadzu UV- 3150). This strong broadband optical absorption is particularly useful for low-cost solar-to-heat conversion. [0069] Stability/durability test: To demonstrate the stability/durability of carbon powder attached on the paper fibers, a CCP sample was cleaned ultrasonically in clean water. The water solution was changed every 30 minutes to visualize the effect of the ultrasonic cleaning. As shown in Figure 20A, the amount of carbon powder washed from the CCP decreased gradually. After 4 hours, no obvious carbon powder was visible in the water. It was noted that there was no apparent change in the shade of the CCP sample (Figure 20B). To evaluate the cleaning effect of the ultrasonic vibration process, the absorption spectrum was characterized using an integration sphere spectroscopy (Thorlabs IS200-4 integrated with Ocean Optics Jaz) and the optical performance was confirmed as was almost unchanged (Figure 20C). This test provided strong evidence to demonstrate the great durability of the CCP sample. [0070] To demonstrate the baseline for solar vapor generation performance, a direct comparison was performed under several different conditions as shown in Figure 14D.
[0071] To measure the water evaporation rate, a 250 mL beaker (open area of the beaker was 35.3 cm<2>) filled with -165 g water was placed under a solar simulator (Newport 69920). The CCP floated on the water surface with or without the EPS foam. The residual water surface was covered by EPS foam to eliminate natural evaporation. Two pieces of Fresnel lens (26 cm<?>17.8 cm, focal length: 300 mm, OpticLens) were used to concentrate solar light. 1-10 times concentrated solar light was calibrated using a powermeter (PM100D, Thorlabs Inc.) equipped with a thermal sensor (S305C, Thorlabs Inc.) The evaporation weight change was measured by an electronic scale every 10 minutes. [0072] In a dark environment (i.e., at room temperature of 21 °C and humidity of 10%), the water weight loss was 0.44 g/h. Therefore, the average evaporation rate in the dark environment was 0.125 kg/(m<2>h), which was subtracted from all subsequent measured evaporation rates to eliminate the effect of natural water evaporation. Under solar illumination using a solar simulator (Newport 69920 with the solar intensity of 1 kW/m<2>, i.e., AMI .5), the weight loss increased to 1.11 g/h. After that, a 4x4 cm<2>white paper and a 4x4 cm<2>CCP were placed on top of the water surface, and the weight change increased to 1.16 g/h and 1.48 g/h, respectively. To interpret the weight change difference, a portable thermal imager (FLIR ONE®) was used to characterize the temperature of these samples. The thermal imaging characterization was confirmed by a direct measurement using a thermocouple sensor probe, indicating a reasonable accuracy (i.e., < 0.4 °C in the 33-35 °C range).
[0073] To demonstrate the accuracy of the thermal imaging used in the experiment, two samples (i.e., black Al foil and CCP sample) were placed on a heat plate (Super-NuovaTM,
HP131725). Figure 21 A shows the thermal image when the temperature of the heat plate was set to 40 °C. The temperature was then measured at three different positions using a thermal couple sensor probe (Signstek 6802 II, see Figure 21B), demonstrating the reasonable accuracy of the thermal imaging (i.e., < 0.4 °C). Therefore, the temperature change over 5-10 °C observed in the subsequent characterization is reliable based on the thermal imaging data. It is noted that accurate measurement of the surface temperature is a technical challenge since it is dependent on many factors, especially the emissivity of the object being observed and the distance to the object. Therefore, thermal imager estimation of the temperature in the literature is usually not accurate.
[0074] To interpret the evaporation rate difference, the IR thermal imager (FLIR ONE, FLIR system) was used to measure the surface temperature of different samples. The vapor and liquid temperatures were also measured by a thermometer equipped with two K-Type thermocouple sensor probes (Signstek 6802 II). One of the probes was placed above the CCP sample and covered by a small piece of white cardboard to eliminate the heating effect of direct illumination (Figure 22A). The other one was placed under the CCP sample to measure the temperature of bulk water (Fig 22B).
[0075] As shown in Figure 14E, the CCP surface temperature increased to the highest degree of 35.4 °C due to the enhanced solar-to-heat conversion. [0076] However, this heating effect was not well isolated from the bulk water (i.e., the bulk water was heated to 31.7 °C), resulting in less efficient vapor generation effect. One can see that the water evaporation speed with the CCP was 1.33 times higher than that of pure water under the 1 kW/m<2>solar illumination.
Efficient vapor generation using thermally isolated CCP
[0077] A thermal-isolating strategy was employed to confine the heating effect at the top surface for more efficient vapor generation. The finite thickness, large contact area and fluid transport of previously studied porous substrates led to relatively poor thermal insulation performance {e.g., in two previous studies, the thermal conductivities were 0.49 W/(m K) and 0.426 W/(m K)). Without being bound by any theory, a strategy was utilized for the test embodiment to make full use of the capillary force of the porous paper to draw fluid up around the support rather than through it, thus minimizing the thermal loss to the bulk fluid below. As shown by the upper panel in Figure 15 A, a 6-mm-thick EPS foam slab was inserted under the CCP to thermally isolate the porous paper from the bulk water. The thermal conductivity of this EPS foam was 0.034-0.04 W/(m K), one of the lowest thermal conductivities available among extremely low cost materials. In this configuration, the only contact area between the water and CCP was at the edges of the porous paper (i.e., a line contact). This significantly reduced the region of fluid transport compared to placing the substrate directly on the water surface (see the lower panel in Figure 15 A). In this case, the paper contacting the water along the sides of the EPS foam transported the water droplets to the upper surface to facilitate evaporation. It should be noted that during testing, the upper surface of the CCP was always wet, indicating that this reduction in transport area did not limit the evaporation rate of the system.
[0078] To eliminate water evaporation from other open areas, the surrounding exposed water surface was covered with EPS foam with a square hole for the CCP (Figure 15B). To demonstrate the thermal isolation effect, the surface temperature was characterized with and without the EPS foam under the CCP, as shown in Figure 2C. Under solar light illumination having an intensity of 1 kW/m<2>, the upper surface temperature of the CCP increased from 32.9 °C (lower panel) to 44.2 °C with the EPS foam insulation (upper panel). The vapor generation performance is shown in Figure 15C. One can see that the water mass change improved to 1.28 kg/(m<2>h), which was 3.0 times greater than that of the pure water case and 2.0 times greater than that of CCP without EPS foam isolation. This evaporation rate was better than the best reported data under 1 sun illumination with no solar concentration using exfoliated graphite (i.e., circles of Figure 15C). In principle, one would only need a -0.2 m<2>structure to produce 2 liters of fresh water to meet an individual' s daily needs assuming 8-hours of non-concentrated solar illumination. Solar concentration enhances this generation rate further. Characterization of the liquid transportation rate of the CCP
[0079] A potential concern for reduced liquid flow cross section would decrease the liquid flow rate to the CCP surface. To characterize this practical upper limit, the liquid transportation capability of the CCP was characterized. The original weight of a CCP sample was measured, and then an edge of the sample was placed into water and the IR imager was used to monitor water flow as the function of time. The 4-cm-long sample was saturated by water in -25 seconds after which the weight of the wet-CCP was measured. It was noted that the flow rate was not a constant when the paper was saturated. By considering the small cross-sectional area of the CCP -layer (i.e., -0.2 mm<?>2 cm), the practical upper limit of the CCP sample was well over 1,500 kg/m<2>/h, which is higher than the theoretical upper limit under ?,???<?>solar concentration. Therefore, the reduced liquid flow rate was not a limitation in the test system under small to moderate solar concentration. High solar thermal conversion efficiency
[0080] In most previously reported work, the sample surface was always wet, indicating that the performance was limited by surface temperature only. Therefore, the ultimate performance can be improved by introducing concentrated solar illumination. Thus, the vapor generation performance was analyzed under moderate solar concentration conditions to better compare with previously reported nanostructures. In this experiment, an inexpensive planar PVC Fresnel lens {e.g., OpticLens®, $2.39/piece with the area of 26 cm<?>17.8 cm) was employed to focus the incident light from the solar simulator. As shown in Figure 16 A, when the solar light was concentrated by 3, 5, 7 and 10 times, the water mass change was increased to 3.66, 6.24, 9.34, and 13.30 kg/(m<2>h), respectively. To characterize the enhanced surface heating effect more accurately, two thermocouple sensor probes were used to measure the temperature of vapor and bulk water (see Figure 22). As shown by solid curves in Figure 16B, the vapor temperature increased sharply within the first 3 minutes and reached a steady state after 10 minutes. In contrast, the temperature of bulk water increased slowly and continuously as shown by dashed lines in Figure 16B. Higher concentration of light led to higher vapor and bulk water temperatures. Using Equation (2) below, a solar conversion thermal efficiency, ??, of 88.6% was obtained under 1 sun illumination, and 94.8% under 10 times solar concentration, as shown in Figure 16C. Compared with previous reports, this CCP-foam structure realized a very high solar thermal conversion efficiency, especially under low optical concentration condition.
However, the test system shows that there is no need to employ large area solar concentrating systems, in contrast to other, more expensive systems.
[0081] To evaluate the solar-vapor generation performance quantitatively, the solar conversion thermal efficiency, ??, was calculated, using Equation (2):
_ rhhLV(2)
where rh is the mass flux, hLVis the total enthalpy of liquid-vapor phase change, Coptis the optical concentration, and qtis the normal direct solar irradiation (i.e., 1 kW/m<2>). Particularly, the calculation of the total enthalpy of liquid-vapor phase change, hLV, should consider both the sensible heat and the temperature-dependent enthalpy of vaporization. [0082] The thermal conversion efficiency, ??, is widely employed in the literature as an important figure of merit in evaluating the performance of solar vapor generation. However, the detailed values for parameters employed in those literature are slightly different. Therefore, it is necessary to explain the calculation in detail to demonstrate that the presently-obtained ??was unambiguously higher than previously reported results.
[0083] The most frequently used equation for thermal conversion efficiency is ]th<= mhlV>(Eq. (2)). The variable parameter employed in different calculation was the total enthalpy of liquid-vapor phase change, hLV, containing two parts: i.e., the sensible heat and the enthalpy of vaporization (i.e., hLV= C X (T— T0) + Ahvap). In the present experiments, T0was the initial temperature of water, i.e., 21 °C. T was the vapor temperature measured by the thermometer, which was in the range of 40 °C to 90 °C (see data listed in Table 3 below). In this temperature range, the specific heat capacity of water, C, was a constant, i.e., 4.18 J/g K.
However, the enthalpy of vaporization, Ahcap, was highly dependent on the temperature, which was larger at lower temperature. Recent literature employed different values of hLVin their calculation, resulting in certain inaccuracies in the resulting calculated ??.
[0084] For instance, a first paper directly employed a constant Ahvapat 100 °C (2260 kJ/kg) as hLVto calculate ???. Another paper employed a temperature-dependent enthalpy of vaporization Ahvapas hLVto calculate ??. These sources did not consider the sensible heat (i.e., C x (T— T0)). In contrast, another paper considered the sensible heat but employed a constant Ahvapat 100 °C (2260 kJ/kg). By considering these two terms more accurately, the solar thermal conversion efficiencies of the presently-disclosed structure under 1, 3, 5, 7, 10 times
concentrated solar illumination were calculated in Table 3. Fortunately, the sensible heat (i.e., C x (T— T0)) was much smaller than Ahvap, especially under small solar concentration conditions, as shown by the data listed in Table 3. Therefore, previously reported values under 1 sun illumination are still reliable but may contain up to > 10% difference under 10x solar concentration.
[0085] Thus, for energy conversion efficiency estimation, the sensible heat should be considered since this energy is actually consumed by the vapor. But if one focuses on vapor generation performance, this term can be neglected since it just results in higher temperature vapor rather than generates more vapor. TABLE 3 : Accurate calculation of the solar thermal conversion efficiency.
Image available on "Original document"
[0086] In addition, this ???actually describes the energy consumption in the vapor and has two major components: the energy used for water-to-vapor phase change and the energy used to heat the water/vapor. A larger ??does not necessarily correspond to a higher vapor generation rate. For a given value of ??, a higher temperature of the generated vapor will actually result in a lower generation rate since more energy is used to heat the water. Therefore, in terms of solar vapor generation rate, it was beneficial to analyze the theoretical upper limit and thermal loss channels in order to estimate the opportunity available for improvement.
Loss channels
[0087] Recently, a strategy was reported to demonstrate the close to 100 °C steam generation under one sun enabled by a floating structure with "thermal concentration." A detailed thermal loss analysis was performed, revealing that radiative loss and convective loss were two major thermal loss channels in the solar vapor generation systems. The radiative and the convective losses per area are expressed by Equations (3) and (4), respectively:
Prad= £°(T* - T ) (3)
Peon = T2- 7\) (4) where e is the emissivity of the CCP (i.e., 0.98), s is the Stefan-Boltzmann constant (i.e., 5.67x 10<8>W/(m<2>K<4>)), T2is the temperature at the surface of the CCP, 7 is the temperature of the adjacent environment, and h is the convection heat transfer coefficient (assumed to be 10 W/(m<2>K)). Using these two equations, it was estimated that the radiative loss from the
100 °C blackbody absorber surface to the ambient environment (20 °C) was -680 W/m<2>and the convective loss was -800 W/m<2>. Following this theoretical estimation, when the absorber surface was 44.2 °C (via experimental observation), the radiative loss to ambient was -147 W/m<2>and the convective loss was -232 W/m<2>, corresponding to a total of 37.9% energy loss (i.e., 14.7%+23.2%). In this case, it seems that an efficiency -90% is impossible. An immediate question is why one can observe a record high vapor generation rate under 1 sun.
[0088] To interpret the unique features and physics of the proposed CCP-foam architecture, the thermal environment and heat transfer diagram was analyzed (Figure 17 A). First, the downwards thermal radiation was suppressed. According to the previously reported experimental characterization, the reflection of a 3-mm-thick EPS foam slice was in the range of 40%-60% over the spectral region of thermal emission with -10% thermal radiation absorption. Therefore, under thermal equilibrium condition, the temperature of the EPS-foam surface was very close to the bottom surface of the CCP layer so that the downwards radiative loss from the CCP layer was significantly suppressed. Without being bound by any theory, it appeared that the EPS foam employed in some embodiments of the present system served as a thermal radiation shield (in addition to its excellent thermal insulation characteristics), which was superior over previously reported double-sided black systems. [0089] In further analysis of the microscopic thermal environment (Figure 17B), one can recognize that the CCP surface was covered by a sheet of water and surrounded by heated vapor. Without being bound by any theory, it is believed that the absorbed solar energy of the CCP layer first exchanges thermal energy with water sheet and vapor in this small region rather than directly emitting thermal radiation and exchanging heat with the surroundings through the convection. In many reported experiments to identify the vapor temperature, a thermocouple was usually placed on top of the absorber surface, further demonstrating that the top surface of the absorber was surrounded by heated vapor. Since the temperature of the adjacent environment on top of CCP absorber was very close to the temperature of CCP surface, the radiative and convective loss should be very small. For instance, according to Eqs. (3) and (4), the radiative loss from the 44.2 °C surface under 1 sun to the -41.6 °C vapor environment was -1.8%) and the convective loss was only -2.6%. Most absorbed solar energy was still used to evaporate the water sheet on top of the absorber surface rather than lost through these two channels. Without being bound by any theory, it is believed that this is a major physical mechanism for the observed high vapor generation rate. This physical mechanism was not detailed in previous reports. [0090] More importantly, in a real enclosed solar steam system, the vapor cannot be released immediately and the environment inside the system is thermally isolated from the cooler surrounding environment. Furthermore, typical acrylic or glass slabs are opaque to mid-infrared radiation. Consequently, thermal radiation cannot be emitted to the environment. Additionally, convective energy transfers are also largely suppressed when the internal environment is heated under near-thermal equilibrium conditions. In this case, the radiative and convective losses in a real system should be even more negligible. Intriguingly, in a recent report, the highest temperature of the generated steam was observed in a vapor chamber, demonstrating the accuracy of our physical picture. Performance for solar desalination and the effect of the bulk water temperature
[0091] Conventional desalination technologies are usually energy demanding {e.g., reverse osmosis membrane technology consumes ~2 kW h/m<3>) with serious environment costs. It was estimated that a minimum energy consumption for active seawater desalination is
~1 kW h/m<3>, excluding prefiltering and intake/outfall pumping. Passive solar desalination technologies, such as that of the present disclosure, are particularly attractive due to the electricity -free operation with minimum negative impacts on the environment.
[0092] To characterize the evaporation performance and reusability of our CCP-foam for desalination, salt water was prepared with 3.5 wt% NaCl and the solar water evaporation experiment was performed repeatedly. For each cycle, two CCP-foam samples were put on the surfaces of salt water and pure water, respectively, and illuminated under 1 kW/m<2>for one hour. After that, the CCP samples were dried completely and reused for the next cycle. As shown in Figure 18 A, the evaporation rates of 10 cycles in pure water and salt water (see the arrows) are both stable {i.e., 1.2-1.3 kg/(m<2>h)), demonstrating the reliability of the proposed CCP-foam. Considering the excellent wet and dry strength and autoclavable features of the fiber-rich nonwoven paper {e.g., TechniCloth™ Wiper TX609, available from Texwipe™), it is particularly useful for long term solar desalination application.
[0093] After the 1-hour recycling test, a millimeter sized salt crystal was observed on the sample surface (see the first panel in Figure 18D). Without being bound by any theory, it appears that these white salt particles introduce scattering (see Figure 18B for SEM image of salt crystal plates on the CCP surface), which should reduce the optical absorption of the CCP sample. An immediate question is whether this salt crystallization will significantly degrade the performance of the vapor generation in practice, which was not mentioned in previous reports.
[0094] To investigate this issue, an 8-hour continuous experiment was performed in pure water and salt water in a beaker, respectively. Intriguingly, one can see that the evaporation speeds increased continuously and saturated at the 4th~5thhour at ~1.32 kg/(m<2>h) and
-1.42 kg/(m<2>h) for salt water and pure water, respectively, as shown by the dots connected by the solid lines in Figure 18C. Since the CCP surface was always wet during the 8-hour test (indicating sufficient water transportation contributed by capillary forces), the salt crystal did not grow further to cover the entire surface. Instead, the salt crystal area even shrank surprisingly, as shown by the photographs of the CCP surface at different time spots (see Figure 18D). When this experiment was repeated (usually on the next day), this evaporation rate increase was still observed under identical experimental conditions starting from the lower rate, indicating the stable and reusable performance for longer term seawater desalination. As shown by thermal images in Figure 18E, the average surface temperature of the CCP sample increased from 44-45 °C gradually and saturated at 53-54 °C at the 4th~5thhour. Therefore, the next question is what introduced this surface temperature change.
[0095] According to the experimental data shown in Figures 14-16, the only observed gradual change is the bulk water temperature, as shown by dashed curves in Figure 16B. To identify this correlation, the bulk temperature was monitored over 8 hours, as shown by the dashed curves in Figure 18C (see the arrows). One can see that the bulk water temperature (from 22 °C to 32-33 °C) and the evaporation rate changed coincidentally. This observation demonstrated that the surface temperature of the CCP-foam is still related to the bulk liquid temperature. The temperature of the bulk water in this experiment reached the thermal equilibrium after -5 hours. This may be due to the excellent thermal insulation of the EPS foam support employed in the presently-disclosed structure. Also, it was observed that the salt crystal shrank as the bulk and surface temperature increased (i.e., Figure 18D). This may be due to the higher solubility of salt in warmer water. This vapor generation performance should improve if better thermal insulation materials are used in the water container for small volume test. On the other hand, if the bulk water temperature change is negligible in larger scale vapor generation applications, one should not expect this obvious evaporation rate change, as is validated in the prototype system demonstration below. A prototype solar still system
[0096] An exemplary desalination solar still system is illustrated in Figure 19A(A): A box made by thermal insulating materials is filled by seawater or salty water. A tilted transparent glass covers the box to collect solar light. For conventional solar vapor generation technology, light absorbing materials were usually placed at the bottom of the basin to heat the entire liquid volume with fairly low thermal efficiency {i.e., 30%-40%).
[0097] To overcome this weakness, a 5x5 CCP array (Figure 19A(B)) was developed wherein the array included a 2x2 cm<2>for each CCP unit with the total area of 100 cm<2>. The array was placed in a polypropylene box (15 cm in diameter with 1500 g water). However, thermal isolating walls were not incorporated in this experiment. According to the thermal distribution measurement, the temperature of CCP surface increased from 18.2 °C (Figure 19A(C) under dark condition) to 44.6 °C (Figure 19A(D) under 1 sun illumination). Without being bound by any theory, it is believed that the slight nonuniformity of the temperature distribution (39.5 °C at the comer) in Figure 19A(D) was introduced by the intensity distribution of the finite size of the light beam. To evaluate its performance, the solar desalination experiment was repeated using this large area sample (Figure 19A(E)). Meanwhile, two control samples were characterized: (1) a layer of black aluminum foil placed at the bottom of the box (Figure 19A(F), its optical absorption spectrum is shown in Figure 23) and (2) salty water with no CCP-foam
(Figure 19A(G)). As shown in Figure 19B(H), the mass change rate for the CCP-foam array was -1.275 kg/(m<2>h) (with the estimated thermal efficiency ??of 88.2%), which is obviously better than those for control samples (i.e., -0.408 kg/(m<2>h) with ??of 28.2% for the bulk heating strategy, and -0.242 kg/(m<2>h) with ??of 16.7% for the bare salt water evaporation). It was noted that the evaporation rate in this large scale CCP array experiment did not appear to increase. Its bulk water temperature change was also relatively small (20-25 °C, as shown by the bottom dashed curve in Figure 19B(H)). It is believed that this is due to the much larger amount of bulk water, without being bound by any theory. In contrast, the evaporation rates of the two control samples increased slightly, corresponding to their bulk temperature changes, as shown by their respective dashed curves in Figure 19B(H) (see Description of the Drawings). The net water mass change produced by this 100 cm<2>CCP-foam structure was 14.5 g after the 5 -hour operation, which was -25 times of that produced by a single unit (i.e., 0.58 g/h, see Figure 3). In this case, it was unnecessary to introduce a solar concentrator to enhance the water evaporation rate, which is different from the case for commercial concentrated photovoltaic systems. Due to the extremely low manufacturing cost of the CCP-foam, large area products can easily be realized using commercial paper printing technologies at a price much lower than those for conventional solar concentrators.
[0098] As shown in Figure 19A(I), a complete portable solar still system was
demonstrated using an open bottom box (with the 0.01 m<2>5x5 CCP-foam array directly in contact with the open water below with buoyancy ensured by foam (represented by dark square visible along the exterior)), shown in the inset of Figure 19A(I)). The clean water was collected by the distillate channel and guided into a collection bag. This system was then placed on a lake together with a commercial solar still product with an effective area of 0.342 m<2>(Aquamate Solar Still® (NATO stock no. 4610-99-553-9955) at the retail price of $225), as shown in
Figure 19A(J). It should be noted that the exemplary CCP-array can take the lake water directly while the Aquamate Solar Still® needs to be actively fed. It is believed that the Aquamate Solar Still® uses the conventional solar still principle of heating bulk water. The Aquamate Solar Still® does not use the presently-disclosed CCP-foam arrangement. It is likely that there are other differences between the systems, but the Aquamate Solar Still® is a closed system, so its contents cannot be readily ascertained. After a 10-hour operation in the outdoor environment on a sunny-cloudy day with varying sun light illumination conditions (see Figure 19B(K) for temperature and sun light intensity distribution), generation productivities of 0.832 kg/(m<2>day) and 0.344 kg/(m<2>day) were obtained for these two systems, respectively. The performance of the CCP-foam system is -2.4 times of the Aquamate Solar Still®. In addition, due to a scattering of mist formed on the cover (Figure 19A(J)), the input light decreased significantly. Performance may be improved by the use of a non-toxic, super-hydrophobic surface treatment on the transparent glass cover of embodiments of the present disclosure. The prototype did not include corrugation or an air gap between the substrate and the support. Cost estimation and comparison
[0099] Considering the key components for solar-to-heat conversion employed in previously-reported literature {e.g., metal nanoparticles or nanorods dispersed in water, metal nanoparticles on nanoporous anodic alumina, exfoliated graphite on porous carbon foam, a selective absorber inserted between a polystyrene foam disk and a bubble wrap), the cost of embodiments of the presently-disclosed structure is the low. In Figure 19, a complete system was demonstrated using low cost plastic plates. It is well-known that the cost for plastic products are extremely low. However, the cost for condensate collection and other components are required by all solar still systems, which was not discussed in recent literature. According to a review article published in 2007, the net cost of materials for conventional solar still is ~$185.2/m<2>. In contrast, the system shown in Figure 19 is only $76.45/m<2>based on the small scale retail price for all materials/components (see Table 4 below). It is noted that the major cost was for the acrylic slabs, and that these slabs can be replaced by lower cost plastic boxes to reduce costs even further. The net cost for mass production will be significantly lower.
[0002] CROSS-REFERENCE TO RELATED APPLICATION
[0003] This application claims the benefit of U. S. Provisional Application 62/456,853, filed on February 9, 2017, the contents of which are hereby incorporated in their entirety.
[0004] STATEMENT OF GOVERNMENT SUPPORT
[0005] This invention was made with government support under Grant no. CMMI 1537894 awarded by the National Science Foundation. The government has certain rights in the invention.
[0006] FIELD OF THE INVENTION
[0007] The invention is directed to materials and methods for efficiently extracting potable water from atmospheric moisture.
[0008] BACKGROUND
[0009] Providing potable water to the world's population remains one of the greatest challenges of our time. It is estimated that over one billion people in the world lack sufficient access to water, and close to 2.7 billion people find access to water scarce. The problem is especially frustrating as water covers over 70% of the earth's surface. However, of all the world's water, only 3% is fresh water; the remainder is non-potable salt water. Furthermore, two-thirds of fresh water supplies is inaccessible, as it is locked away in glaciers. There have been numerous attempts to convert ocean water to drinking water. Systems include reverse osmosis and solar desalinization. However, these solutions are only practical in coastal environments. Many of the world's water-starved regions are far inland, away from the oceans. Strategies other than desalinization have also been explored, for instance moisture extraction from the air. Conventional atmospheric moisture harvesting devices include condensing and cooling devices. However, these devices can be difficult and expensive to operate, and typically require electrical inputs to function. Such devices are not ideal for many of the most water-starved regions. Moreover, many moisture harvesters only function well in high humidity environments. Many regions lacking water security, however, are arid and dry throughout the year. More recently, researchers have explored hydrogels and various polymers to extract water from the air. However, while many materials that readily absorb moisture are known, substantially less common are those materials that will also readily release the absorbed water. Thermodynamically, a material that absorbs water under particular conditions will not release water under the same conditions without an additional energy input. Conductive hydrogels have been proposed that absorb/release moisture depending on the charge applied to the system. However, like conventional condensers, such systems require external electrical inputs.
[0010] There remains a need for water harvesters capable of efficiently extracting moisture from the atmosphere, even in low humidity environments. There remains a need for water harvesters that do not include a complex array of engineered parts, and that are operable without electrical energy inputs.
[0011] SUMMARY
[0012] Disclosed herein are compositions and methods which address one or more of the foregoing needs. In particular are disclosed water harvesting polymer networks capable of absorbing atmospheric moisture, including in low humidity conditions. Also disclosed are water harvesting polymer networks capable of absorbing and release moisture without electrical energy inputs. The water harvesting polymer networks can include one or more thermoresponsive water storage polymers, permitting operation using solar energy.
[0013] The details of one or more embodiments are set forth in the descriptions below. Other features, obj ects, and advantages will be apparent from the description and from the claims.
[0014] BRIEF DESCRIPTION OF THE FIGURES
[0015] Figure 1 includes a depiction of the harvester system prepared in Example 1 in the dehydrated and hydrated states.
[0016] Figure 2 includes a depiction of SEM images of the harvester system prepared in
[0017] Example 1 in dehydrated form.
[0018] Figure 3 includes a depiction of the moisture absorption of the harvester system prepared in Example 1 at different humidity levels (mass of absorbed water relative to weight of the network).
[0019] Figure 4 includes a depiction of the moisture absorption of a chloride-doped polypyrrole (FIG. 4A) and poly-N-isopropylacrylamide (FIG. 4B) at 60% RH.
[0020] Figure 5 includes a depiction of SEM images of a sample prepared by mixing preformed polypyrrole and poly(N-isopropyl)acrylamide. Figure 6 includes a depiction of the moisture absorption the harvester system prepared in Example 1 at different humidity levels (mass of absorbed water relative to weight of the harvester system).
[0021] Figure 7 includes a depiction of the moisture absorption of an exemplary harvester system at different ionic doping levels (mass of absorbed water relative to weight of the harvester system).
[0022] Figure 8 includes a depiction of water absorption/release cycles for the harvester system prepared in Example 1.
[0023] Figure 9 depicts an FT-IR spectrum of NIP AM alone, PPyCl alone and the interpenetrating network prepared in Example 1.
[0024] Figure 10 depicts (a) the storage modulus (G') and (b) loss modulus (G") of poly- NIPAM gel, poly -NIP AM/PPy-Cl gel and the SMAG tested in a frequency sweep mode.
[0025] Figure 11 depicts the water absorption isotherms of SMAG networks at different relative humidities.
[0026] Figure 12 depicts the moisture capturing behavior of freestanding SMAG networks, and SMAG networks pinned to either meshed nylon or glass sheets.
[0027] Figure 13 depicts the moisture releasing behavior of SMAG networks with differing water content under 1 kW/m2 solar irradiation.
[0028] Figure 14 depicts outdoor AWH powered by natural sunlight. A, Schematic illustration of (1) the water harvester based on SMAGs for (2) the water collector. B and C, Photograph of SMAG bags during (B) water capturing in natural environment and (C) water releasing under solar radiation. The obvious volume change of SMAGs indicates a large water yield. D, Representative outdoor water capturing process in the early morning, where ambient temperature, dew point temperature and ambient RH were presented. E,
[0029] Representative outdoor water releasing process in noontime, where the surficial temperature of SMAG (red curve), condenser temperature, internal air temperature, internal RH and solar flux were presented.
[0030] DETAILED DESCRIPTION
[0031] Before the present methods and systems are disclosed and described, it is to be understood that the methods and systems are not limited to specific synthetic methods, specific components, or to particular compositions. It is also to be understood that the terminology used herein is for the purpose of describing particular embodiments only and is not intended to be limiting. As used in the specification and the appended claims, the singular forms "a," "an" and "the" include plural referents unless the context clearly dictates otherwise. Ranges may be expressed herein as from "about" one particular value, and/or to "about" another particular value. When such a range is expressed, another embodiment includes-1 from the one particular value and/or to the other particular value. Similarly, when values are expressed as approximations, by use of the antecedent "about," it will be understood that the particular value forms another embodiment. It will be further understood that the endpoints of each of the ranges are significant both in relation to the other endpoint, and independently of the other endpoint.
[0032] "Optional" or "optionally" means that the subsequently described event or circumstance may or may not occur, and that the description includes instances where said event or circumstance occurs and instances where it does not.
[0033] Throughout the description and claims of this specification, the word "comprise" and variations of the word, such as "comprising" and "comprises," means "including but not limited to," and is not intended to exclude, for example, other additives, components, integers or steps. "Exemplary" means "an example of and is not intended to convey an indication of a preferred or ideal embodiment. "Such as" is not used in a restrictive sense, but for explanatory purposes.
[0034] Disclosed are components that can be used to perform the disclosed methods and systems. These and other components are disclosed herein, and it is understood that when combinations, subsets, interactions, groups, etc. of these components are disclosed that while specific reference of each various individual and collective combinations and permutation of these may not be explicitly disclosed, each is specifically contemplated and described herein, for all methods and systems. This applies to all aspects of this application including, but not limited to, steps in disclosed methods. Thus, if there are a variety of additional steps that can be performed it is understood that each of these additional steps can be performed with any specific embodiment or combination of embodiments of the disclosed methods.
[0035] The moisture harvesting networks include interpenetrating networks of hygroscopic polymers and thermoresponsive water storage polymers. Interpenetrating networks include those formed by forming one of the polymers (by polymerization) in the presence of the already -formed other polymer. The hygroscopic system absorbs moisture from the air, which is stored and selectively released by the thermoresponsive water storage system. As used herein, a moisture harvesting network can be designated a "super moisture absorbent gels," or "SMAG." The storage modulus (G') and loss modulus (G") values can be used to determine if a network includes interpenetrating polymers. For instance, the interpenetrating networks disclosed herein will have lower G', lower G", or both lower G' and G" values than either the pure hygroscopic polymer, pure thermoresponsive water storage polymer, or simple mixtures of hygroscopic polymer and thermoresponsive water storage polymer. A simple mixture refers to the combination of two separately formed polymers. In certain embodiments, the storage modulus of the interpenetrating network will be less than the storage modulus of a simple mixture of the same polymers, in the same amounts. For instance, the storage modulus of the interpenetrating network can be 10% less, 25% less, 50% less, or 75% less than the storage modulus of the equivalent simple mixture of the same polymers. In certain embodiments, the loss modulus of the interpenetrating network will be less than the loss modulus of a simple mixture of the same polymers, in the same amounts. For instance, the loss modulus of the interpenetrating network can be 10% less, 25% less, 50% less, or 75% less than the loss modulus of the equivalent simple mixture of the same polymers.
[0036] Hygroscopic polymer systems include those capable of extracting water from the atmosphere. Hygroscopic polymers include those that can absorb at least 50%, at least 100%, at least 150%, at least 200%, at least 250%, at least 300%, at least 350%, at least 400%, at least 450%, at least 500%, at least 550%, at least 600%, at least 650%, at least 700%, at least 750%, at least 800%, at least 850%, at least 900%, at least 950%, or at least 1000% by weight of water, relative to the dry weight of the polymer. Hygroscopic polymers include those having a mass average molar mass of less than 500,000, less than 450,000, less than 400,000, less than 350,000, less than 300,000, less than 250,000, less than 200,000, less than 175,000, less than 150,000, less than 125,000, less than 100,000, less than 75,000, or less than 50,000. Exemplary hygroscopic polymers include polyesters, polycarbonates, poly(meth)acrylates, polyacrylonitriles (e.g., ABS resins), poly(meth)acylamides, polysaccharides,
[0037] polyheterocycles, and polysiloxanes.
[0038] In some instances, the hygroscopic polymer can include one or more ionically charged polymers, for instance, polyacrylic acids, functionalized poly(meth)acrylates and poly(meth)acrylamides such as aminoalkyl (meth)acrylates and (meth)acrylamides.
[0039] Exemplary conductive polymers include polypyrroles, polyanilines, polycarbazoles, polyindoles, polyazepines and copolymers thereof. Copolymers include polymers derived from two or more monomers including pyrroles, anilines, carbazoles, indoles, azepines, acrylic acids, functionalized (meth)acrylates and (meth)acrylamides. The copolymer can be a random copolymer, such as formed when two or more monomers are polymerized together. The copolymer can be a block copolymer, such as when individual monomers are polymerized and subsequently joined together.
[0040] In some instances, the conductive polymer can include one or more doped conductive polymers. Doped polymers include polymers that have been oxidized (p-doping) or reduced (n-doping). In some instances, conductive polymers containing basic atoms can be doped under non-redox conditions, for instance by reaction with an acid. Exemplary acids include mineral acids such as hydrochloric acid, hydrobromic acid, hydroiodic acid, sulfuric acid, nitric acid, phosphoric acid, perchloric acid, and tetrafluoroboric acid. Other acids include organic acids such as sulfonic acids (e.g., toluenesulfonic acid, camphorsulfonic acid, benzenesulfonic acid, methanesulfonic acid, and trifluorosulfonic acid), as well as carboxylic acids (e.g., trifluoroacetic acid and trichloroacetic acid). The use of such compounds leads to doped polymers including one or more anions such as chloride, bromide, iodide, sulfate, phosphate, nitrate, perchlorate, tetrafluoroborate, sulfonate, acetates, and mixtures thereof.
[0041] Doped polymers may be characterized by the number of holes per monomer. In some embodiments the doping level is at least 0.010, 0.025, 0.050, 0.075, 0.100, 0.125, 0.150, 0.175, 0.200, 0.225, 0.250, 0.275, 0.300, 0.325, 0.350, 0.375, 0.400, 0.425, 0.450, 0.475, 0.500, 0.525, 0.550, 0.575, 0.600, 0.625, 0.650, 0.675, 0.700, 0.725, 0.750, 0.775, 0.800, 0.825, 0.850, 0.875, 0.900, 0.925, 0.950, or 0.975 holes per monomer. In some
[0042] embodiments, the doping level can be from 0.010-1.0; from 0.10-1.0; from 0.20-1.0; from 0.30-1.0; from 0.40-1.0; from 0.50-1.0; from 0.60-1.0; from 0.70-1.0; from 0.80-1.0; from 0.90-1.0; from 0.10-0.75; from 0.20-0.75; from 0.30-0.75; from 0.40-0.75; from 0.50-0.75; from 0.10-0.50; from 0.20-0.50; from 0.30-0.50; or from 0.40-0.50.
[0043] In certain embodiments, the hygroscopic polymer can be a poly(pyrrole), poly(aniline), a mixture thereof, or a copolymer thereof. Exemplary dopants include chloride, bromide, phosphate and tetrafluoroborate. In some embodiments, the hygroscopic polymer can have a mass average molar mass of less than 100,000, less than 90,000, less than 80,000, less than 70,000, less than 60,000, or less than 50,000. The hygroscopic polymer can have a mass average molar mass from 35,000-100,000, from 50,000-100,000, from 50,000- 90,000, from 50,000-80,000, from 50,000-70,000, from 50,000-60,000, from 35,000-50,000, or from 35,000-75,000.
[0044] Thermoresponsive polymers include those which selectively retain or release water based on temperature. Such systems exhibit a volume phase transition at a certain temperature, resulting in a sudden change of the solvation state. Polymers that become less soluble (or insoluble) in water as temperature increases are characterized by a Lower Critical Solution Temperature (LCST). Thermoresponsive polymers that can be used in water harvesting systems can have an LCST from about 10-80° C, 20-70° C, 25-70° C, 30-70° C, 30-65° C, or 30-60° C.
[0045] In some instances, the thermoresponsive water storage polymer can include one or more poly(N-alkylacrylamides), poly(N,N dialkylacrylamides), poly(acrylic acids), poly(vinyl ethers), or poly(vinylcaprolactams). Thermoresponsive water storage polymers can be derived from one or more monomers including N-alkylacrylamides, N,N- dialkylacrylamides, vinyl ethers, acrylic acid, and vinylcaprolactam. The thermoresponsive water storage polymer can further include monomers such as acrylic acid and/or acrylamide. The N-alkylacrylamide can be an N-Ci-C4alkylacrylamide, the N,N-dialkylacrylamide can be an N,N-di(Ci-C4)alkylacrylamide. The alkyl groups in in the N,N-dialkylacrylamides can be the same, or can be different. When the thermoresponsive polymer is a copolymer, it can be a random copolymer or block copolymer. Exemplary thermoresponsive storage polymers can be derived from N-alkylacrylamide and/or N,N-dialkylacrylamide monomers, and may further be derived from acrylic acid, including salts thereof, and/or acrylamide. The thermoresponsive storage polymer can be derived from one or more monomers such as methylacrylamide, ethylacrylamide, n-propylacrylamide, iso-propylacrylamide, n- butylacrylamide, iso-butylacrylamide, sec-butylacrylamide, tert-butylacrylamide, dimethylacrylamide, diethylacrylamide, di-n-propylacrylamide, di-iso-propylacrylamide, N- methyl-N-ethylacrylamide, N-methyl-N-n-propylacrylamide, N-ethyl-N-n-propylacrylamide, N-methyl-N-iso-propylacrylamide, and N-ethyl-N-iso-propylacrylamide. In some instance, the thermoresponsive polymer is derived from monomers including N-isopropylacrylamide or N,N-diethylacrylamide, and can further include monomers of acrylamide and/or acrylic acid. In certain embodiments, the thermoresponsive polymer can include block copolymers of polyethylene oxide and polypropylene oxide.
[0046] The thermoresponsive water storage polymer can be a crosslinked polymer.
[0047] Crosslinked polymers can be obtained by polymerizing the monomers in the presence of one or more crosslinking monomers. Crosslinked polymers can be derived from one or more monomers having two or more vinyl groups. In some instance, the crosslinking monomer will contain two, three, four, five or six vinyl groups. Exemplary crosslinking monomers include (Ci-Cioalkylene) bisacrylamide, such as N,N-methylenebisacrylamide, N,N- ethylenebisacrylamide, N,N-propylenebisacrylamide, and functionalized acrylamides including mono and di-(C3-Cioalkenyl) acrylamide such as N-allylacrylamide or N,N- diallylacrylamide. The molar ratio of crosslinking monomers to other monomers can be from 1: 10,000 to 1: 100, from 1 :5,000 to 1 : 100, from 1:2,500 to 1: 100, from 1:2,000 to 1: 100, from 1: 1,500 to 1 : 100, from 1: 1,000 to 1 : 100, from 1 :750 to 1 : 100, from 1 :500 to 1: 100, from 1:250 to 1: 100, from 1:5,000 to 1 :500, from 1 :5,000 to 1: 1,000, from 1 :5,000 to 1 :2,500, from 1 :5,000 to 1 :3,000, from 1 :4,000 to 1,1000, from 1:4,000 to 1 :2000, from 1:7,500 to 1:2,500, or from 1 : 10,000 to 1:5,000.
[0048] The water harvesting networks can be characterized according to the (dry) weight ratio of the hygroscopic polymer to thermoresponsive polymer. For instance, the ratio of hygroscopic polymer to thermoresponsive water storage polymer can be from about 1 :0.05 - 1: 1, 1 :0.1 - 1: 1, 1 :0.25 - 1 : 1, 1:0.50 - 1: 1, 1 :0.75 - 1 : 1, 1:0.05 - 1:0.75, 1 :0.1 - 1:0.75, 1:0.25 - 1 :0.75, 1 :0.50 - 1 :0.75, 1 :0.05 - 1 :0.50, 1 :0.10 - 1 :0.50, 1 :0.25 - 1 :0.50, or 1 :0.25 - 1:0.75. In some instances, the weight fraction of the hygroscopic polymer can be at least 50%, at least 60%, at least 70%, at least 80%, at least 85%, at least 90%, or at least 95%, relative to the total weight of the polymer network.
[0049] The interpenetrating water harvesting networks can be prepared by polymerizing one component of the network in the presence of the already formed polymer of the other component. For instance, monomer precursors of the thermoresponsive water storage polymer can be combined with a hygroscopic polymer, and then subjected the conditions suitable to form the thermoresponsive water storage polymer. In other embodiments, monomer precursors of the hygroscopic polymer can be combined with a thermoresponsive water storage polymer, and then subjected the conditions suitable to form the hygroscopic polymer.
[0050] Because the water harvesting networks disclosed herein include thermoresponsive water storage polymers, they can be utilized without the use of electricity or other artificial energy outputs. For instance, the water harvested can be placed in a cool environment, for instance in the shade or overnight, to absorb water. The hydrated harvester can be placed in a collector and exposed to sunlight. As the sun heats the network, the thermoresponsive polymer undergoes a phase transition, releasing water into the collector. For instance, the network can be heated to a temperature of at least 30° C, at least 35° C, or at least 40° C, at which time the absorbed water will be rapidly released from the network. Generally, at least 50% of the water will be released in less than 60 minutes, less than 45 minutes, less than 30 minutes, less than 20 minutes, or less than 10 minutes when the network is heated to a temperature greater than the Lower Critical Solution Temperature (LCST) of the
[0051] thermoresponsive water storage polymer.
EXAMPLES
[0052] The following examples are set forth below to illustrate the methods and results according to the disclosed subject matter. These examples are not intended to be inclusive of all aspects of the subject matter disclosed herein, but rather to illustrate representative methods, compositions, and results. These examples are not intended to exclude equivalents and variations of the present invention, which are apparent to one skilled in the art.
[0053] Efforts have been made to ensure accuracy with respect to numbers (e.g., amounts, temperature, etc.) but some errors and deviations should be accounted for. Unless indicated otherwise, parts are parts by weight, temperature is in °C or is at ambient temperature, and pressure is at or near atmospheric. There are numerous variations and combinations of reaction conditions, e.g., component concentrations, temperatures, pressures, and other reaction ranges and conditions that can be used to optimize the product purity and yield obtained from the described process. Only reasonable and routine experimentation will be required to optimize such process conditions.
[0054] Example 1: Interpenetrating network formed by polymerizing a thermoresponsive polymer in the presence of a hygroscopic polymer
[0055] Pyrrole monomer, ammonium persulfate ("APS") and LiCl (molar ratio 1 : 1 : 1) was gradually added into an aqueous HC1 solution (3.7 % wt). The polymerization reaction was stopped by vacuum filtering and washing. The obtained black product was dispersed in DI water by sonication. The resulting PPyCl polymer (50 μg), N-isopropylacrylamide
[0056] ("NIP AM") monomers (567 mg), N, N-tetramethylenediamine (10 μΐ) and deionized water (10 mL) were mixed together and purged with nitrogen for ten minutes, followed by centrifugation for five min with a speed of 7000 rpm. Then Ν',Ν'-methylenebisacrylamide (0.3 mg) and APS (0.56 mg) were added into the solution. The polymerization was carried out for 12 h. The obtained hydrogel was immersed into DI water overnight to remove unreacted monomers. As shown in Figure 8, the resulting material showed good water absorbing/releasing properties over multiple cycles. Figure 9 depicts an FT-IR spectrum of NIP AM alone, PPyCl alone and the interpenetrating network.
[0057] Example 2: Interpenetrating network formed by polymerizing a hygroscopic polymer in the presence of a thermoresponsive polymer
[0058] N-isopropylacrylamide monomers (567 mg), N, N-tetramethylenediamine (10 μί) acting as accelerator and deionized (DI) water (10 mL) were mixed together and purged with nitrogen for 10 min (Solution E). The bubbles in the solution E was removed by centrifugation for 5 min at a speed of 7000 rpm. Then the N', N'-methylenebisacrylamide and solution (100 μί, 30 mg/mL) acting as the cross linker and ammonium persulfate solution (APS, 50 228 mg/mL) acting as the initiator were added into 1 mL solution E under sonication. The polymerization was carried out for 12 h. The obtained poly-NIPAM hydrogel was immersed into hot DI water (ca. 80 °C) for 12 h to remove unreacted monomers.
[0059] Poly-NIPAM hydrogel (ca. 1 cm3 ) was immersed in hot DI water (80 °C) to be completely shrunk and then transferred into pyrrole solution (volume ratio of pyrrole and water is 1 : 10) overnight. The swollen hydrogel was washed with DI water. Then, the poly -NIP AM/Py hydrogel was immersed into a solution of ammonium persulfate (228 mg), lithium chloride (127 mg), 37% hydrochloride (85 uL) and 10 mL DI water. The hybrid gel was formed overnight by in situ polymerization within the poly-NIPAM hydrogel. Finally, the obtained poly-NIPAM/PPy-Cl was immersed into hot DI water (ca. 80 °C) for 3 h to remove unreacted monomers. The purification step was repeated 3 times.
[0060] The G' and G" values of pure poly-NIPAM gel, poly -NIP AM/PPy-Cl gel and SMAG are shown in fig. S2. Their gel states are revealed by the wide linear viscoelastic region in the dynamic frequency sweep experiments and further confirmed by the fact that the value of storage modulus is higher than that of the loss modulus in each case. The poly-NIP AM/PPy- Cl gel sample shows identical G' and G" values with those of pure poly-NIPAM gel, which is attributed to the similar skeleton structure brought by the continuous and flexible polymeric network of the poly-NIPAM. On the contrary, the G' and G" values of SMAG are significantly lower than that of the poly -NIP AM/PPy-Cl gel, indicating a weakened skeleton. Moreover, the G" of SMAG and all the control samples based on poly-NIPAM show identical trend (e.g. inflection point at -50 Hz), indicating that the framework of SMAG was established by the poly-NIPAM network.
[0061] Example 3: Water harvesting evaluation
[0062] The RH can be stabilized to a required value by a certain super-saturated salt solution. To evaluate the hygroscopicity, the obtained samples were attached in the nylon mesh bag, which was suspended above the super-saturated salt solution in an enclosed container (without air convection) at a temperature of 25 °C (achieved by constant temperature oven) to create required RH level. Additionally, since the RH is related to the air pressure, a needle was used to connect internal space and atmosphere, maintaining an ambient air pressure. A series of RH can be achieved by specially selected salts.
[0063] The network prepared in Example 2 was cut into sheets with thickness of ~5 mm were cut into small pieces with area of 1 cm2 . The obtained tablets were completely dried in vacuum oven at 100 °C. The dried network (50 g) was bagged by meshed nylon and exposed to moisture air at certain relative humidity (RH). After that the hydrated tablets were heated by the solar radiation (lkW m"2 ) to release the containing water in a closed transparent container. The volume of collected water was directly measured by a graduated cylinder. For a typical AWH cycle at RH of 60% and 90 %, the time of water capturing and releasing were 50 min and 10 min, respectively. For a typical AWH cycle at RH of 30 %, the time of water capturing and releasing were 280 min and 80 min, respectively. Figure 11 depicts the water absorption isotherms of SMAG networks at different relative humidities.
[0064] Example 4: Atmospheric water harvesting (AWG)
[0065] Small SMAG tablets (Fig. 14 A) were packaged in transparent nylon mesh bags (Fig.14A I and II), which were exposed to air for water capturing and placed on the upper layer of a closed container for water releasing, demonstrating a scalable, potentially low-cost atmospheric water harvester. The solar vaporized water (i.e. normal mode) was condensed on the transparent condenser (Fig. 14A III) and flowed to the bottom, converging with the directly released water upon the express mode (Fig. 14A IV). As shown in Fig 14 B and C, upon exposure to the moist air, the original dry SMAG bags display a visible swelling after several hours, indicating that the moisture can be captured by the SMAGs. The subsequent water releasing of swollen SMAGs was processed by placing the container under natural sunlight.
[0066] The AWH experiment was carried out from 5:00 a.m. (ca. 1 hour before sunrise) to 9:00 a.m. under a sunshade, where the ambient temperature, RH and dew point temperature were traced (Fig. 14D). In the early -morning, the RH was around 85 %, indicating an ideal environment for rapid water harvesting. However, the comparison of ambient temperature (Fig. 14D) and dew point temperature (Fig. 14D) eliminated the possibility of spontaneous water condensation. Upon exposure to the ambient, the water uptake of SMAG tablets can be increased to 5.4 g g"1 in four hours with an average water capturing rate of ca. 1.3 g g"1 h"1 . Subsequently, the hydrated SMAGs were retrieved and exposed to the sunlight (ca. 0.7 kW m"2 ) from 10:00 a.m. to 2:00 p.m. (Fig. 14E). The water adsorbed at the surface of SMAG tablets can be evaporated by the solar heating, increasing the internal RH of the container (to a saturated state). When the SMAGs were heated to ca. 40 °C, its surface temperature variation was slowed down (Fig. 14E), indicating a stimulated water releasing in the express mode. The quantitative monitoring of water uptake (Fig. 14E) further confirmed a major water release of 3.9 g g"1 from 10:40 to 11 :20. After that, the surface temperature of SMAG gradually increased to ca. 63 °C, which was an equilibrium temperature upon evaporation cooling and solar heating due to the water release in the normal mode. It still contributed to a continuous water release (ca. 0.4 g g"1 h"1 ) after 11 :20. Moreover, the condenser maintained a low temperature (Fig. 14E), enabling a steady condensation of vaporized water. The internal air temperature went beyond 40 °C after 12:00 a.m. (Fig. 14E), suggesting that the main water releasing process was finished. It was worth noting that, although the environmental RH is fluctuant and the natural sunlight is relatively weak compared with most of drought regions around the world, the SMAG presents efficient water production. These results indicate that the SMAGs enables a flexible AWH adapting to the varying environment, revealing its potential for practical applications.
[0067] The compositions and methods of the appended claims are not limited in scope by the specific compositions and methods described herein, which are intended as illustrations of a few aspects of the claims and any compositions and methods that are functionally equivalent are intended to fall within the scope of the claims. Various modifications of the compositions and methods in addition to those shown and described herein are intended to fall within the scope of the appended claims. Further, while only certain representative compositions and method steps disclosed herein are specifically described, other combinations of the compositions and method steps also are intended to fall within the scope of the appended claims, even if not specifically recited. Thus, a combination of steps, elements, components, or constituents may be explicitly mentioned herein or less, however, other combinations of steps, elements, components, and constituents are included, even though not explicitly stated. The term "comprising" and variations thereof as used herein is used synonymously with the term "including" and variations thereof and are open, non-limiting terms. Although the terms "comprising" and "including" have been used herein to describe various embodiments, the terms "consisting essentially of and "consisting of can be used in place of "comprising" and "including" to provide for more specific embodiments of the invention and are also disclosed. Other than in the examples, or where otherwise noted, all numbers expressing quantities of ingredients, reaction conditions, and so forth used in the specification and claims are to be understood at the very least, and not as an attempt to limit the application of the doctrine of equivalents to the scope of the claims, to be construed in light of the number of significant digits and ordinary rounding approaches.
Page 21
[0002] CROSS-REFERENCE TO RELATED APPLICATIONS[0003] [0001] This application claims priority to U.S. Provisional Patent Application No. 62/576,251 , filed on October 24, 2017, entitled "METHOD AND DEVICES FOR ENHANCED WATER EVAPORATION FROM SALTY AQUEOUS SOLUTION BY USING SUNLIGHT AS ENERGY SOURCE," the disclosure of which is incorporated here by reference in its entirety.
[0004] BACKGROUND
[0005] TECHNICAL FIELD
[0006] [0002] Embodiments of the subject matter disclosed herein generally relate to methods and devices for water evaporation, and more specifically, to methods and systems for enhancing water evaporation from salty aqueous solutions using sunlight as energy source.
[0007] DISCUSSION OF THE BACKGROUND
[0008] [0003] Sunlight is the most abundant and accessible renewable energy source. The annual solar energy incident on the Earth's surface is 10
4 times the current annual global energy consumption. One of the promising options to utilize solar energy is the solar-driven water evaporation, also known as solar steam generation. This method is widely utilized in various applications. The most important application is the solar distillation, which uses solar-driven water evaporation to produce steam and then collects the condensate as fresh water. [0004] Solar distillation is able to effectively deal with a variety of water sources, including seawater, industrial wastewater, brine, brackish water, etc. Unlike other water-related technologies, solar distillation does not involve any moving parts, electronic devices and high pressure operations, which makes it attractive and economical especially for small to medium scale applications. The solar-driven water evaporation process also has a great potential for many types of water removal processes, such as in wastewater treatment, to reduce the volume of the wastewater and to incidentally obtain fresh water, especially in oil and energy sectors.[0009] [0005] A conventional passive solar still (see "Renewables: Wind, Water, and Solar," A comprehensive decade review and analysis on designs and performance parameters of passive solar still, December 2015) 100, as illustrated in Figure 1 , has a container 102 that holds water 104. A black photothermal paint 106 that absorbs sunlight 108 is coated on the bottom of the container 102. The top of the container 102 is covered with a glass 1 10 for allowing the sunlight to enter inside the container and heat the water. The water source 104 sits on top of the photothermal paint 106. The sunlight 108 enters through the glass cover 1 10 and hits the water surface first, before reaching the bottom photothermal layer 106. The entire water source 104 is slowly heated up during daytime due to the direct exposure to the sunlight and also due to the heat released by the photothermal paint 106.
[0010] [0006] Part of the water source 104 evaporates forming vapors 1 12, which move upward and arrive at the glass cover 1 10. Because the glass cover is cooler than the water vapors, the water vapors condensate on the glass cover, forming a condensate 1 14. The condensate 1 14 includes pure (distilled) water. All the impurities and/or salts from the water source 104 are left with the water source. The purified water 1 14 falls due to the gravity (the glass cover is tilted) to an output 1 16. In this way, pure water is separated from the water source 104. Note that the water source 104 may be a mixture of water and any other substances.
[0011] [0007] This is not a zero-liquid-discharge process as the concentrated source water 104 has to be disposed before the formation of salt crystal on top of the photothermal layer 106 to avoid a cleaning operation. Furthermore, in a conventional solar still as illustrated in Figure 1 , as the water evaporation goes on, the salt concentration increases in the water source 104, which undesirably decreases the water evaporation rate and therefore degrades the system's performance.
[0012] [0008] In industrial practice, disposal of brine water is chosen instead of drying out the source water completely in solar-still based operations. Disposal of a small quantity of brine is not a problem, but brine disposal at a large scale is a great challenge because a continuous disposal of highly concentrated brine on land or sea would cause soil salinization, affect vegetation, and impact the health of marine life. Most of the current clean water production technologies, such as reverse osmosis (RO), membrane distillation (MD), ion exchange, etc., generate a large quantity of brine wastewater and the water production plants using these technologies are all facing great challenge in brine disposal management.
[0013] [0009] In the last decade, the interfacial heating idea was introduced to the solar-driven water evaporation processes to reduce heat loss and to ensure a fast response in steam generation by concentrating all of the heat that is generated by the photothermal materials within a thin top surface water layer (see, "The emergence of solar thermal utilization: solar driven steam generation," J. Mater. Chem. A, 2017, 5, 7691 -7709). Unlike in the conventional solar still method discussed above, the photothermal material 106 is placed on top of the water surface in this method. In some of the variations of this method, the source water is pulled up from a bulk water body by capillary effect, in a confined water path, to diminish the heat loss by decreasing the heat transfer from the top water layer to the water body. The advantage of this type of design is that the energy utilization efficiency is greatly increased.
[0014] [0010] In all of the existing interfacial heating photothermal system designs, there is one commonality: the light adsorption surface of the photothermal material is physically/geometrically the same as the water evaporation surface. In these designs, the photothermal material is located right at the water/air interface and the water evaporates directly above/from the photothermal material surface and into the overlying air.
[0015] [0011] However, there is an intrinsic problem as a result of these designs. Salt crystallization and solid precipitation appear on the surface of the photothermal material as water evaporates, leaving behind solid deposits on the surface of the photothermal material. As the amount of crystallized salt and other solids
[0016] accumulates on the photothermal material surface, the light capture capability of the photothermal material is suppressed considerably, which would necessitate frequent physical cleaning and rinsing of the salt/solid off the surface.
[0017] [0012] It has been reported that as the salt accumulated on a graphene-oxide (GO) photothermal membrane, the water evaporation rate was reduced from 2.0 to 0.5 kg.m
2 /h, representing a 75% decrease in performance (see, Environmental Science & Technology 2017 Sep 27, doi: 10.1021/acs.est.7b03040). [0013] Thus, the existing methods and devices are limited in the sense that their efficiency decreases over time as the salt accumulates on the light absorbent material. Therefore, there is a need for a method and device for water purification that overcomes the limitations noted above.[0018] SUMMARY
[0019] [0014] According to an embodiment, there is a solar-powered system that includes a support portion and an evaporation portion having a pumping layer and a photothermal layer. The support portion pumps a fluid to the evaporation portion, the pumping layer evaporates the fluid based on solar power; and the photothermal layer is insulated from the pumping layer.
[0020] [0015] According to another embodiment, there is a solar-powered system that includes a support portion and an evaporation portion having a pumping layer and a transparent non-porous, layer covering a first face of the pumping layer. The support portion pumps a fluid to the evaporation portion, and the pumping layer evaporates the fluid at a second face, opposite the first face, based on solar power.
[0021] [0016] According to yet another embodiment, there is a method for evaporating water from a source, the method including a step of placing a solar- powered system into a water source, and a step of evaporating water from the water source with the solar-powered system. The solar-powered system includes a support portion and an evaporation portion having a pumping layer and a
[0022] photothermal layer. The photothermal layer is insulated from the pumping layer.
[0023] BRIEF DESCRIPTON OF THE DRAWINGS
[0024] [0017] The accompanying drawings, which are incorporated in and constitute a part of the specification, illustrate one or more embodiments and, together with the description, explain these embodiments. In the drawings:
[0025] [0018] Figure 1 illustrates a traditional solar still;
[0026] [0019] Figure 2 illustrates a solar-powered system that has a pumping and evaporation layer separated from a photothermal layer;
[0027] [0020] Figure 3 illustrates another solar-powered system that has a pumping and evaporation layer separated from a photothermal layer;
[0028] [0021] Figure 4 illustrates a variation of the solar-powered system of Figure 2;
[0029] [0022] Figure 5 illustrates another variation of the solar-powered system of Figure 2;
[0030] [0023] Figure 6 illustrates yet another variation of the solar-powered system of Figure 2;
[0031] [0024] Figure 7 illustrates still another variation of the solar-powered system of Figure 2;
[0032] [0025] Figure 8 illustrates another variation of the solar-powered system of Figure 2;
[0033] [0026] Figure 9 illustrates yet another variation of the solar-powered system of Figure 2;
[0034] [0027] Figure 10 illustrates various shapes of an evaporation portion of the solar-powered system; and
[0035] [0028] Figure 1 1 is a flowchart of a method for evaporating water with a solar- powered system. DETAILED DESCRIPTION
[0036] [0029] The following description of the embodiments refers to the
[0037] accompanying drawings. The same reference numbers in different drawings identify the same or similar elements. The following detailed description does not limit the invention. Instead, the scope of the invention is defined by the appended claims. The following embodiments are discussed, for simplicity, with regard to a solar- powered system that is used to evaporate water from a water source. However, the invention is not limited to this scenario, but it may be used to evaporate water of another fluid from a fluid source.
[0038] [0030] Reference throughout the specification to "one embodiment" or "an embodiment" means that a particular feature, structure or characteristic described in connection with an embodiment is included in at least one embodiment of the subject matter disclosed. Thus, the appearance of the phrases "in one embodiment" or "in an embodiment" in various places throughout the specification is not necessarily referring to the same embodiment. Further, the particular features, structures or characteristics may be combined in any suitable manner in one or more
[0039] embodiments.
[0040] [0031] According to an embodiment, the photothermal material part, which is responsible for light adsorption, and the water evaporation surface of a solar- powered system are physically separated from each other so that the salt that is formed due to the water evaporation does not contaminate the photothermal material part. This novel configuration provides a rational solution to the long-standing problem of salt solid-accumulation-led-performance-degradation in solar distillation systems. This novel design offers a variety of options to suits varying application purposes, including solar desalination with zero-liquid discharge, salt recovery from wastewater, salt mineral extraction from salt lakes, and brine treatment for all kind of water plants.
[0041] [0032] Figure 2 shows a schematic of a solar-powered system 200 that prevents the accumulation of salt/solid on the surface of the photothermal material by separating the water evaporation interface, where the salt/solid accumulation takes place, from the light incident interface of the photothermal material. According to this embodiment, the solar-powered system 200 (called system herein) has a support portion 201 A and an evaporation portion 201 B. The evaporation portion 201 B, as discussed later, is responsible for absorbing and transforming the sunlight into heat and using the heat to evaporate the water. The evaporation portion 201 B is shown in Figure 2 as being cup-shaped and being attached and supported by the support portion 201 A. The evaporation portion 201 B has an inner surface 200A that bears the function of absorbing light 202 and generating heat while the outer surface 200B is endowed with the function of evaporating the water at a water evaporation interface 204. The water evaporation interface 204 is defined as a border or boundary between the water in the liquid phase and water in the vapor phase (the vapors happen because of the evaporation of the water in the liquid phase). The water evaporation interface may coincide or not with the outer surface 200B of the evaporation portion 201 B.
[0042] [0033] With the solar-powered system 200 of Figure 2, the salt crystallization and solid accumulation occurs only on the outer surface 200B and therefore, it will not affect the light absorption capability of the inner surface 200A. Results of a few experiments carried on for the system shown in Figure 2 have shown that in this case, the salt 224 is loosely accumulated on the outer surface 200B, at the water evaporation interface 204, and thus does not significantly affect the water evaporation rate therein.
[0043] [0034] The wall of the evaporation portion 201 B of the system 200 is a multilayered structure that includes, in this embodiment, a support layer 210, a pumping layer 212, and a photothermal layer 214. The support layer 210 may be made of any material (e.g., metal or composite or plastic, etc.) that has enough mechanical strength to support the pumping and photothermal layers. As the entire system may have a height H between 5 cm to 10 m (even higher), it is up to the support layer 210 to maintain the cup shape of the system. Note that the support layer 210 in this embodiment extends in both the support portion 201 A and the evaporation portion 201 B, i.e., the support layer 210 extends all the way through the system 200. However, in one embodiment, it is possible that the support layer 210 extends only from the top of the system to point A (only in the evaporation portion 201 B), and the pumping layer 212 acts as a support element for the top (cup) portion of the system. The support layer 210 also has the scope of physically separating the pumping layer 212 from the photothermal layer 214. For transferring the heat from the heat photothermal layer 214 to the pumping layer 212, the support layer 210 may have a thermal conductivity of at least 1 W nr
1 k-1 .[0044] [0035] The pumping layer 212 is configured to "pump" (or supply) water from a source 220 (the source may be a container, the sea, ocean, lake, etc.) to the evaporation portion 201 B of the system. The pumping may be passive or active. A passive pumping is achieved by using capillarity, i.e., the pumping layer may have plural small channels (or may be porous) 213 (only two are illustrated for simplicity, but one skilled in the art would understand that there are many small channels that extend all the way from the source 220 to the top of the pumping layer). In this way, the fluid (typically water) 222 from the solution 224 (e.g., brine) stored by source 220 is transported (pumped) to a proximity of the photothermal layer 214.
[0045] [0036] In other words, the solar-powered system 200 includes a support portion 201 A and an evaporation portion 201 B having a pumping layer 212 and a photothermal layer 214. The support portion 201 A pumps the water 222 to the evaporation portion 201 B and the pumping layer 212 evaporates the water 222 based on the solar power. The photothermal layer 214 is insulated from the pumping layer 212 either by another layer 210, or by other means, as discussed later.
[0046] [0037] An active pumping may be achieved by using a motor 230 and one or more pipes 232, as illustrated in Figure 3, for mechanically pumping the fluid 222 from the solution 224 to the proximity of the photothermal layer 214. Other mechanisms may be used for actively pumping the fluid 222 to the proximity of the photothermal layer 214.
[0047] [0038] The photothermal layer 214 is located on the support layer 210, opposite to the pumping layer 212. The photothermal layer 214 is configured to capture sunlight 202 from the sun and convert it to heat. The photothermal layer 214 can be a porous or nonporous material. The photothermal layer 214 is directly exposed to the sunlight.
[0048] [0039] In case that the photothermal layer 214 is porous, the other functionality of the support layer 210 is to keep the water from the pumping layer 212 from getting into the photothermal layer 214, i.e., the support layer 210 has to be non-porous in this case to not transport the water from the outer surface 200B to the inner surface 200A. In case that the photothermal layer 214 is non-porous, the support layer 210 can be porous or non-porous or can be omitted. As discussed above, the pumping layer 212 is a porous layer for water evaporation. The pore size of this layer should be less than 1 mm to ensure a strong capillary force to pull water from the solution 224.
[0049] [0040] The water from the solution 224, which may be the salty source water of interest, such as sea water, brine water, and wastewater, spontaneously moves from the source 220 to the interface 204 (porous water evaporation layer) due to the capillary force and transpiration effect. Under the sunlight illumination, the photothermal layer 214 captures the sunlight 202 and converts the solar energy to heat. The heat energy is transferred to the support layer 210 and then to the pumping layer 212 and the interface 204 to accelerate the water evaporation rate there. The salt 224 will precipitate on the surface of the pumping layer 212 with a loosely stacked structure and it will drop off the outer surface from time to time without the need of manual intervention. In some cases, some additives need to be added to the source brine to control the structure of the salt crystal and thus, to make the salt easily removable. In some cases, the pumping layer 212 may need to be cleaned after a long time operation. After the water 222 evaporates, the water vapors 226 (see Figure 2) may be recovered with known mechanisms (e.g., condensation mechanisms).
[0050] [0041] The entire system 200 may be attached with a support mechanism 230 to the source 220. In one application, the support mechanism includes a flange and bolts. In another application, the support mechanism 230 may be a floating platform (for example, a barge or a boat) that floats in the ocean (the source 220) and the system 200 extracts independently and autonomously distilled water from the sea water. In still another application, more than one system 200 is attached to the source 220. In yet another application, the system 200 is a small system (e.g., in the order of cm) and many such systems are released on a salty source (e.g., sea, ocean or a brine storage container) for separating the water from the salt. Those skilled in the art would understand that the device described in Figures 2 and 3 may be used for other chemical processes that require a source of energy.
[0051] [0042] The system 200 may be modified to have a different shape than the cup shape shown in Figure 2. For example, as illustrated in Figure 4, a similar system 400 may have only one arm 401 . Although Figure 2 shows the system 200 having two straight arms (in fact the system 200 in Figure 2 has the evaporation portion shaped as a cup and the two arms correspond to a cross-section through the cap) and Figure 4 shows the system 400 having a single straight arm, those skilled in the art would understand that these arms may be curved or more arms may be used (for example, plural arms that open up as the petals of a flower).
[0052] [0043] Different configurations for the layers discussed above may be used. For example, Figure 5 shows a configuration in which system 500 includes the photothermal layer 214 and the pumping layer 212, but no support layer 210. For this configuration, the photothermal layer 214 is nonporous. In this case, the support layer 210 is not needed. In other words, the photothermal layer 214 directly contacts with the pumping layer 212. The photothermal layer 214 or the pumping layer 212 or both is selected to provide the mechanical strength to support the entire structure. [0044] Figure 6 shows a system 600 for which the pumping layer 212 is porous and it acts as both the photothermal layer 214 and water evaporation layer at the same time. A nonporous transparent layer 602 is coated/covered on the sun receiving side of the pumping layer 212 to prevent water evaporation from and off the sun receiving side of the pumping layer and thus, to prevent the possibility of salt precipitation on the inner surface of the pumping layer. The nonporous transparent layer 602 can also be given the role of co-photothermal material to absorb sunlight to certain extent in this case. The mechanical strength of the system can be provided by the nonporous transparent layer 602, or by the pumping layer 212, or by both of them.
[0053] [0045] Figure 7 shows a system 700 in which the pumping layer 212 is porous and acts as both the photothermal layer and the water evaporation layer at the same time. A transparent nonporous film or plate 702 (top cover) covers the top of the cup structure as shown in the figure and the top cover 702 keeps the water vapor from escaping from the cup structure. In other words, the top cover 702 forms a cavity 704 with the inner surface of the pumping layer 212. Thus, this structure prevents continuous water evaporation at the inner surface 212A of the pumping layer 212. The system 700 effectively stops the salt precipitation on the inner surface 212A of this structure and allows water evaporation and precipitation only on the outer surface 212B of the pumping layer 212.
[0054] [0046] Figure 8 shows a system 800 having a solid cone structure. In this embodiment, the top layer is the photothermal layer 214, which captures sunlight and converts solar energy to heat. The middle layer 802 is a thermal conducting layer, which passes the heat from the photothermal layer 214 to the pumping layer 212. The pumping layer 212 may have a porous structure and acts as the water evaporation layer. The thermal conducting layer 802 fills in the entire cup of the evaporation portion.
[0055] [0047] Figure 9 shows a system 900 in which the cup structure of the previous systems is changed to a solid disk structure. In this embodiment, the top layer is the photothermal layer 214, which captures sunlight and converts solar energy to heat. The middle layer is a thermal conducting layer 210, which passes the heat from photothermal layer 214 to the pumping layer 212. The pumping layer 212 possess a porous structure, acting as the water evaporation layer, as in the previous embodiments.
[0056] [0048] A common idea of these embodiments is to stop the continuous water evaporation on the surface of the photothermal layer facing the sunlight, and therefore, to prevent the surface of the photothermal layer from being covered by salts/solid. Thus, those skilled in the art, having the advantage of this document, would be able to design other systems that separate the water evaporation from the photothermal layer so that no salt is deposited on this layer.
[0057] [0049] The photothermal layer used in these embodiments may include all types of existing and potentially possible materials that have strong light absorption capability in the solar spectrum range, such as metal nanoparticle (gold, silver, copper, cobalt, iron, nickel, aluminum, and there alloys), carbon based materials (carbon black, carbon nanotubes, graphene, graphene oxide, reduced graphene oxide, etc.), black metal oxides (C03C , Mn02, T12O3, Fe30
4 , CuCr204 , FeCr204 , CuMn204 , MnFe204 , ZnFe204 , MgFe204 , etc.), black metal chalcogenides (M0S2, MoSe2, WSe2, CdS, CdTe, etc.), black paint and black cement materials, and black polymer materials. The spectrally selective absorber materials of the photothermal layer are especially desired, which may provide best performance.[0058] [0050] The water evaporation material of the pumping layer should be porous and hydrophilic to ensure strong water absorption capability and to make sure there is a strong capillary force to pull and spread water onto the entire water evaporation interface. This material may be paper, quartz glass fibrous membrane, carbon paper, copper foam, carbon foam, polymer foam, macroporous silica, etc. A thickness of any of these layers may be in the nanometer to centimeter range, except for the support layer, which may be thick enough to support the other layers.
[0059] [0051] During solar distillation application, a device incorporating any of the systems discussed above may be placed directly on top of the salty source water of interest and/or self-float there. The device may also be physically away from the salty water surface, with a water supply path (as shown in Figure 2) provided by a hydrophilic porous materials (e.g., cotton, silica, polymer, metal oxides, carbon, etc.) to continuously deliver water to the water evaporation surfaces (e.g., the outer surface in all structures).
[0060] [0052] Although same of the embodiments discussed above disclose a cup- shaped evaporation portion of the system, one skill in the art would understand that other shapes may be implemented for these systems. For example, as illustrated in Figure 10, the evaporation portion of the system may have, instead of the conical shape 1000 used for the embodiment of Figure 2, a semi-spherical shape 1002, a cylindrical shape 1004 or a cubical shape 1006.
[0061] [0053] A method for evaporating water from a source, based on one of the systems discussed above, includes a step 1 100 of placing a solar-powered system 200 into a water source 220 and a step 1 102 of evaporating water 222 from the water source 220 with the solar-powered system. The solar-powered system includes a support portion 201 A, and an evaporation portion 201 B having a pumping layer 212 and a photothermal layer 214. The photothermal layer 214 is insulated from the pumping layer 212.
[0062] [0054] One or more of the advantages of the systems discussed above are as follow: (1 ) no water evaporation occurs on the light adsorption surface of the photothermal material and thus, no salt accumulates on the surface of the photothermal material that is facing the sunlight. Thus, the light adsorption performance in these systems is not affected by water evaporation and salt accumulation and therefore, there is no need for regular maintenance, which is expensive. A constant and non-degrading solar energy harvesting is thus achieved for the embodiments discussed above, which none of the current solar distillation systems is able to do. Thus, the rational of separating the light adsorption surface from the water evaporation surface would offer benefit for any practical solar distillation application. (2) The systems discussed above allow for the crystallized salt or other solids on the water evaporation surface to leave the surface on its own gravity, which minimizes human intervention. (3) Given the loose nature of the surface-water-evaporation-induced salt accumulation, the effect of the surface accumulated salt solid on the surface water evaporation rate is insignificant. (4) The surface of the water evaporation interface can be further modified to be salt-resistant so that salt crystal or other solids, once formed, would leave the surface
[0063] immediately, leaving behind no solid residue. This would further improve the photothermal material's long-term operation performance. [0055] Thus, the solar-distillation structures discussed above promise a constantly high photothermal performance, reduce the maintenance requirement of the system during applications, and extends the system's operation longevity, all leading to much reduced operational cost for the same level of products delivered.
[0064] [0056] The solar-driven water evaporation process used by the systems discussed above has three emerging application directions: (1 ) solar-driven seawater desalination, which, with its unmatched energy efficiency (i.e., 80% at lab scale), is regarded as having a potential of becoming the next-generation seawater desalination technology, especially for small scale plants; (2) brine treatment - brine disposal is a long-lasting problem in many industrial processes, including SWRO, mineral extraction, solar distillation, etc. These devices can be placed on top of the conventional evaporation pond to accelerate the water evaporation efficiency; and (3) salt extraction out of salty water for the purpose of metal salts mining from salt lakes or sea water and salt resource recovery from some waste salt water. This is a largely uncharted territory for solar-driven water evaporation, but represents a future growth point of the utilization of solar energy.
[0065] [0057] The disclosed embodiments provide methods and mechanisms for separating an evaporation interface from a photothermal layer. It should be understood that this description is not intended to limit the invention. On the contrary, the embodiments are intended to cover alternatives, modifications and equivalents, which are included in the spirit and scope of the invention as defined by the appended claims. Further, in the detailed description of the embodiments, numerous specific details are set forth in order to provide a comprehensive understanding of the claimed invention. However, one skilled in the art would understand that various embodiments may be practiced without such specific details.
[0066] [0058] Although the features and elements of the present embodiments are described in the embodiments in particular combinations, each feature or element can be used alone without the other features and elements of the embodiments or in various combinations with or without other features and elements disclosed herein.
[0067] [0059] This written description uses examples of the subject matter disclosed to enable any person skilled in the art to practice the same, including making and using any devices or systems and performing any incorporated methods. The patentable scope of the subject matter is defined by the claims, and may include other examples that occur to those skilled in the art. Such other examples are intended to be within the scope of the claims.
US2015353385 -- HYDROPHOBIC PHOTOTHERMAL MEMBRANES, DEVICES INCLUDING THE HYDROPHOBIC PHOTOTHERMAL MEMBRANES, AND METHODS FOR SOLAR DESALINATION
US9358750 -- METHOD OF PRODUCING NANOPATTERNED ARTICLES, AND ARTICLES PRODUCED THEREBY
US2017267577 -- COMPOSITIONS AND METHODS FOR MICROPATTERNING SUPERHYDROPHOBIC SURFACES
US10307716 -- Grafted membranes and substrates having surfaces with switchable superoleophilicity and superoleophobicity and applications thereof
https://phys.org/news/2017-01-academics-ultimate-solar-powered-purifier.html
by Cory Nealon

From the top left corner, moving clockwise, the four images depict: University at Buffalo students performing an experiment, clean drinking water, water evaporating, and black carbon wrapped around plastic in water with evaporated vapor on …more
You've seen Bear Grylls turn foul water into drinking water with little more than sunlight and plastic.
Now, academics have added a third element — carbon-dipped paper — that may turn this survival tactic into a highly efficient and inexpensive way to turn saltwater and contaminated water into potable water for personal use.
The idea, which could help address global drinking water shortages, especially in developing areas and regions affected by natural disasters, is described in a study published online today (Jan. 30, 2017) in the journal Global Challenges.
"Using extremely low-cost materials, we have been able to create a system that makes near maximum use of the solar energy during evaporation. At the same time, we are minimizing the amount of heat loss during this process," says lead researcher Qiaoqiang Gan, PhD, associate professor of electrical engineering in the University at Buffalo School of Engineering and Applied Sciences.
Additional members of the research team are from UB's Department of Chemistry, Fudan University in China, the University of Wisconsin-Madison and the lab of Gan, who is a member of UB's New York State Center of Excellence in Materials Informatics and UB's RENEW Institute, an interdisciplinary institute dedicated to solving complex environmental problems.
Solar vapor generator
To conduct the research, the team built a small-scale solar still. The device, which they call a "solar vapor generator," cleans or desalinates water by using the heat converted from sunlight. Here's how it works: The sun evaporates the water. During this process, salt, bacteria or other unwanted elements are left behind as the liquid moves into a gaseous state. The water vapor then cools and returns to a liquid state, where it is collected in a separate container without the salt or contaminants.
"People lacking adequate drinking water have employed solar stills for years, however, these devices are inefficient," says Haomin Song, PhD candidate at UB and one of the study's leading co-authors. "For example, many devices lose valuable heat energy due to heating the bulk liquid during the evaporation process. Meanwhile, systems that require optical concentrators, such as mirrors and lenses, to concentrate the sunlight are costly."
The UB-led research team addressed these issues by creating a solar still about the size of mini-refrigerator. It's made of expanded polystyrene foam (a common plastic that acts as a thermal insulator and, if needed, a flotation device) and porous paper coated in carbon black. Like a napkin, the paper absorbs water, while the carbon black absorbs sunlight and transforms the solar energy into heat used during evaporation.
The solar still coverts water to vapor very efficiently. For example, only 12 percent of the available energy was lost during the evaporation process, a rate the research team believes is unprecedented. The accomplishment is made possible, in part, because the device converts only surface water, which evaporated at 44 degrees Celsius.
Efficient and inexpensive
Based upon test results, researchers believe the still is capable of producing 3 to 10 liters of water per day, which is an improvement over most commercial solar stills of similar size that produce 1 to 5 liters per day.
Materials for the new solar still cost roughly $1.60 per square meter—a number that could decline if the materials were purchased in bulk. (By contrast, systems that use optical concentrators can retail for more than $200 per square meter.) If commercialized, the device's retail price could ultimately reduce a huge projected funding gap — $26 trillion worldwide between 2010 and 2030, according to the World Economic Forum — needed for water infrastructure upgrades.
"The solar still we are developing would be ideal for small communities, allowing people to generate their own drinking water much like they generate their own power via solar panels on their house roof," says Zhejun Liu, a visiting scholar at UB, PhD candidate at Fudan University and one the study's co-authors.
DOI: 10.1002/gch2.201600003
Passive solar vapor generation represents a promising and environmentally benign method of water purification/desalination. However, conventional solar steam generation techniques usually rely on costly and cumbersome optical concentration systems and have relatively low efficiency due to bulk heating of the entire liquid volume. Here, an efficient strategy using extremely low-cost materials, i.e., carbon black (powder), hydrophilic porous paper, and expanded polystyrene foam is reported. Due to the excellent thermal insulation between the surface liquid and the bulk volume of the water and the suppressed radiative and convective losses from the absorber surface to the adjacent heated vapor, a record thermal efficiency of ≈88% is obtained under 1 sun without concentration, corresponding to the evaporation rate of 1.28 kg (m2 h)−1. When scaled up to a 100 cm2 array in a portable solar water still system and placed in an outdoor environment, the freshwater generation rate is 2.4 times of that of a leading commercial product. By simultaneously addressing both the need for high-efficiency operation as well as production cost limitations, this system can provide an approach for individuals to purify water for personal needs, which is particularly suitable for undeveloped regions with limited/no access to electricity.
http://www.sciencemag.org/news/2017/02/sunlight-powered-purifier-could-clean-water-impoverished
DOI: 10.1126/science.aal0699
By Robert Service

One-tenth of the world’s population lacks clean water. Now, researchers report they have developed a cheap solar still, which uses sunlight to purify dirty water up to four times faster than a current commercial version. The raw materials cost less than $2 per square meter. The technology will “allow people to generate their own drinking water much like they generate their own power via solar panels on their house roof,” says Zhejun Liu, a visiting scholar at the State University of New York (SUNY) in Buffalo and one of the study’s co-authors.
Solar stills have been around for millennia. Most are simple black-bottomed vessels filled with water, and topped with clear glass or plastic. Sunlight absorbed by the black material speeds evaporation, which is trapped by the clear topping, and funneled away for drinking water. Most pollutants don’t evaporate, and so are left behind. But much of the sun’s energy is wasted in the slow heating of a full vessel of water. Even the best stills need to be about 6 square meters in size to produce enough water for a single person for a day.
In recent years, researchers have improved stills using two approaches. First, they design their stills so that only the very top layer of water in the vessel is heated and evaporated, which means less energy is lost. Second, they’ve turned to nanomaterials to absorb more of the sun’s rays. But efficient light-absorbing nanomaterials can cost hundreds of dollars per gram, making them unrealistic for widespread use in developing countries where the technology is needed most.
Qiaoqiang Gan, an electrical engineer at SUNY Buffalo, saw that problem firsthand. His lab was already developing new nanomaterials as absorbers for solar power cells, and wanted to also use them in a solar still. But it quickly became apparent that the material’s cost would never allow the technology to be viable. So Gan began looking for cheap alternatives.
His team’s new device has three main components. Gan and his colleagues start with a fiber-rich paper—sort of like the paper used to make currency. They coat this with carbon black, a cheap powder left over after the incomplete combustion of oil or tar. Next, they take a block of polystyrene foam—the stuff used to make coffee cups—and cut slices through it making 25 connected sections. The foam floats on the untreated water and acts as an insulating barrier to prevent sunlight from heating up too much of the water below. The researchers then layer pieces of their paper over each section, folding the ends down so that they dangle into the water. The paper wicks water upward, wetting the entire top surface of each of the 25 sections. Finally, a clear acrylic housing sits on top.
During operation, evaporated water from the carbon paper is trapped by the acrylic and funneled to a collection vessel, and the paper wicks up additional water to replace it. Gan and his colleagues report this week in Global Challenges that the setup not only works, but that it’s 88% efficient at channeling the energy in sunlight into evaporating water. This allows a 1-square-meter-sized device to purify 1 liter of water per hour, which is about four times faster than commercially available versions, Gan says.
Equally important Gan adds, is that the still is cheap. He estimates the materials needed to build it cost roughly $1.60 per square meter, compared with $200 per square meter for commercially available systems that rely on expensive lenses to concentrate the sun’s rays to speed evaporation. At that price, providing the minimal water needed for a family of four might cost as little as $5 for the raw materials per device. That cheap cost may not only help people in impoverished regions, but also help aid workers deploy cheap water purifiers to people affected by natural disasters that wipe out safe drinking water sources. “We think this is an immediate application,” Gan says.
The new work is “good progress,” says Gang Chen, a mechanical engineer at the Massachusetts Institute of Technology in Cambridge, who has developed his own version of the technology in recent years, which uses slightly different materials. The new setup not only uses cheaper starting materials than anything on the market, but makes freshwater much more quickly, he notes. “This is really important in solving many water challenges.”
The authors of the report have formed a company — Suny Clean Water — to commercialize the work and are already in discussions with other companies around the world to make the new technology available.
http://onlinelibrary.wiley.com/doi/10.1002/gch2.201600003/full
DOI: 10.1002/gch2.201600003
Zhejun Liu, e tal.
Abstract
Passive solar vapor generation represents a promising and environmentally benign method of water purification/desalination. However, conventional solar steam generation techniques usually rely on costly and cumbersome optical concentration systems and have relatively low efficiency due to bulk heating of the entire liquid volume. Here, an efficient strategy using extremely low-cost materials, i.e., carbon black (powder), hydrophilic porous paper, and expanded polystyrene foam is reported. Due to the excellent thermal insulation between the surface liquid and the bulk volume of the water and the suppressed radiative and convective losses from the absorber surface to the adjacent heated vapor, a record thermal efficiency of ≈88% is obtained under 1 sun without concentration, corresponding to the evaporation rate of 1.28 kg (m2 h)−1. When scaled up to a 100 cm2 array in a portable solar water still system and placed in an outdoor environment, the freshwater generation rate is 2.4 times of that of a leading commercial product. By simultaneously addressing both the need for high-efficiency operation as well as production cost limitations, this system can provide an approach for individuals to purify water for personal needs, which is particularly suitable for undeveloped regions with limited/no access to electricity.
1 Introduction
Efficient solar energy-to-heat conversion for vapor/steam generation is essential for various applications ranging from large scale absorption chillers, desalination systems to compact and portable applications including drinking water purification and sterilization systems.[1-5] Conventional solar steam generation techniques usually rely on costly and cumbersome optical concentration systems to heat a bulk liquid.[6] Even though some highly absorbing materials are utilized to enhance solar absorption, such as charcoal,[7] sponge,[8] or cotton cloth,[9] the energy conversion efficiency is still relatively low (e.g., 30–40%[10]) due to the heat dissipation in the entire liquid volume. Therefore, there is a significant need to develop more efficient, self-powered, and highly portable solar energy harvesting systems for vapor/steam generation. Low-cost and broadband light absorbing micro/nanomaterials show promise in this regard.
In recent years, plasmonic nanoparticles (NPs) and their assemblies have been widely studied because of their unique light and heat localization properties. In particular, it was revealed that the localized heat effect can be used for new solar vapor/steam generation that cannot be addressed using conventional technologies that heat the entire fluid volume. For instance, plasmonic metallic NPs[11-13] and nanorods[14, 15] dispersed in aqueous solutions can generate vapor bubbles. However, due to limited solar absorption bands, the resulted solar thermal conversion efficiencies of these earlier works are relatively low. For example, it was reported that Au NPs dispersed in water obtained a solar thermal conversion efficiency of 24% (i.e., only 24% of the solar energy was transferred to generate vapor).[16] To overcome this bandwidth limitation, broadband dark metallic nanostructures (e.g., Au and Al-based NPs[17-20]) were developed to enhance the overall solar-to-heat conversion efficiency (e.g., 57.3% under illumination of 20 kW m−2 (i.e., 20 sun concentration)[17] and 92.6% under illumination of 6 kW m−2 (i.e., 6 sun concentration) using ultrabroadband black gold membrane structures,[18] 77.8% under illumination of 4.5 kW m−2 (i.e., 4.5 sun concentration) using airlaid-paper-based Au NP structure[19]). However, the intrinsically high cost of Au-based nanomaterial (e.g., retail price of $395 mg−1 for Au nanoshells[21]) is a significant bottleneck for practical applications using these systems. This is especially true when absorbing NPs are dispersed throughout the bulk of a liquid (e.g., ref. [16]) and a significant number is effectively wasted due to absorption and scattering of the incident light by the NPs above.
To overcome this issue, floating substrates such as carbon foam,[22] paper,[19] and nanoporous anodic alumina[17, 18, 20] have been employed to localize the absorbing material at the surface of water for more efficient and cost-effective solar steam generation. In these platforms, the substrates functioned as thermally insulating layers that reduce the heat transfer between the vaporization region (i.e., the water surface) and the bulk liquid. Due to the capillary action of these porous supports, localized evaporation was realized with improved thermal efficiencies (e.g., 64% under 1 kW m−2 illumination using exfoliated graphite on carbon foam[22]). Additionally, it was reported that the use of solar concentrators further improved the thermal efficiencies of these systems, up to 85–90% (e.g., ref. [17-20, 22]). However, in order to achieve these high efficiencies, these platforms still require specialized fabrication of highly absorbing, structured nanomaterials (e.g., black gold or aluminum NPs on nanoporous anodic alumina[17, 18, 20]) and/or porous hydrophilic supports (e.g., porous carbon foams at the retail price of ≈$1.5 in.−3[23]), as well as costly solar concentrating systems. These requirements impose prohibitively high costs for practical applications over large areas.
In this work, we report an efficient carbon-based solar vapor generation system based on carbon-coated paper (CP) affixed to expanded polystyrene (EPS) foam. Due to the superior absorption, heat conversion, and insulating properties of our CP-foam structure, most of the absorbed energy can be used to evaporate surface water with significantly reduced thermal dissipation compared with previously reported architectures.[24-26] Remarkably, we realized a record solar thermal conversion efficiency of >88% under illumination of 1 kW m−2 with no solar concentration. Furthermore, seawater desalination was also demonstrated with reusable stable performance. By utilizing extremely low-cost materials, and circumventing the need for solar concentrators, economically viable large area systems will be possible with no energy input required for operation. This prospect is particularly attractive for addressing global freshwater shortages, especially for individuals to purify water for personal needs (i.e., ≈2 L d−1) in developing regions.
2 Results
2.1 CP for Solar Vapor Generation
In previously reported pioneering works based on porous materials (e.g., ref. [17-20, 22]), capillary force is essential to assist the enhanced vapor generation process since it is much easier to vaporize small droplet diffused into the pores than to heat and vaporize the bulk volume. In principle, hydrophilic porous materials are generally suitable for this purpose.[27] In this work, we selected a fiber-rich nonwoven paper (Texwipe TX609[28]) as our support since it is extremely low-cost (i.e., retail price of ≈$1.05 m−2), chemical-binder-free, and has excellent water transport properties. Its microstructure is shown in Figure 1A, consisting of 10–20 μm wide paper-fiber bundles. We then dye it using low-cost carbon black powders (e.g., Sid Richardson Carbon & Energy Co., retail price of $2.26 lb−1; see Section S1 in the Supporting Information for fabrication details and stability/durability test results). As a result, the paper fibers were coated with carbon nanoparticles, as shown in Figure 1B. The direct comparison between the white paper and the carbon-coated paper is shown in the inset of Figure 1C. The optical absorption of the CP is very strong with the average absorption of ≈98% throughout the visible to near IR domain (from 250 nm to 2.5 μm, measured by a spectrophotometer equipped with an integration sphere, Shimadzu UV-3150). This strong broadband optical absorption is particularly promising for low-cost solar-to-heat conversion. It should be noted that although the latest reported Al-nanoparticle structure is also inexpensive if implemented in yield productions,[20] the inflammability of 5–30 nm sized Al-NPs imposes a potential safety issue (see the Safety Data Sheet of Al NPs,[29] code H261[30]). Therefore, the proposed CP structures are also superior since they are environmentally benign and safe to handle during production.


To demonstrate the baseline for solar vapor generation performance, we first performed a direct comparison under several different conditions as shown in Figure 1D (see Section S2 in the Supporting Information for experiment details). In this experiment, the open area of the beaker is 35.3 cm2, containing ≈165 g water. In the dark environment (i.e., at room temperature of 21 °C and humidity of 10%), the water weight loss is 0.44 g h−1. Therefore, the average evaporation rate in the dark environment is 0.125 kg (m2 h)−1, which will be subtracted from all subsequent measured evaporation rates to eliminate the effect of natural water evaporation. Under the solar illumination using a solar simulator (Newport 69920 with the solar intensity of 1 kW m−2, i.e., AM1.5), the weight loss increased to 1.11 g h−1. After that, we put a 4 × 4 cm2 white paper and a 4 × 4 cm2 CP on top of the water surface, the weight change increased to 1.16 and 1.48 g h−1, respectively. To interpret the weight change difference, we employed a portable thermal imager (FLIR ONE) to characterize the temperature of these samples. The thermal imaging characterization was confirmed by a direct measurement using a thermocouple sensor probe (see Section S3 in the Supporting Information), indicating a reasonable accuracy (i.e., ≤0.4 °C in the 33–35 °C range). As shown in Figure 1E, the CP surface temperature increased to the highest number of 35.4 °C due to the enhanced solar-to-heat conversion. However, this heating effect is not well isolated from the bulk water (i.e., the bulk water was heated to 31.7 °C), resulting in the inefficient vapor generation effect. One can see that the water evaporation speed with the CP is 1.33 times higher than that of pure water under the 1 kW m−2 solar illumination, which is only an incremental improvement. Next, we will discuss the thermal-isolating strategy to confine the heating effect at the top surface for more efficient vapor generation.
2.2 Efficient Vapor Generation Using Thermally Isolated CP
One of the most attractive features claimed by previously reported nanomaterials for solar-vapor generation is the surface heating effect with no need to heat the bulk volume of the water (e.g., ref. [17-20, 22]). According to pioneering studies employing carbon foams,[22] nanoporous alumina,[17, 18, 20] and floating paper,[19] porous supports transport small water droplets to the upper surface directly through the structure of the support. Although they are also designed to serve as thermal insulators, the finite thickness, large contact area, and fluid transport of the porous substrates lead to relatively poor thermal insulation performance (e.g., the thermal conductivities are 0.49 W (m K)−1 in ref. [19] and 0.426 W (m K)−1 in ref. [22]], respectively). Therefore, a better thermal isolation will improve the solar vapor generation performance. In this work, we propose a better strategy to make full use of the capillary force of the porous paper to draw fluid up around the support rather than through it, and thus minimize the thermal loss to the bulk fluid below. As shown by the upper panel in Figure 2A, we inserted a 6 mm thick EPS foam slab under the CP to thermally isolate the porous paper from the bulk water. The thermal conductivity of this EPS foam is 0.034–0.04 W (m K)−1,[31] one of the lowest thermal conductivities available among extremely low-cost materials. In this configuration, the only contact area between the water and CP is at the edges of the porous paper (i.e., a line contact). This significantly reduces the region of fluid transport compared to placing the paper[19] or carbon foam[22] directly on the water surface (see the lower panel in Figure 2A). In this case, the paper contacting the water along the sides of the EPS foam transports the water droplets to the upper surface to facilitate evaporation. It should be noted that during testing, the upper surface of the CP was always wet, indicating that this reduction in transport area does not limit the evaporation rate of the system. A more detailed characterization of the liquid transportation capability of the CP is shown in Section S4 (Supporting Information).
To eliminate the water evaporation from other open areas, the surrounding exposed water surface was covered with EPS foam with a square hole for the CP (Figure 2B). To demonstrate the thermal isolation effect, we then characterized the surface temperature with and without the EPS foam under the CP, as shown in Figure 2C. Under the solar light illumination with the intensity of 1 kW m−2, the upper surface temperature of the CP increased from 32.9 °C (lower panel) to 44.2 °C with the EPS foam insulation (upper panel). The vapor generation performance is shown in Figure 2D. One can see that the water mass change is improved to 1.28 kg (m2 h)−1, which is 3.0 times greater than that of the pure water case and 2.0 times greater than that of CP without EPS foam isolation. This evaporation rate is better than the best reported data under 1 sun illumination with no solar concentration using exfoliated graphite (i.e., circles taken from Figure 2D of ref. [22]). In principle, one would only need a ≈0.2 m2 structure to produce 2 L of freshwater to meet an individual's daily needs assuming 8 h of nonconcentrated solar illumination. Solar concentration will enhance this generation rate further, as will be discussed next.
2.3 High Solar Thermal Conversion Efficiency
In most previously reported works,[17-20, 22] the sample surface is always wet, indicating that the performance is limited by surface temperature only. Therefore, the ultimate performance can be improved by introducing concentrated solar illumination. Next, we will analyze the vapor generation performance under moderate solar concentration conditions to better compare with previously reported nanostructures. In this experiment, an inexpensive planar PVC Fresnel lens (e.g., OpticLens, $2.39 per piece with the area of 26 cm × 17.8 cm) was employed to focus the incident light from the solar simulator. As shown in Figure 3A, when the solar light was concentrated by 3, 5, 7, and 10 times, the water mass change was increased to 3.66, 6.24, 9.34, and 13.30 kg (m2 h)−1, respectively. To characterize the enhanced surface heating effect more accurately, we then employed two thermocouple sensor probes to measure the temperature of vapor and bulk water (see Figure S3 in the Supporting Information). As shown by solid curves in Figure 3B, the vapor temperature increased sharply within the first 3 min and reached a steady state after 10 min. In contrast, the temperature of bulk water increases slowly and continuously as shown by dashed lines in Figure 3B. To evaluate the solar-vapor generation performance quantitatively, we then calculate the solar conversion thermal efficiency, ηth, which is described by Equation (1)[22]
display math(1)
where math formula is the mass flux, hLV the total enthalpy of liquid-vapor phase change, Copt the optical concentration, and qi the normal direct solar irradiation (i.e., 1 kW m−2). Particularly, the calculation of the total enthalpy of liquid-vapor phase change, hLV, should consider both the sensible heat and the temperature-dependent enthalpy of vaporization (see Section S4 for details of this equation and calculation in the Supporting Information). Using Equation (1), we obtained the solar conversion thermal efficiency, ηth, of 88.6% under 1 sun illumination, and 94.8% under 10 times solar concentration, as shown in Figure 3C. Compared with previously reported exfoliated graphite,[22] Au NPs,[18] and black gold membranes,[17] this CP-foam structure realized a very high solar thermal conversion efficiency especially under low optical concentration condition (see direct comparison in Figure 3D calculated by similar data processing procedures; see Section S5 in the Supporting Information for more details). It should be noted that since the reported CP structure does not require any special micro/nanofabrication process, the system is extremely low-cost (cheaper than that of the concentrator) and amenable to scaling up over large or huge areas for real applications. Therefore, there is no need to employ large area solar concentrating systems for real applications.
Figure 3.

In addition, this ηth actually describes the energy consumption in the vapor, and has two major components: the energy used for water-to-vapor phase change, and the energy used to heat the water/vapor. A larger ηth does not necessarily correspond to a higher vapor generation rate. For a given value of ηth, a higher temperature of the generated vapor will actually result in a lower generation rate since more energy is used to heat the water. Therefore, in terms of solar vapor generation rate, it is necessary to analyze the theoretical upper limit and thermal loss channels in order to estimate the opportunity available for improvement.
2.4 Theoretical Upper Limit
The ideal condition for solar vapor generation is to convert liquid water to vapor at ambient temperature with no energy used to heat either the bulk or evaporated water. Radiative loss and convective loss are both assumed to be zero. In this case, based on our experimental conditions (i.e., at ambient temperature of 21 °C), the ideal vapor generation rate is 1.466 kg (m2 h)−1 assuming ηth = 1 and hLV in Equation (1) is 2455.6 kJ kg−1 at ambient temperature.[32] Based on this ideal vapor generation rate, we can straightforwardly estimate ηth obtained in our experiment, i.e., 1.28/1.466 = 87.3%, which only considers the energy used to produce vapor at room temperature. Detailed thermal loss mechanisms are automatically excluded in this simple estimation. However, this theoretical upper limit is unlikely realized since thermal losses are inevitable in these systems. Additionally, under 10× solar concentration, this theoretical maximum is 14.66 kg (m2 h)−1. Therefore, even if these theoretical upper limits can somehow be further approached using advanced (and likely expensive) nanomaterials in the near future, the opportunity for improvement is relatively limited. As a result, the more pressing issue in developing technologies for high performance solar vapor generation is cost, which is the primary advantage of our proposed structure and system.
2.5 Loss Channels
Recently, a new strategy was reported to demonstrate the close to 100 °C steam generation under 1 sun enabled by a floating structure with “thermal concentration.”[33] A detailed thermal loss analysis was performed, revealing that radiative loss and convective loss are two major thermal loss channels in the solar vapor generation systems. The radiative and the convective losses per area are expressed by Equations (2) and (3), respectively
where ε is the emissivity of the CP (i.e., 0.98), σ the Stefan–Boltzmann constant (i.e., 5.67 × 10−8 W (m2 K4)−1), T2 the temperature at the surface of the CP, T1 the temperature of the adjacent environment, and h the convection heat transfer coefficient (assumed to be 10 W (m2 K)−1[33]). Using these two equations, it was estimated that the radiative loss from the 100 °C blackbody absorber surface to the ambient environment (20 °C) is ≈680 W m−2 and the convective loss is ≈800 W m−2. Following this theoretical estimation, when the absorber surface is 44.2 °C (our experimental observation), the radiative loss to ambient is ≈147 W m−2 and the convective loss is ≈232 W m−2, corresponding to a total of 37.9% energy loss (i.e., 14.7 + 23.2%). In this case, it seems that an efficiency ≈90% is impossible. But why can we observe a record high vapor generation rate under 1 sun?
To interpret the unique features and physics of the proposed CP-foam architecture, the thermal environment and heat transfer diagram is analyzed in Figure 4A. First, the downward thermal radiation is suppressed. According to the previously reported experimental characterization, the reflection of a 3 mm thick EPS foam slice is in the range of 40–60% over the spectral region of thermal emission with ≈10% thermal radiation absorption.[34] Therefore, under thermal equilibrium condition, the temperature of the EPS-foam surface is very close to the bottom surface of the CP layer so that the downward radiative loss from the CP layer is significantly suppressed. In this case, the EPS foam employed in our system actually serves as a thermal radiation shield (in addition to its excellent thermal insulation characteristics), which is superior over previously reported double-sided black systems (e.g., ref. [22, 35]).
Figure 4.
A) Energy balance and heat transfer diagram in the CP-foam architecture during the vapor generation process. B) Zoom-in diagram near the surface of the CP structure during the vapor generation process.

If we further analyze the microscopic thermal environment (Figure 4B), one can recognize that the CP surface is covered by a sheet of water and surrounded by heated vapor. The absorbed solar energy of the CP layer will first exchange thermal energy with water sheet and vapor in this small region rather than directly emit thermal radiation and exchange heat with the surroundings through the convection. In particular, in many reported experiments to identify the vapor temperature, a thermocouple was usually placed on top of the absorber surface (e.g., ref. [18, 20, 22, 33]), further demonstrating that the top surface of the absorber is surrounded by heated vapor. Since the temperature of the adjacent environment on top of CP absorber is very close to the temperature of CP surface, the radiative and convective loss should be very small. For instance, according to Equations (2) and (3), the radiative loss from the 44.2 °C surface under 1 sun to the ≈41.6 °C vapor environment is ≈1.8% and the convective loss is only ≈2.6%. Most absorbed solar energy is still used to evaporate the water sheet on top of the absorber surface rather than being lost through these two channels. This is the major physical mechanism for the observed high vapor generation rate. This is also applicable to other reported solar vapor generation systems (e.g., ref. [17, 19, 22, 27, 36-38]) since they are also covered by a film of water and/or surrounded by heated vapor. However, this physical mechanism was not detailed in previous reports.
More importantly, in a real enclosed solar steam system, the vapor cannot be released immediately and the environment inside the system is thermally isolated from the cooler surrounding environment. Furthermore, typical acrylic or glass slabs are opaque to mid-infrared radiation. Consequently, thermal radiation cannot be emitted to the environment. Additionally, convective energy transfers are also largely suppressed when the internal environment is heated under near-thermal equilibrium conditions. In this case, the radiative and convective losses in a real system should be even more negligible, demonstrating the potential to develop practical solar steam systems using extremely low-cost materials. Intriguingly, in the latest report,[33] the highest temperature of the generated steam was observed in a vapor chamber, demonstrating the accuracy of our proposed physical picture. In the next section, we will continue to demonstrate its application for seawater desalination, a process to remove salts and minerals to generate freshwater, representing a key solution to address the emerging water scarcity faced by this world.[3-5]
2.6 Performance for Solar Desalination and the Effect of the Bulk Water Temperature
Conventional desalination technologies are usually energy demanding (e.g., reverse osmosis membrane technology consumes ≈2 kW h m−3[5]) with serious environment costs. It was estimated that a minimum energy consumption for active seawater desalination is ≈1 kW h m−3,[3] excluding prefiltering and intake/outfall pumping. Passive solar desalination technology is particularly attractive due to the electricity-free operation with minimum negative impacts on the environment. To characterize the evaporation performance and reusability of our CP-foam for desalination, here we prepared salt water with 3.5 wt% NaCl and performed the solar water evaporation experiment repeatedly. For each cycle, two CP-foam samples were put on the surfaces of salt water and pure water, respectively, and illuminated under 1 kW m−2 for 1 h. After that, the CP samples were dried completely and reused for the next cycle. As shown in Figure 5A, the evaporation rates of ten cycles in pure water and salt water are both stable (i.e., 1.2–1.3 kg (m2 h)−1), demonstrating the reliability of the proposed CP-foam. Considering the excellent wet and dry strength and autoclavable features of the fiber-rich nonwoven paper (Texwipe TX609[28]), it is particularly attractive for long term solar desalination application, which is still under test.
Figure 5.
A) The evaporation rate of CP-foam samples on salt water (blue spheres) and pure water (red spheres) as the function of cycle number. The two solid lines are guide for the eye to show the stable performance. B) The SEM image of a CP sample after 1 h evaporation in salt water. C) The evaporation rate of CP sample in salt water over an 8 h evaporation period as a function of illumination time. D) Photographs and E) thermal images of a CP-foam on salt water at times corresponding to the blue spheres in Figure 5C.

Noticeably, after the 1 h recycling test, a millimeter sized salt crystal can be observed on the sample surface (see the first panel in Figure 5D). Obviously, these white salt particles will introduce scattering (see Figure 5B for scanning electron microscope (SEM) image of salt crystal plates on the CP surface), which should reduce the optical absorption of the CP sample. An immediate question is whether this salt crystallization will significantly degrade the performance of the vapor generation in practice, which was not mentioned in previous reports (e.g., ref. [19, 20] performed their experiments for 1–4 h only). To clarify this issue, we then performed an 8 h continuous experiment in pure water and salt water in a beaker, respectively. Intriguingly, one can see that the evaporation speeds increased continuously and saturated at the fourth to fifth hour at ≈1.32 and ≈1.42 kg (m2 h)−1 for salt water and pure water, respectively, as shown in Figure 5C. Since the CP surface is always wet during the 8 h test (indicating sufficient water transportation contributed by capillary forces), the salt crystal did not grow further to cover the entire surface. Instead, the salt crystal area even shrank surprisingly, as shown by the photographs of the CP surface at different time spots (see Figure 5D). When we repeated this experiment (usually on the next day), this evaporation rate increase can still be observed under identical experimental conditions starting from the lower rate, indicating the stable and reusable performance for longer term seawater desalination. As shown by thermal images in Figure 5E, the average surface temperature of the CP sample increased from 44 to 45 °C gradually and saturated at 53–54 °C at the fourth to fifth hour. Therefore, the immediate next question is what introduced this surface temperature change?
According to the experimental data shown in Figures 1-3, the only observed gradual change is the bulk water temperature, as shown by dashed curves in Figure 3B. To identify this correlation, we monitored the bulk temperature over 8 h, as shown by dotted curves in Figure 5C. One can see that the bulk water temperature (from 22 to 32–33 °C) and the evaporation rate changed coincidentally. This observation demonstrated that the surface temperature of the CP-foam is still related to the bulk liquid temperature. Due to the excellent thermal insulation of the EPS foam support employed in our structure, the temperature of the bulk water in this experiment reached the thermal equilibrium after ≈5 h. Also, due to the higher solubility of salt in warmer water, we observed that the salt crystal shrank as the bulk and surface temperature increases (i.e., Figure 5D). This vapor generation performance should be improved if better thermal insulation materials are used in the water container for small volume test. On the other hand, if the bulk water temperature change is negligible in larger scale vapor generation applications, one should not expect this obvious evaporation rate change, as will be further validated in the prototype system demonstration below.
2.7 A Prototype Solar Still System
A typical desalination solar still system is illustrated in Figure 6A: A box made by thermal insulating materials is filled by seawater or salty water. A tilted transparent glass covers the box to collect solar light. For conventional solar vapor generation technology, light absorbing materials were usually placed at the bottom of the basin to heat the entire liquid volume with fairly low thermal efficiency (i.e., 30–40%[7]). To overcome this weakness, we developed a 5 × 5 CP array as shown in Figure 6B (i.e., 2 × 2 cm2 for each CP unit with the total area of 100 cm2), which was placed in a polypropylene box (15 cm in diameter with 1500 g water). However, thermal isolating walls have not been incorporated in this experiment. According to the thermal distribution measurement, the temperature of CP surface increased from 18.2 °C (Figure 6C under dark condition) to 44.6 °C (Figure 6D under 1 sun illumination). The slight nonuniformity of the temperature distribution in Figure 6D was introduced by the intensity distribution of the finite size of the light beam. To evaluate its performance, we repeated the solar desalination experiment using this large area sample (Figure 6E). Meanwhile, two control samples were characterized: (1) a layer of black aluminum foil placed at the bottom of the box (Figure 6F, its optical absorption spectrum is shown in Figure S4 in the Supporting Information) and (2) salty water with no CP-foam (Figure 6G). As shown in Figure 6H, the mass change rate for the CP-foam array is ≈1.275 kg (m2 h)−1 (with the estimated thermal efficiency ηth of 88.2%), which is obviously better than those for control samples (i.e., ≈0.408 kg (m2 h)−1 with ηth of 28.2% for the bulk heating strategy, and ≈0.242 kg (m2 h)−1 with ηth of 16.7% for the bare salt water evaporation). It should be noted that the evaporation rate in this large scale CP array experiment did not increase obviously. Its bulk water temperature change is also relatively small (20–25 °C, as shown by the red dashed curve in Figure 6H) due to the much larger amount of bulk water. In contrast, the evaporation rates of those two control samples increased slightly, corresponding to their bulk temperature changes, as shown by green and blue dashed curves in Figure 6H. The net water mass change produced by this 100 cm2 CP-foam structure is 14.5 g after 5 h operation, which is ≈25 times of that produced by a single unit (i.e., 0.58 g h−1, see Figure 3). In this case, it is unnecessary to introduce a solar concentrator to enhance the water evaporation rate, which is different from the case for commercial concentrated photovoltaic systems. Due to the extremely low manufacturing cost of the CP-foam, huge area products can easily be realized using commercial paper printing technologies at the price much lower than those for solar concentrators. Therefore, portable or large scale systems directly floating on seawater surfaces are possible to meet some low-end freshwater generation needs, as will be demonstrated next. In this case, the costs for seawater intake and pretreatment for conventional reverse osmosis processes are largely avoided, which provides a potential solution to low-cost freshwater generation applications.
Figure 6.
A) Schematic illustration of a conventional desalination solar still. B) Photograph of a 5 × 5 CP array with a total area of 100 cm2. C,D) Thermal images of CP array before (C) and after (D) solar illumination. E–G) Photographs of experimental systems with (E) the CP-foam array on salt water, (F) bare salt water with a layer of black aluminum foil placed at the bottom, and (G) bare salt water with no CP-foam. H) Hourly water weight change with the CP-foam array on the water surface (red dots), black aluminum foil at the bottom (green triangles), and salt water (blue squares) as a function of illumination time. I) The photograph of a prototype system paced on Lake Lasalle at the University at Buffalo. J) The photograph of a control experiment with a commercial product (left) and our system (right) during the experiment. Obvious mist can be seen at the inner surfaces of the covers. K) The solar intensity (upper panel) and outdoor temperature curves (lower panel) from 8:00 a.m. to 6:00 p.m. on May 6, 2016 at the University at Buffalo.
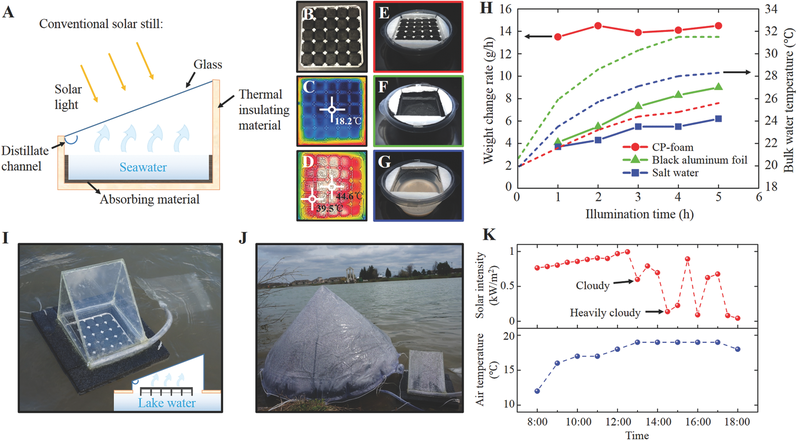
As shown in Figure 6I, a complete portable solar still system was demonstrated by covering an open bottom box with a transparent acrylic slab (with the 0.01 m2 5 × 5 CP-foam array directly in contact with the open water below, see the inset of Figure 6I). The clean water is collected by the distillate channel and guided into a collection bag. We then placed this system on Lake Lasalle at the University at Buffalo together with a commercial solar still product with an effective area of 0.342 m2 (Aquamate Solar Still at the retail price of $225), as shown in Figure 6J. It should be noted that our CP-array can take the lake water directly, while the commercial system needs to be actively fed. After a 10 h operation in the outdoor environment on a sunny–cloudy day at Buffalo with varying sunlight illumination conditions (see Figure 6K for temperature and sunlight intensity distribution), we obtained the generation productivities of 0.832 and 0.344 kg (m2 d)−1 for these two systems, respectively. The performance of the CP-foam system is ≈2.4 times of the commercial product. In addition, due to the scattering of the mist formed on the cover (Figure 6J), the input light decreased significantly, which is the next technical issue to optimize the performance of a real system. A nontoxic superhydrophobic surface treatment for antimist on the transparent glass cover[39] will improve the system performance, which is still under investigation but beyond the scope of this work. Consider the low-cost of the core elements for solar-to-heat conversion, the solar still system can be developed at a very low-cost (see Section S7 in the Supporting Information), which is particularly promising for the distribution in developing regions and in areas affected by natural disasters where drinking water supply is temporarily interrupted.
3 Conclusion
In summary, we have developed an extremely cost-effective and efficient carbon-based solar vapor generation system based on CP supported by floating EPS foam. Due to the efficient solar absorption of the CP, the superior thermal insulation of the EPS foam support and suppressed radiative and convective loss in the heated vapor environment, most of the absorbed solar energy is confined within a thin surface layer of liquid, resulting in efficient heat conversion and vapor generation. As a result, our system achieved a record thermal conversion efficiency of ≈88% under nonconcentrated solar illumination of 1 kW m−2. This corresponds to an optimized vapor generation rate that is ≈3 times greater than that of natural evaporation. In addition, stable and repeated seawater desalination tests were performed in a portable prototype both in the laboratory and an outdoor environment, and achieved a water generation rate that was 2.4 times that of a commercial product. Furthermore, by analyzing the theoretical upper limit for solar vapor generation rates, we show that the opportunity for improvement in vapor generation rates is relatively limited. This indicates that the area that offers the most potential for improvement is in the reduction of cost. Compared with previously reported advanced nanostructures, this CP–EPS system is extremely low-cost in terms of both materials and fabrication, environmentally benign, and safe to handle during production. These attributes enable this system to be easily expanded to large scales, something that is of particular interest in regions where access to freshwater is limited. It should be noted that activated carbon structures and materials are widely used in water and gas treatment applications (e.g., ref. [40]). These functionalities are inherently compatible with our CP structure, which may enable simultaneous freshwater generation and treatment from heavily contaminated source water. Considering the challenges in contaminated/waste water treatment and reuse, the development of low-cost, electricity-free, and multifunctional technologies represents new research avenues in carbon-based solar vapor generation.
The shortage of freshwater and sanitation is one of the most pervasive challenges afflicting people throughout the world. It was predicted that by 2025, over half the nations in the world will face freshwater stress, and by 2050, ≈75% of the world's population could face water scarcity. Therefore, it is essential to develop technologies for disinfection and decontamination of water, and to increase water supplies through economic and sustainable ways (i.e., at lower cost, smaller energy consumption, and smaller environmental impacts). Membrane-based separations for water purification and desalination are dominant technologies, which, unfortunately, are usually energetically demanding with serious environmental costs. There is emerging global interest in developing new technologies to address these issues. Successful demonstration of the portable solar steam generation system represents a revolutionary product to beat the conventional products both in performance and retail price, which is particularly attractive for addressing global freshwater shortages, especially in developing regions.
https://phys.org/news/2017-01-academics-ultimate-solar-powered-purifier.html
Related :
http://www.sciencemag.org/news/2016/08/solar-still-made-bubble-wrap-could-purify-water-poor
By Robert F. Service
Solar stills can make tainted water or seawater fit to drink. But to produce more than a trickle, devices typically require expensive lenses or other equipment. Not anymore. Today, researchers report that they’ve created a cheap solar still from bubble wrap and other simple materials.
Solar stills have been used for thousands of years. The most basic versions are water-filled vessels with black bottoms that absorb the sun’s rays, increasing evaporation of the water inside. Glass or other clear material on top captures the vapor, and the condensate drips into a collection vessel. To speed up this process, modern versions use lenses or mirrors to collect about 100 times more sunlight. But the high cost of these solar concentrators, typically on the order of $200 per square meter, makes them unaffordable for many people.
Two years ago, researchers led by Gang Chen, a mechanical engineer at the Massachusetts Institute of Technology in Cambridge, unveiled an efficient solar absorber made from a layer of graphite on floating carbon foam. The two layers were perforated, allowing the water below to wick up to the graphite, where it was warmed by the sun. The device worked, but much of the energy in the sunlight radiated away. To boil water, the still needed additional devices to concentrate 10 times the ambient sunlight to overcome the infrared losses.
Chen and his colleagues wanted to do away with the extras. They kept their idea of a spongy insulator floating on water. For their current experiment, the researchers replaced the graphite solar absorber with a thin layer of a bluish metal and ceramic composite material used in commercial solar water heaters. This material selectively absorbs visible and ultraviolet rays from the sun, but it doesn’t radiate heat in the infrared. Between this layer and the foam, they placed a thin sheet of copper, an excellent heat conductor. The researchers then punched holes through the sandwichlike layers as before.
A problem remained. Much of the energy absorbed by the composite was being swept away by convection, heat lost to the air moving above the still’s top surface. The fix came from Chen’s 16-year-old daughter, who was designing a cheap greenhouse for a science fair experiment. She found that a top layer of bubble wrap acted as an excellent insulator. So Chen and his student George Ni covered their solar still in bubble wrap. And in today’s issue of Nature Energy they report that their setup allowed them to boil and distill water with no extra solar concentrator. Down the road, Chen estimates that this will allow them to make large-area solar stills for about one-twentieth the cost of conventional technology.
“This work certainly represents a key step forward,” write materials scientists Wen Shang and Tao Deng from Shanghai Jiao Tong University in China in a commentary accompanying the report. Chen believes the low-cost apparatus could help purify wastewater near fracking sites, for example. Typically, companies work to evaporate water from wastewater ponds to concentrate and remove the contaminants. A cheap solar sponge could speed the cleanup.
To be useful for desalination or other drinking water applications, the device needs another plastic or glass layer on top to collect the water vapor. This could increase the system’s efficiency by trapping more heat and boosting evaporation, Chen says.
Creating a purification system would be no small task. Chen estimates it would require 20 to 40 square meters of the solar still material to provide 50 liters of water per day, the minimum that United Nations says a person needs for daily life.
Related :
http://onlinelibrary.wiley.com/doi/10.1002/adma.201500135/full
Yanming Liu, et al.
Abstract
A bioinspired, reusable, paper-based gold-nanoparticle film is fabricated by depositing an as-prepared gold-nanoparticle thin film on airlaid paper. This paper-based system with enhanced surface roughness and low thermal conductivity exhibits increased efficiency of evaporation, scale-up potential, and proven reusability. It is also demonstrated to be potentially useful in seawater desalination.