
rexresearch.com
Linnard GRIFFIN
Hydrogen Generator
Hydrogen Generator
http://www.overunity.com/index.php?topic=3633.0
Griffin Electrolysis Forum
http://www.youtube.com/watch?v=va81PNJzYIg&feature=player_embedded#at=13
PbSn fireworks reaction from Dr. Linnard Griffin
http://www.youtube.com/watch?v=lnj5Hp2W2FA&feature=relmfu
Dr. Linnard Griffin go cart run on hydrogen from an Iron-acid reaction with catalyst
http://www.youtube.com/watch?v=S24mfAZzn8Q&feature=relmfu
Aluminium Iron Al Fe electrolysis by Dr. Linnard Griffin
http://www.overunity.com
An Extended Time Operrational Study of a Hydrogen
Electricity Generator Based Off a Modification of the
Linnard Griffin Electrolysis Patent
[ PDF ]
by David B. Rybarczyk
20 Nov. 2007[ PDF ]
by David B. Rybarczyk


US2010181204
Nickel-Zinc-Aluminum-Hydrogen Production Reactor and Methods of Use
Nickel-Zinc-Aluminum-Hydrogen Production Reactor and Methods of Use
Inventor: GRIFFIN LINNARD GENE
EC: C25B5/00 IPC: C25B1/02; C25B9/00
Abstract -- The technology provides apparatus and methods for generating hydrogen without applying electrical energy from an outside source. An exemplary apparatus has an outer housing having an interior divided into an upper portion and a lower portion separated by a septum. The lower portion contains an electrolyte and a composite electrode at least partially immersed in the electrolyte. The electrolyte includes zinc hydroxide dissolved therein. The composite electrode has an aluminum tube enclosing at least one magnet. An outer surface of the electrode housing is at least partially covered with nano-particles held in place by magnetic attraction of the at least one magnet to form the electrode. The magnetically-adherent nano-particles form a second electrode, in direct contact with the first electrode. The generator apparatus has a vent in communication with the upper portion of the interior of the outer housing for removal of generated hydrogen.
BACKGROUND
[0001] 1. Technical Field
[0002] The technology relates to the production of hydrogen gas in a generator that includes a pair of electrodes and an electrolyte, and more particularly relates to the production of hydrogen without applying an external source of electrical energy to the electrodes, wherein at least one electrode comprises magnetic nano-particles.
[0003] 2. Description of the Related Art
[0004] Hydrogen gas is a valuable commodity with many current uses and potentially wide ranging future uses. Currently many countries are evaluating the installation of a "hydrogen highway" that would provide hydrogen refueling stations for a national fleet of hydrogen-powered vehicles. Currently, several auto manufacturers (e.g., BMW and Honda) are demonstrating hydrogen powered vehicles.
[0005] Aside from the potential for large scale uses of hydrogen to power automobiles, hydrogen also potentially provides a clean fuel from which to generate electricity for other purposes. This is especially desirable if the production of hydrogen does not generate greenhouse gasses, or otherwise has a "small carbon footprint" so that it has potential environmental benefits over fossil fuels.
[0006] One of the methods of generating hydrogen is by the electrolysis of water in an electrolysis cell. However, this method requires an input of electrical energy that might be generated by combustion of fossil fuels thereby releasing carbon dioxide and other greenhouse gasses into the environment.
SUMMARY
[0007] An exemplary embodiment provides an apparatus for generating hydrogen. The apparatus includes an outer housing having an interior divided into an upper portion and a lower portion separated by a septum. The lower portion contains an electrolyte comprising zinc hydroxide dissolved therein, and nano-particles comprising nickel. The lower portion also contains a first electrode at least partially immersed in the electrolyte. The first electrode has several features including a non-ferrous, conductive electrode housing enclosing at least one magnet, with the electrode housing at least partially covered with nano-particles of nickel, tungsten, cobalt, or alloys of these. In addition, the lower portion of the outer housing contains a second electrode of aluminum that is at least partially immersed in the electrolyte. The generator also has a vent in communication with the upper portion of the interior of the housing for removal of generated hydrogen.
[0008] Another exemplary embodiment provides an apparatus for generating hydrogen that has an outer housing having an interior divided into an upper portion and a lower portion separated by a septum. The lower portion contains an electrolyte and a composite electrode at least partially immersed in the electrolyte. The electrolyte includes zinc hydroxide dissolved therein. The composite electrode has several features including a non-ferrous, conductive electrode housing enclosing at least one magnet. An outer surface of the electrode housing is at least partially covered with nano-particles held in place by magnetic attraction of the at least one magnet to thereby form another electrode in direct contact with the first electrode. The nano-particles may be of nickel, iron, tungsten, cobalt, or alloys of these. The generator apparatus has a vent in communication with the upper portion of the interior of the outer housing for removal of generated hydrogen.
[0009] Another exemplary embodiment provides a method of generating hydrogen gas without applying electrical energy from an outside source. The method includes the steps of providing an electrolyte comprising zinc hydroxide, and disposing a first electrode comprised of aluminum in the provided electrolyte. It also includes disposing a second electrode comprised of a non-ferrous housing in the electrolyte. The non-ferrous housing contains at least one magnet and the outer surface of the housing is at least partially covered with nano-particles of nickel, tungsten, iron, cobalt, or alloys of these. In addition, the steps include producing hydrogen gas at the first electrode without applying a current from an external source to the first electrode or to the second electrode, and collecting the hydrogen gas produced.
[0010] A further exemplary embodiment provides yet another method of generating hydrogen gas without applying electrical energy from an outside source. The method includes the steps of providing an electrolyte that includes zinc hydroxide, and disposing a first electrode in the provided electrolyte. The first electrode is comprised of aluminum and has a cavity formed therein that contains at least one magnet. An outer surface of the first electrode is at least partially covered with nano-particles that form a second electrode in contact with the first electrode. The steps further include producing hydrogen gas without applying an external current to the electrode, and collecting the hydrogen gas produced in the generator.
BRIEF DESCRIPTION OF THE DRAWINGS
[0011] For a more complete understanding of the present technology, reference is now made to the following descriptions taken in conjunction with the following drawings that are not to scale, in which:
[0012] FIG. 1 illustrates a simplified, exemplary embodiment of a hydrogen-producing cell that has two electrodes; and

[0013] FIG. 2 illustrates an alternative exemplary embodiment of a hydrogen-producing cell.

DETAILED DESCRIPTION
[0014] The exemplary embodiments provide hydrogen generators that do not require the input of energy from an external source. More particularly, the consumables for the exemplary embodiments of hydrogen generators include aluminum electrodes and water only. At least one electrode has a non-ferrous housing containing at least one magnet, and nano-particles adhered thereto by magnetic forces. In another feature, a coating of magnetic nano-particles is either used to form an electrode or to form an integral part of an electrode. In addition, the initiation, termination and rate of hydrogen generation may be controlled by relatively simple mechanisms.
[0015] FIG. 1 is a drawing of an exemplary two-electrode hydrogen generator 100, which does not require the application of an external electrical current. The configuration and materials may vary and those skilled in the art will appreciate that actual configurations may be influenced by capacity for hydrogen generation, electrode size, electrode materials, and other parameters.
[0016] Briefly, the generator 100 of FIG. 1 includes a housing 110 that is divided horizontally into an upper portion 112 and a lower portion 114 by a septum 116. The lower portion contains two electrodes 130 and 150. The electrodes 130, 150 are electrically connected by a conductive element 160.
[0017] Generator 100 commences operation when electrolyte 125 is supplied through electrolyte feeder tube 118 from the upper portion 112 of the housing 110 to the lower portion 114. When the electrolyte 125, described below, enters the lower portion 114 through the feeder tube 118, a chemical reaction begins and the aluminum electrode 150 is consumed as the reaction proceeds. The chemical reactions are described below. The chemical reactions, and hydrogen production from the reactions, can be terminated by the removal of the electrolyte 125 through the feeder tube 118, or by another means including, but not limited to, a drain line at the base of housing 110, not shown. Hydrogen gas produced at electrode 130 is exhausted through vent tube 120. The production of hydrogen continues until all the consumables are consumed. The consumables include water and the electrode 150.
[0018] The exemplary generator of FIG. 1 includes an electrode 150 that is composed of aluminum. The other electrode, electrode 130, is a composite structure and is composed of three elements. In this exemplary embodiment, composite electrode 130 includes firstly a non-ferrous tube electrically-conductive element, such as a copper tube 132. Copper tube 132 encloses in its annular cavity either a single magnet or a plurality of magnets 134. Electrode 130 secondly includes one or more cylindrical magnets 134. These magnet(s) 134 may be diametrically polarized rather than axially polarized, to enhance performance, but either will suffice to the task. Diametric polarization may provide greater efficiency in hydrogen generation. Thirdly, the electrode 130 includes nano-particles 140 attracted by magnet(s) 134 that adhere by magnetic force to at least a portion of the outer surface of tube 132. While these nano-particles are shown schematically as spaced from the tube 132, for reasons of clarity, they are in fact held to the outer surface of tube 132 to thereby complete the structure of electrode 130. The nano-particles 140 may be selected from magnetic particles such as nickel, iron, tungsten, cobalt, and the like, and their alloys. Because of its multiple structural features, electrode 130 may be regarded as a "composite electrode."
[0019] Because of their high surface area to volume ratio, the nano-particles provide a very large surface area from which the electrode 130 releases hydrogen, when the two electrodes 130, 150 are connected to each other electrically via connector 160. To be operative, the conductive electrical connection 160 connects electrodes 130 and 150 to complete a circuit. Accordingly, hydrogen production may be stopped by opening this electrical connection but chemical reaction with the electrolyte and erosion of the aluminum electrode 150 will continue for some time. Hydrogen production may also be controlled by controlling the electrical resistance of connector 160 either through material selection, or through dimensions, or by adding a variable, controllable resistance element to it.
[0020] The exemplary electrolyte 125 is aqueous and is produced from a liquid mixture that includes colloidal silver, colloidal magnesium, and sodium hydroxide and potassium hydroxide dissolved in distilled water. Zinc is placed in this liquid mixture along with a nickel electrode. The zinc is allowed to digest and the resulting liquid mixture, after removal of any excess undigested zinc, is the electrolyte 125.
[0021] In another exemplary embodiment, that may be scaled up or down as to volumes and weights, the exemplary electrolyte includes:
[0022] 50 ml colloidal silver
[0023] 50 ml colloidal magnesium
[0024] 50 ml distilled water
[0025] 20 grams sodium hydroxide
[0026] 20 grams potassium hydroxide
[0027] This mixture may be placed in a container that includes a nickel electrode and a zinc electrode of about 7 grams of elemental zinc. The zinc is allowed to digest. After digestion, the remaining zinc is removed. The liquid mixture produced is an example of an electrolyte.
[0028] It is theorized, without being bound, that in the generator 100 of FIG. 1, an exchange reaction takes place on the surface of the aluminum electrode 150 with the zinc hydroxide in the electrolyte solution. This reaction forms metallic zinc on the surface of the aluminum. This metallic zinc in turn reacts with the nano-particles 140 producing hydrogen gas at electrode 130.
[0029] It is further theorized, without being bound, that during hydrogen production, the zinc hydroxide of the electrolyte is reduced to zinc on the aluminum electrode. The zinc reacts with the nano-nickel (or nano-particles of iron, cobalt, tungsten, and the like) in the strong base electrolyte, thereby producing hydrogen on the nano-particle covered electrode 130.
[0030] It was observed that there is some hydrogen produced off the surface of the aluminum electrode 150. It is theorized, without being bound, that this results in an apparent greater hydrogen production than might be expected from stoichiometry. This hydrogen, it is believed without being bound, results from a further reaction that converts ZnOH to Zn and a reaction converting the aluminum to form Al2O3. It is theorized, without being bound, that the following reactions A, B take place:
[0000] 6ZnOH+4Al=6Zn+2Al2O3+3H2 [A]
[0000] 2Zn+NaOH/KOH (in presence of Nickel)+2H2O=2ZnOH+NaOH/KOH+H2 [B]
[0031] Regardless of any theory, the exemplary hydrogen generator of FIG. 1 provides a controlled rate of hydrogen production.
[0032] FIG. 2 illustrates an alternative exemplary embodiment. In this embodiment, the generator 100 also includes a housing 110 divided into upper 112 and lower 114 portions by a horizontal septum 116. In comparison with the example of FIG. 1, the non-ferrous tube 132 is eliminated. Instead, composite electrode 150 includes a housing with a cavity, such as an aluminum tube 154 that houses one or more cylindrical magnets 134 in its annular space. As in the embodiment of FIG. 1, nano-nickel particles 140 in the electrolyte 125 are attracted to the outer surface of the aluminum tube 154 of an electrode 150 and form a coat on the surface held in place by magnetic fields. Once the outer surface of the tube 132 is at least partially coated with magnetically-adhering nano-particles, the nano-particles effectively form the second electrode, which is in direct contact with the aluminum tube 154 that is the first electrode. Hydrogen is produced from this nano-particle-coated surface. Since the nano-particles 140 are in direct electrical communication with the aluminum tube 154 of electrode 150, an electrical connector 160 is not required to connect the nano-particles to the aluminum electrode housing 154.
[0033] Hydrogen production rate and volume is similar to the embodiment of FIG. 1, but the overall generator complexity and cost is reduced. To control hydrogen production, the extent of the immersion of the electrode 150 in the electrolyte 125 may be controlled. In one mode of operation, the electrode 150 is lowered or raised in the solution to control the hydrogen production rate.
EXAMPLES
[0034] A number of experiments were performed to determine the hydrogen production based on the consumption of aluminum. One gram of aluminum will produce 1.23 liters of hydrogen. The results appear to indicate producing hydrogen in an amount greater than might be expected. In all of these experiments, the generator was in accordance with FIG. 2, and the electrolyte was produced as follows. The following components were mixed together:
[0035] 50 ml colloidal silver
[0036] 50 ml colloidal magnesium
[0037] 50 ml distilled water
[0038] 20 grams sodium hydroxide
[0039] 20 grams potassium hydroxide
[0040] This mixture was placed in a beaker containing a nickel electrode. To this was added 7 grams of elemental zinc, connected to the nickel electrode, and the zinc was allowed to digest, thereby producing electrolyte 125. The nickel electrode and any remaining zinc were then removed. The resulting liquid was used as the electrolyte.
Experiment 1
[0041] 7.5 grams of aluminum produced 10.19 liters of hydrogen @ STP. Based on stoichiometry, 7.5 grams should produce only 9.2 liters of hydrogen.
Experiment 2
[0042] 2.9 grams of aluminum produced 4.163 liters of hydrogen @ STP. Based on stoichiometry, 2.9 grams of aluminum should produce 3.567 liters of hydrogen.
Experiment 3
[0043] 4.1 grams of aluminum produced 8.7 liters of hydrogen @ STP. Based on stoichiometry, 4.1 grams of aluminum should produce 5.041 liters of hydrogen.
Experiment 4
[0044] 2.6 grams of aluminum produced 3.57 liters of hydrogen @ STP. Based on stoichiometry, 2.6 grams of aluminum should produce 3.198 liters of hydrogen.
[0045] The average hydrogen production was 1.5 liters per gram of aluminum. All of the experiments were performed by water displacement using a calibrated column, the temperature and atmospheric pressure were recorded and the volume of hydrogen corrected to standard pressure and temperature.
[0046] While several exemplary embodiments have been presented in the foregoing detailed description of the invention and in the foregoing non-limiting examples, it should be appreciated that a multiplicity of variations exists. It should also be appreciated that the exemplary embodiment or exemplary embodiments are only examples, and are not intended to limit the scope or applicability of the technology in any way. Rather, the foregoing detailed description will provide those skilled in the art with a convenient roadmap for implementing an exemplary embodiment of the invention, it being understood that various changes may be made in the specific components described in an exemplary embodiment without departing from the scope of the invention, as set forth in the appended claims and their legal equivalents.
US2010108498
Hydrogen Production Systems Utilizing Electrodes Formed From Nano-Particles Suspended in an Electrolyte
Hydrogen Production Systems Utilizing Electrodes Formed From Nano-Particles Suspended in an Electrolyte
Inventor: GRIFFIN LINNARD GENE [US] Applicant:
EC: C25B1/02; C25B9/16
Abstract -- An electrolytic system for generating hydrogen gas includes a pair of electrodes and an electrolyte. The electrolyte includes colloidal silver, colloidal magnesium, and a nano-metal comprising nano-nickel, nano-iron or a nano-nickel-iron alloy. The electrodes include a first electrode of a non-magnetic material. A second electrode includes an electrode precursor of a magnetic material or an electro-magnet. When in its magnetic state, the electrode precursor exerts a magnetic force of sufficient strength to pull the nano-metal of the electrolyte onto at least a portion of its surfaces, to form the second electrode.
Description
STATEMENT OF RELATED APPLICATIONS
[0001] This application claims priority from provisional U.S. Application No. 61/111,991, filed Nov. 6, 2008.
BACKGROUND
[0002] 1. Technical Field
[0003] The technology relates to the production of hydrogen, and more particularly to the use of chemical reaction to produce hydrogen in a system that includes an electrode formed from metallic nano-particles suspended in an electrolyte.
[0004] 2. Description of the Related Art
[0005] There is a growing demand for sources of energy other than from the combustion of fossil fuels. The combustion of these fuels has long been associated with the production of undesirable combustion gas products, such as sulfur dioxide. In more recent years, it has also become a matter of concern that the combustion of fossil fuels releases carbon dioxide into the atmosphere. The growing concentration of carbon dioxide has been implicated in the phenomenon variously known as "global warming" or "climate change." Accordingly, there is a desire to develop other sources of energy, or to find ways to utilize fossil fuels which may entail technologies that either sequester or otherwise remove the potential for carbon dioxide release into the atmosphere.
[0006] Among the proposed alternatives to fossil fuels as a source of energy that do not release carbon dioxide are solar power, wind power, nuclear power, marine (wave) power and hydrogen. Each of these power sources poses challenges and each may occupy a niche in a long term energy strategy aimed at minimizing the release of carbon dioxide into the atmosphere. Hydrogen is a plentiful elemental gas but is usually chemically bound or in the atmosphere in a relatively small percentage. Accordingly, the large scale use of hydrogen requires technologies that will produce hydrogen from its chemically bound state and permit its capture in a form useful for conversion to energy, by combustion or otherwise. Much attention has been devoted to fuel cell technology, and the use of hydrogen as a potential automotive fuel is also being explored.
SUMMARY
[0007] An exemplary embodiment provides a controlled electrolysis system for generating hydrogen gas by creating an electrode with a magnetic field and controlling the magnetic field strength to control a rate of hydrogen production. The system includes a first electrode and an electrolyte in contact with it that includes colloidal silver, colloidal magnesium, and nano-metal particles. The system also has a conductive body portion in contact with the electrolyte. Further, it includes a magnetic element having a magnetic field at least partially encompassing the conductive body portion. The magnetic field pulls nano-metal particles from the electrolyte to at least partially coat a surface of the conductive body portion to form a second electrode. The strength of the magnetic field is controllable to either increase or decrease a rate of hydrogen production by controlling an extent of the surface of the conductive body portion coated with nano-metal particles.
[0008] A further exemplary embodiment provides a system for controlled generation of hydrogen gas by creating an electrode with a magnetic field and controlling the magnetic field strength to control a rate of hydrogen production. The system includes a first non-magnetic electrode and, in contact with it, an electrolyte that includes colloidal silver, colloidal magnesium, and nano-metal particles. In addition, it has a hollow body having a conductive portion and an insulated portion. The hollow body is in contact with the electrolyte. Further, it has a magnetic element having a magnetic field. The magnetic field at least partially encompasses the hollow body and pulls nano-metal particles from the electrolyte to at least partially coat an outer surface of the conductive portion to form a second electrolyte and produce hydrogen. The extent of influence of the magnetic field on the conductive portion is controlledly variable to control the rate of hydrogen production.
[0009] Another exemplary embodiment provides a system for controlled generation of hydrogen gas by creating an electrode with a magnetic field and controlling the magnetic field strength to control a rate of hydrogen production. The system includes a cell that has a first non-magnetic electrode, an electrolyte in contact with it, and a hollow body that forms a second electrode, when coated with nano-metal particles, under influence of a magnetic field. The electrolyte may include colloidal silver, colloidal magnesium, and nano-metal particles. The nano-metal particles may include at least one of nano-nickel, nano-iron or a nano-nickel-iron alloy. The hollow body has a conductive portion and an insulated portion and is in contact with the electrolyte. The hollow conductive body is coated with nano-metal from the electrolyte to form a second electrode, when the system is in hydrogen production mode. Further, the system includes at least one controlled magnetic element located within the hollow body and pulling nano-metal particles from the electrolyte to at least partially coat an outer surface of the hollow body to form the second electrode to produce hydrogen by electrolysis. The magnetic element controls a rate of hydrogen production by controlling the strength of the magnetic field at the conductive portion of the hollow body. The system also includes a gas-tight end cover enclosing contents of the cell, the end cover having an outlet therein for removal of produced hydrogen.
BRIEF DESCRIPTION OF THE DRAWINGS
[0010] For a more complete understanding of the present technology, and the advantages thereof, reference is now made to the following description taken in conjunction with the accompanying schematic, not-to-scale drawings in which:
[0011] FIG. 1 illustrates an exemplary embodiment of a system including a magnetic electrode;

[0012] FIG. 2 illustrates another exemplary embodiment of a system including a magnetic electrode in the OFF state;

[0013] FIG. 3 illustrates another exemplary embodiment of a system including a magnetic electrode in the ON state; and

[0014] FIG. 4 illustrates another exemplary embodiment of a system including an electro-magnetic electrode.

DETAILED DESCRIPTION
[0015] In the following description, numerous details may be set forth to provide a thorough understanding of the present technology. However, it will be apparent to those skilled in the art that the present technology may be practiced without some of these specific details. For the most part, details considering alternate material choices and design configurations and the like have been omitted inasmuch as details are not necessary to obtain complete understanding of the present technology and are within the skills of persons of ordinary skill in the relevant art.
[0016] In the specification, the term "exemplary embodiment" means a non limiting example of an embodiment of the technology.
[0017] FIG. 1 illustrates a simplified exemplary embodiment of a system that is a single cell hydrogen generator 5 that includes a chemical inert container 10, in this instance an elongate container, of a non-magnetic material, typically a chemically inert material. Container 10 may vary in configuration. Container 10 includes a hollow electrical (copper or any other conductive material which is non-reactive) conductor 20 and a zinc electrode 30 abuts one end of the conductor 20. Exemplary embodiments may have either zinc electrodes or aluminum electrodes when the electrolyte contains zinc hydroxide so that zinc will plate out onto the aluminum electrode. Other non-magnetic electrode materials may also be used. The conductor 20 may be of any configuration that is suitable. In this example, conductor 20 is composed of a hollow, copper tube. Conductor 20 is divided into two sections (insulated portion 40 and conductive portion 45), the outer surface of conductor 20 exposed to the electrolyte 65. The second electrode is formed by nano-metal particles, such as nano-nickel and iron particles, attracted to and coated over the non-insulated area, conductive portion 45, of conductor 20, in the illustrated example. The hollow interior of conductor 20 is accessible from outside of container 10 through a port in the end seal 80, which also has an outlet 90 for produced hydrogen gas. This allows the movable magnetic element 50 to be selectively positioned within conductor 20 to control hydrogen production. Hydrogen production is at a maximum when the magnetic element 50 is fully inserted into the conductive portion 45 of the conductor and the maximum area of this conductive portion 45 is coated with attracted nano-metal particles. As the magnetic element 50 is withdrawn, the area of the conductor 20 that is coated with nano-metal is reduced (and hydrogen production is also reduced) until the magnet is completely shielded within insulated portion 40. When magnetic element 50 is completely shielded within insulated portion 40, the magnetic field strength at conductive portion 45 is weak or non-existent and the conductive portion 45 is substantially free of magnetically attracted nano-metal particles. At this point, hydrogen production is minimized or terminated. Thus, the movement of magnetic element 50, which affects the magnetic field strength at the conductive portion 45, acts to control hydrogen production.
[0018] The second electrode (conductive portion 45 as coated with nano-metal) is produced by the magnetic field effects of a movable magnetic element 50 and nano-particles 60 of the electrolyte 65. Thus, when the magnetic element 50 is in the insulated portion 40 of conductor 20, as illustrated in FIG. 2, the cell 5 is inactive. In this "off" mode, the presence of the magnetic field of magnetic element 50 attracts metallic nano-particles 60 of the electrolyte 65 to the outer surfaces of the insulated portion 40, resulting in no hydrogen production. When the magnetic element 50 is moved into the conductive portion 45 of conductor 20, the attracted nano-particles follow the magnetic field, thereby forming a metallic nano-particle coating on the outside surface of the conductive portion 45, thereby forming the second electrode. In this "on" mode, the hydrogen generator cell 5 is active and produces hydrogen. Thus, the magnetic element 50 should be in a position to exert a sufficiently strong magnetic field strength on the conductive portion 45 of conductor 20 to attract nano-metal particles to it to form the second electrode. Once the second electrode is formed, hydrogen production commences. As the magnetic field moves to cover a greater portion of the area of conductive portion 45, the extent of the proportion of the area of conductive portion 45 coated with nano-metal particles increases, and hydrogen production increases. Likewise, as the magnetic element 50 retreats and the magnetic field encompasses less of the area of conductive portion 45, the area of nano-metal coating is reduced, and consequently hydrogen production is reduced.
[0019] An exemplary embodiment of a movable magnetic element 50 may selected, for example, from the rare earth magnets, or any other magnetic material that will attract magnetic nano-particles, such as nickel and nano-iron, so strongly as to cause these particles to move through electrolyte 60 to attach to insulated surfaces of conductor 20 (off position) or to the non-insulated portion of conductor 20 (on position) forming the second electrode. These magnetic nano-particles may be selected from nano-nickel, nano-iron, nano-alloys of nickel and iron, or other nano-metals, such as tungsten, tungsten carbide, platinum, etc.
[0020] An exemplary embodiment of the electrolyte 65 may include colloidal silver, colloidal magnesium, sodium hydroxide, potassium hydroxide and distilled water. Into this electrolyte solution is placed nano-nickel and nano-iron particles. For example, a 100 ml solution might be composed of 10 ml of colloidal silver, 10 ml of colloidal magnesium, 80 ml of distilled water, and 33 grams of the hydroxide. To this may be added 0.5 grams of nano-nickel and 0.5 grams of nano-iron particles.
[0021] FIG. 2 shows an exemplary embodiment of a hydrogen generator cell 5 with the magnetic element 50 in the "off" position, when no hydrogen is produced. The magnetic element 50 is within the insulated layer 40 and this attracts the nano-metal particles to the surface of the insulated layer 40. In an exemplary embodiment, the magnetic field pulls substantially all the nano-nickel and nano-iron particles onto the outer surface of hollow conductor 20. No second electrode is formed, because the nano-metal particles coat an insulated portion 40, and thus there is no hydrogen production.
[0022] In FIG. 3, in contrast, the magnetic element 50 is moved all the way into conductor 20 (i.e. inside conductive portion 45) to the proximity of the zinc electrode 30. The nano-metal particles are pulled onto the surface of conductive portion 45 which is in close proximity of the zinc electrode 30, thereby allowing electrolysis to commence by making an electrical connection. As a result, electrolytic hydrogen production begins. The hydrogen is produced from the nano-metal electrode formed on the conductive portion 45 of hollow conductor 20. The production of hydrogen can be reduced or terminated by moving the magnetic element 50 toward the "off" position until it is within the insulated portion 40, as in FIG. 2.
[0023] In an exemplary embodiment, the extent of insertion of the magnetic element 50 within the conductor 20, in other words, its location relative to the "on" and "off" positions described above, may be used to control the rate of hydrogen gas production from the hydrogen generator cell 5. Alternatively, the second electrode (which is formed by magnetically attracted nano-metal particles on conductive portion 45) may be sized for a particular hydrogen output by a predetermined sizing of the area of conductive portion 45, or through application to the conductive portion 45 of a variable magnet permeable coating which will change the strength of the magnetic field. The production rate of hydrogen may also be controlled by temperature: increasing electrolyte temperature increases the rate of hydrogen generation.
[0024] FIG. 4 illustrates an alternative exemplary embodiment wherein the magnetic element 50 is an electro-magnet movable laterally as shown by arrow 55. When power is supplied to the windings of the electro-magnet 50, it becomes magnetic. Thus, when fully inserted into the hollow conductor 20, the electro-magnetic element 50 pulls nano-particles onto the outer surface of conductive portion 45 of conductor 20 to form a second electrode.
[0025] The electro-magnetic material of the electro-magnet(s) may be selected from any suitable material, such as electro-magnetic alloys of iron or steel. Operation of the hydrogen generation cell 5 is similar to the above description using permanent magnets, but electro-magnets provide some additional flexibility and ease of control. For example, an electro-magnet readily permits control of hydrogen production by controlling magnetic field strength. Magnetic field strength may be controlled to some extent by controlling electrical current supplied to the electro-magnet.
[0026] An electrode for electrolysis of water using an electrical current may be constructed by forming a coating of nano-material around a conductive magnet, thereby producing a cathode of one nano-material and an anode of a second nano-material.
[0027] While at least one exemplary embodiment has been presented in the foregoing detailed description of the invention, it should be appreciated that a wide range of variations exist. It should also be appreciated that the exemplary embodiment or exemplary embodiments are only examples, and are not intended to limit the scope, applicability, or configuration of the invention in any way. Rather, the foregoing detailed description will provide those skilled in the art with a convenient road map for implementing an exemplary embodiment of the invention, it being understood that various changes may be made in the function and arrangement of elements described in an exemplary embodiment without departing from the scope of the invention as set forth in the appended claims and their legal equivalents.
US2009152126
Gas Production Through Pulsed Electrolysis
Gas Production Through Pulsed Electrolysis
Inventor: GRIFFIN LINNARD GENE
EC: C25B1/04; C25B15/00; IPC: C25B1/02; C25B9/00
Abstract -- Cells and methods of producing hydrogen and oxygen from an aqueous solution at about 90% of the Faraday Limit are provided. An exemplary method includes the steps of placing a graphite electrode and a nickel electrode in an alkaline solution comprising colloidal silver, colloidal magnesium and a powdered metal such as aluminum, and applying a constant positive voltage to the nickel electrode. Further, the example includes cyclically applying a negative voltage potential to the graphite electrode by turning on the negative applied voltage for a first time period and switching off the negative voltage for a second time period. The second time period should be sufficient to permit removal of substantially all or at least some of any aluminum or zinc deposited on the graphite electrode. Graphite-containing electrodes may be pretreated to infuse with a precious metal.
Description
STATEMENT OF RELATED APPLICATIONS
[0001] This application claims priority from U.S. provisional patent application No. 61/107,197 filed Oct. 21, 2008, and from U.S. provisional patent application No. 61/005,961 filed on Dec. 10, 2007.
BACKGROUND
[0002] 1. Technical Field
[0003] The present technology relates to the field of electrolysis, and more particularly to the use of electrolysis techniques to produce hydrogen and oxygen gasses.
[0004] 2. Description of the Related Art
[0005] In the field of electrolysis it is generally regarded as highly desirable to be able to produce hydrogen (and oxygen) at or near the Faraday Limit of 10.5873 Joules per ml, which is the power presumed necessary to be 100% efficient. While many systems have been proposed to meet this goal, it appears that electrolysis may often require exotic chemicals and complex electrical switching systems to exceed even a modest 70% of the Faraday Limit. Exceeding this limit of 10.5873 J/ml generally requires replenishing or replacing some consumables, for example, replacing electrodes due to dissolution and/or the replenishing of consumed chemical(s).
SUMMARY
[0006] An exemplary embodiment of an electrolysis cell and process produces hydrogen and oxygen gasses, by electrolysis of water, at efficiencies ranging above about 80% of the Faraday Limit and approaching and often exceeding about 90% of the Faraday Limit. An embodiment of the technology employs a minimum of two electrodes per cell to provide a system that requires little maintenance, such as electrode repair or maintenance of chemicals, other than addition of make up water to compensate for water consumed in the process. Another exemplary embodiment provides an electrolysis system that may use DC power rather than complex alternating current waveforms and fixed non-replaceable elements.
[0007] An exemplary embodiment provides a method of producing hydrogen and oxygen from an aqueous solution. The method includes the steps of placing a graphite electrode and a nickel electrode in an alkaline electrolyte comprising colloidal silver, colloidal magnesium and aluminum powder, and applying a constant positive voltage to the nickel electrode. Further, the method includes cyclically applying a negative voltage potential to the graphite electrode by turning on the negative applied voltage for a first time period and switching off the negative voltage for a second time period. The second time period should be sufficient to permit removal of substantially all or at least some of any aluminum deposited on the graphite electrode.
[0008] An exemplary embodiment provides a cell producing hydrogen and oxygen gas from an aqueous solution. The cell includes an alkaline electrolyte having colloidal silver, colloidal magnesium, and a metal powder comprising aluminum powder. It also has at least one positive electrode disposed at least partially in the alkaline solution and at least one switching negative electrode that includes graphite, infused with a precious metal. The switching negative electrode is also disposed at least partially in the electrolyte. In addition, the cell has a first chamber configured and located to capture gas produced at the positive electrode; and a second chamber configured and located to capture gas produced at the at least one switching negative electrode. Further, it includes an automatic controller cyclically applying negative voltage potential to the switching negative electrode according to a predetermined sequence. In an alternate embodiment, the aluminum powder may be substituted with finely divided zinc hydroxide. In this instance, the positive electrode comprises nickel and is pre-treated with zinc, as explained here below
[0009] An additional exemplary embodiment provides a cell producing hydrogen and oxygen gas from an aqueous electrolyte. The cell includes an alkaline electrolyte comprising colloidal silver, colloidal magnesium, and a metal powder comprising aluminum. The cell has at least one positive electrode disposed at least partially in the alkaline electrolyte; and a plurality of switching negative electrodes. The switching electrodes each comprise graphite, infused with a precious metal, and the plurality of switching negative electrodes is disposed at least partially in the alkaline electrolyte. The cell has a first chamber configured and located to capture gas produced at the positive electrode; and a second chamber configured and located to capture gas produced at the plurality of switching negative electrodes. The cell also includes an automatic controller cyclically applying negative voltage potential to each of the plurality of switching negative electrodes for a predetermined time and according to a predetermined sequence. In an alternate embodiment, the aluminum powder may be substituted with finely divided zinc hydroxide. In this instance, the positive electrode comprises nickel and is pre-treated with zinc, as explained here below
BRIEF DESCRIPTION OF THE DRAWINGS
[0010] For a more complete understanding of the present technology, and the advantages thereof, reference is now made to the following description taken in conjunction with the accompanying schematic, not-to-scale drawings in which:
[0011] FIG. 1 illustrates an exemplary embodiment that has 1+n switched electrodes; where n=6;
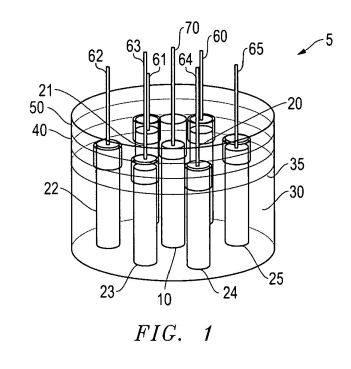
[0012] FIG. 2 illustrates a schematic of an embodiment of a switching relay;

[0013] FIG. 3 is a block diagram illustrating features of an exemplary embodiment of a controller;

[0014] FIG. 4 is a schematic of an exemplary embodiment of a controller showing additional detail;

[0015] FIG. 5 illustrates an exemplary generalized embodiment of an electrolysis cell;

[0016] FIGS. 6-7 illustrate exemplary embodiments of electrolysis cells with differing polarities on the electrodes;

[0017] FIG. 8 illustrates TABLE 1;

[0018] FIG. 9 is an exemplary embodiment of a solar home power application; and

[0019] FIG. 10 is an exemplary embodiment of a transportation application.

DESCRIPTION
[0020] In the following description, numerous details may be set forth to provide a thorough understanding of the present technology. However, it will be apparent to those skilled in the art that the present technology may be practiced without these specific details. In other instances, well-known circuits have been shown in block diagram form in order not to obscure the present technology in unnecessary detail. For the most part, details considering alternate material choices and design configurations and the like have been omitted inasmuch as details are not necessary to obtain complete understanding of the present technology and are within the skills of persons of ordinary skill in the relevant art.
[0021] In the appended drawings, depicted features are not necessarily shown to scale. Further, like or similar features are designated by the same reference numeral through the several views, as far as possible.
[0022] FIG. 1 illustrates an exemplary embodiment of an electrolysis cell 5 that includes a chemical bath 30, which is cylindrical in this case, but which may be of another geometric configuration, such as square, hexagonal, etc., in cross section. The chemical bath 30 has a central electrode 10 to which is applied a fixed voltage. The central electrode is surrounded by six switched voltage electrodes 20, 21, 22, 23, 24, 25 in this embodiment, although another number of electrodes may also be used. Each of these electrodes 20-25 are subjected to a pulsed or cyclical applied negative voltage. Thus, each switched electrode 20-25 may be "on" (voltage applied) or "off" (voltage not applied). Thus, for example, each of switched electrodes 20-25 has a connector 60, 61, 62, 63, 64, 65, respectively. As shown schematically in FIG. 2, a switch relay 90 includes a series of switches 80-85. Each of the switches 80-85 is able to close a circuit with a corresponding one of the connectors 60-65. When a switch is closed, a voltage is applied via the closed switch through the connector to the electrode. A constant voltage is applied via line 91 through connector 70 to the central electrode 10. Thus, the switched electrodes 20-25 may be switched on in any pre-determined sequence such that one of the electrodes 20-25 is on while the others are off. FIG. 3 illustrates an example of a controller system 100 that may be used to achieve the switching automatically. Of course, other types of controllers are also useful as long as they can "pulse" the switched electrodes 20-25 on and off and maintain a constant voltage at the central electrode 10. Further, the chemical bath 30 has separate gas chambers 40, 50 that are isolated from each other. The gas chamber 50 surrounding an upper portion of central electrode 10 is an oxygen chamber, while the gas chamber surrounding upper portions of the switched electrodes 20-25 is a hydrogen chamber. These chambers are used to capture off-gasses from the electrolysis process produced at the electrodes, and the off gasses may be siphoned off from these chambers by conduits (not shown) for any useful purpose. More than two chambers may also be useful, depending upon the configuration of the chemical bath 30, the number of fixed electrodes 10 per chemical bath 30, the number of switched electrodes 20-25, and other factors. Chemical bath 30 contains an electrolysis solution 35, indicated by its upper surface level in FIG. 1.
[0023] A "switching cycle" is the time period between when a switching electrode is first turned on (or off) and when it is next turned on (or off). The switching electrodes are "pulsed" by a pulsing period that is the time period from being turned on to being turned off. Referring to FIG. 3, exemplary controller 100 includes a variable timer 110 for setting the switching cycle time. The variable timer 110 is coupled to a decoder 130 through a divider 120. The decoder 130 sends signals to relay drivers 140-145 (shown generically as 140-140n in FIG. 3, for example) that are each in communication with a corresponding relay switch 80-85. The relay switches 80-85 are each coupled to a corresponding connector 60-65. As explained above, these connectors 60-65 are each in turn connected to a corresponding switched electrode 20-25.
[0024] FIG. 5 is a schematic illustration of an exemplary and generalized embodiment of an electrolysis system 5 that has a single fixed voltage electrode 10 and n switched voltage electrode(s) 20, 20n. The illustrated embodiment shows a case where "n" equals 2, but of course n may be any integer. The positive electrode 10 is located at or near the center of the chemical bath 30 surrounded by a circular array of n electrodes 20, 20n. These n electrodes 20, 20n may be in either a negatively charged state or off, as described above.
[0025] In an exemplary embodiment, the positively charged electrode 10 may be a nickel electrode. Another electrolytically equivalent electrode, such as nickel alloy, Incalloy(TM), tungsten, tungsten carbide, and the like, may also be useful. An exemplary embodiment of switched electrode element 20 is a proprietary carbon-based electrode that is available from Global Hydrogen, Inc. of Bertram, Tex. The chemical bath 30 utilized with this electrode combination may suitably include finely divided aluminum.
[0026] In another exemplary embodiment, the switched negative electrodes 20, 20n may include nickel, or nano nickel, or nano nickel and iron, or tungsten, or tungsten carbide. The positively charged electrode 10 may be graphite. In this embodiment, the chemical bath may contain finely divided zinc hydroxide, instead of aluminum.
[0027] According to the example of FIG. 5, a fixed positive voltage is connected to connection line 91 and a negative voltage is connected to connection line 90. The fixed electrode 10 is connected directly to the positive voltage connection line 91. The electrode elements 20, 20n each include a connector 60-60n, respectively, and these connectors 60-60n may each be sequentially coupled to the negative voltage connection line 90 through a corresponding relay switch 80-80n.
[0028] The example of a chemical bath 30 shown in FIG. 5 includes two isolated gas chambers 40 and 50 to separate H2O (liquid), hydrogen (gas) and oxygen (gas). Details of the electrolysis solution 35 in the chemical bath 30 are described below.
[0029] As used herein, the term "out-gasses" refers to gasses produced in the processes of embodiments including production through electrolysis and production through the reaction of metals with components of the electrolysis liquid.
[0030] The exemplary embodiment of the system 5 shown in FIG. 5 is in a quiescent state with all negative electrodes 20, 20n off. External power may be supplied to electrodes 20-20n through corresponding relay switches 80-80n from connection line 90, and to electrode 10 via line 91. Electrode elements 20, 20n are always either (a) at negative voltage potential when switched on or (b) disconnected (or "off"). Electrode element 10 is always positive. However, reverse voltage may be applied with different results. In the quiescent state, all relays 80, 80n are in the normally open state and no gasses are emitted from either electrode 10 or 20, 20n. Relays 80, 80n are operated sequentially (i.e. for the configuration in FIG. 5, connecting line 90 may rotate).
[0031] Referring to FIG. 6, when relay 80 closes, it applies a (-) negative voltage to electrode 20 and electricity flows between electrode 20 and positive electrode 10 through the electrolytic solution of chemical bath 30. Electrolysis takes place in the chemical bath 30, so that oxygen out-gasses at electrode 10 while hydrogen out-gasses at electrode 20. This gas production continues for a time period that extends beyond the time when the controller 100 relay switch 80 opens (disconnects from electrode 20) and closes relay 81 (not shown). The controlled process of timed opening and closing of relays according to a predetermined schedule is repeated sequentially for each electrode from 20 through 20n, and then the cycle repeats starting at electrode 20.
[0032] During the process, electrode 10 continues producing O2 (gas) as long as the controller is sequencing and any of electrodes 20 through 20n produces H2 (gas) as long as it is live and for a period immediately after applied voltage is removed and it is dead. According to an exemplary embodiment, immediately after any electrode 20n is deselected by the controller and is dead, it will continue to produce H2 (gas) for a period of time, despite being disconnected. After a predetermined period of time, the controller 100 via the relay switching mechanism, for example as described above, switches the applied voltage from one electrode (e.g. electrode 20 (n-1)) to the next electrode (e.g. electrode 20n). The process repeats and continues to cycle until it is stopped, when the system returns to the quiescent state. Note that hydrogen is emitted from the (-) electrode and oxygen is emitted from the (+) electrode.
[0033] Referring to exemplary FIG. 7, the polarity of the electrodes is reversed relative to that of FIG. 6. For example, in FIG. 7, the central electrode 10 is negatively charged while the switched electrodes 20-20n are positively charged, when activated. Hydrogen is then produced at the electrodes 20-20n, while oxygen is produced at the central electrode 10.
Electrode Preparation
[0034] According to an exemplary embodiment, the system uses two types of electrodes: a metal electrode, desirably nickel or an alloy of nickel, and graphite. These electrodes are treated before being used. It is theorized, without being bound, that the treatment saturates the pores of the electrodes with colloidal material and that it also increases the effective surface area of the electrodes by etching their surfaces.
[0035] In an exemplary embodiment, a solution that includes colloidal platinum, available from Purest Colloids, Inc. of Westhampton, N.J., USA, may be used in pre-treating the electrodes. The treatment solution may be prepared by adding about 75 ml of colloidal platinum to 75 ml distilled water and 10 ml of 98% sulfuric acid. Of course, for larger batches, these volumes may be increased proportionately. The colloidal metal is not restricted to platinum but may be any colloidal precious metal such as gold, palladium, rhenium, ruthenium, and the like. The electrodes are immersed at least partially in the solution and a positive terminal of a power source may be connected to the nickel electrode and a negative to the graphite electrode. Upon current flow, the graphite electrode becomes impregnated with platinum. This may be carried out, for example, at 4 volts dc and a 5 amp current. Then, after elapse of a time period, typically but not necessarily in the range 10-15 seconds, polarity is reversed, applying the same current and voltage potential, to plate platinum onto the nickel electrode's surfaces. This reversing of polarity may be carried out several times, desirably while heating the electrolysis solution to about 108[deg.] F. The process may be carried out for about 10 minutes, and then the electrodes may be removed and washed in distilled water.
[0036] An alternative embodiment of a method of electrode treatment includes using a palladium or platinum anode and a graphite or metal cathode to create a metal colloid and carrying out the infusing and plating in one step.
Cell Operation
[0037] As explained above with reference to FIG. 6, electrode element 10 has a positive voltage at all times and electrode elements 20, 20n are always either negative when connected, or otherwise disconnected. In the quiescent state, electrode elements 20, 20n are disconnected by relays 80, 80n, respectively, and no gas is emitted from electrode elements 10 or 20, 20n. In the active state, the controller 100 closes and opens relays 80, 80n in a predetermined scheduled sequence around the illustrated circular cell until the process is terminated. When the controller 100 closes relay element 80, a negative voltage is applied to electrode 20, for example, while electrode 10 is always positive. While relay 80 is closed, copious amounts of hydrogen are emitted from electrode element 20 and oxygen is emitted from electrode element 10. Due to electrolysis reactions, metal hydroxide present in the chemical bath solution plates out onto electrode 20 as a metallic coating while the electrolysis generates hydrogen and oxygen.
[0038] In an exemplary embodiment, the voltage controller 100 is preset or pre-programmed for a "relay closed" time period of three seconds for each electrode 20, 20n. Thus, it automatically closes a relay for each negative electrode sequentially for three seconds. As a consequence, the metal is plated onto the electrode for which the relay is closed (i.e. the "live" negative electrode) for three seconds. When the controller opens the relay, components of the chemical bath 30 solution commence reducing the plated out metal on the now dead electrode, thereby releasing copious hydrogen due to the reduction of metal to metal hydroxide, until substantially all the metal is dissolved back into element 30, or until a negative voltage potential is again applied to the electrode, when metal plating will recommence. In general, after the negative potential is disconnected from the electrode, the disconnected electrode 20 will continue producing gas at a gradually reducing rate and will substantially return to the quiescent state in about seven seconds. In the illustrated example embodiment shown in FIG. 1, there are six electrodes 20 which the controller 100 may sequentially connect to the negative connection lines 90 for three seconds each. Thus, the initial negative electrode 20 produces gas for a minimum of about ten seconds (three seconds while connected and seven seconds after disconnection). The electrode 20 will be selected for re-connection to line 90, according to the controller sequence, fifteen seconds after it was disconnected. The controller 100 establishes a three-seconds "on" and fifteen-seconds "off" sequence for each electrode 20, 20n. The production rate of this system averages over about 90% of the hydrogen that stoichiometry would predict (see FIG. 8, Table 1).
[0039] The gas-producing electrochemical process (metal plating onto the electrode when it is live and metal converting back to metal hydroxide when the electrode is dead) is believed, without being bound, to be of a catalytic nature and should continue as long as H2O lost through conversion to hydrogen and oxygen is replenished. It is believed, without being bound, that the colloidal silver and colloidal magnesium along with palladium infused in the graphite electrode have a catalytic effect.
[0040] When the controller 100 activates an electrode with an applied negative potential, for example electrode 20, electrical current will flow through the electrolyte chemical bath solution 30 between positive electrode 10 and negative electrode 20. Metal is plated on electrode 20, and if the process is allowed to continue, electrode 20 would become increasingly heavily plated, thus changing its electrical potential. As a result, its rate of hydrogen gas production would decline and finally cease. However, according to embodiments of the present technology, electrode 20 is disconnected (the applied potential is removed) after a predetermined period of time. In the non-limiting example described above, this time period was three seconds, although other longer or shorter time periods may be useful as well. In general, the time period may be selected based on several factors, one of which is to permit sufficient "electrode off" time to permit the chemical bath components to oxidize substantially all or so much of the metal from the electrode surface so that the amount of plated metal does not build up to the point of adversely affecting the rate of hydrogen gas production during the time period that the electrode is on. Thus, the chemicals in the electrolyte 35 may begin reacting with the metal deposited on electrode 20, thereby releasing more gas, and may substantially completely remove the deposited metal by the time electrode 20 is again selected by the controller 100 for activation. The total amount of gasses produced includes both (a) gas produced while an electrode is live and (b) gas produced when the electrode is dead. Accordingly, the electrical energy input needed to produce the total amount of gasses is less than would be the case if the gas produced under condition (b) also required energy (electricity) input.
[0041] The cycle time of the controller 100 may be set or pre-programmed to take into account various factors, including, but not limited to, power supplied to the electrodes, the voltage, the current, the electrode active surface area, the number of cells (a cell includes a negative electrode, the positive electrode and the chemical bath solution), and the cell configuration.
The Controller
[0042] It is understood that the illustrated exemplary embodiment of controller 100 in FIGS. 3 and 4 is merely one of many potentially useful controllers provided for explanatory purposes. Other forms of electronic controllers, such as Asics, or software controlled devices or micro-processors may be substituted, for example.
[0043] A more detailed drawing of an embodiment of an exemplary controller 100 is shown in FIG. 4. For purposes of description, element 110 may be a variable LM324 Op Amp oscillator generating square waves controlled by the RC time constant of R1, R16 and C11. Element 110 may, for example, be adjusted for a three-minute output of Divider Q5. Divider 120 may be a seven stage CMOS Divider. Since the exemplary circuit has six switched elements 20-20n, a four bit divider would suffice. The unused stages Q1-Q4 buffer the clock for more accurate timing, if required. The outputs Q5-Q7 are input to an analog sixteen bit decoder 130. A digital decoder may also be used. The decoder drives a Bipolar NPN transistor(s) MOSFET substitutable and has an LED to indicate which element is selected. Output X6 130 returns the counter to reset, causing the divider 120 to reset starting the count over after every sixth count. The number of counts are thus=n, up to n=8 for this example of a chip, or n=16 by using additional logic or a 4:16 bit decoder. The base input of the transistors 140-140n are normally disconnected and grounded turning the transistor(s) "OFF." When the counter 120 selects the channel on Output X6 130, a positive voltage is applied to the selected transistor turning the selected coil on. Coils 80-80n apply voltage on line 90 to the appropriate electrode. A diode is used to suppress high voltage induced by switching of the coils.
[0044] The controller system 100 shown in FIGS. 3 and 4 operates the relays 80, 80n at precise predetermined times. A first LED (D2), which may be any color, such as red, pulses at the Adjusted Oscillator frequency and a second LED (D7), which may be any color (conveniently a different color from the first LED), pulses at the electrode timing frequency, which, in this example, is three seconds "live." Of course, timing can be set by monitoring the state changes of the LEDs (D8-D13).
[0045] There are potentially a wide range of applications for the present technology. For example, FIG. 9 illustrates a residential application and FIG. 10 illustrates an automotive application. Other applications may become apparent to one of skill in the art who has read this disclosure. From the foregoing disclosure, it will be appreciated by those skilled in the art that the techniques described herein may be applied to a wide variety of systems for the production of hydrogen and oxygen that utilizes a system of electrolysis and chemical reduction.
[0046] The following examples merely illustrate aspects of the technology and are not limiting of the invention which is defined by the claims here below.
EXAMPLES
[0047] Hydrogen Production with Aluminum Powder
[0048] A chemical bath was prepared with a solution that included the following solutes:
[0049] 50-ml colloidal silver
[0050] 50 ml colloidal magnesium
[0051] 50 ml distilled water
[0052] 20 grams sodium hydroxide
[0053] 20 grams potassium hydroxide
[0054] 7 grams of aluminum
[0055] This solution 35 was placed in a 250 ml beaker 30. To this, 7 grams of aluminum were added and allowed to digest before the electrodes 10, 20 were inserted. The power supply was connected to the switching circuit, with the output voltage set at 2.0 volts DC (see FIG. 8, Table 1, Col. 1) at 0.25 amps (see Table 1, Col. 2). The positive wire 70 was connected to the nickel electrode 10 and negative lead 60 to the graphite electrodes 20. During the hydrogen/oxygen production, the aluminum hydroxide was reduced to aluminum on the graphite electrode and the aluminum reacted with the strong base electrolyte, thereby producing hydrogen on the graphite electrodes. Oxygen was produced on the nickel electrode as a result of the metal reduction. The gas flow was tested for the presence of hydrogen and oxygen by piping it through soapy water and then lighting the bubbles, which exploded very loudly, strongly indicating the presence of both hydrogen and oxygen.
[0056] It should also be appreciated that the illustrated exemplary embodiments are only examples, and are not intended to limit the scope, applicability, or configuration of the invention in any way. Rather, the foregoing detailed description will provide those skilled in the art with a convenient road map for implementing the exemplary embodiment or exemplary embodiments. It should be understood that various changes can be made in the function and arrangement of elements without departing from the scope of the invention as set forth in the appended claims and the legal equivalents thereof.
[0000] Hydrogen Production with Zinc
[0057] A chemical bath was prepared with a solution that included the following solutes:
[0058] 50-ml colloidal silver
[0059] 50 ml colloidal magnesium
[0060] 50 ml distilled water
[0061] 20 grams sodium hydroxide
[0062] 20 grams potassium hydroxide
[0063] 7 grams of zinc
[0064] This solution is placed in a 250 ml beaker 30 to pre-treat a nickel electrode. To this, 7 grams of zinc are connected to a nickel electrode and allowed to digest, depositing zinc onto the nickel. The nickel electrode is then removed. The power supply is connected to the switching circuit, with the output voltage set at 2.0 volts DC, as in the above example. The positive wire is connected to the graphite electrode and negative lead to the pre-treated nickel electrode. During the hydrogen/oxygen production, the zinc hydroxide is reduced to zinc on the nickel electrode and the zinc reacts with the nickel in the strong base electrolyte, thereby producing hydrogen on the nickel electrodes. Oxygen is produced on the graphite electrode as a result of the metal reduction. The gas flow is tested for the presence of hydrogen and oxygen by piping it through soapy water and then lighting the bubbles, which explode very loudly, strongly indicating the presence of both hydrogen and oxygen.
US2006188436
APPARATUS AND METHOD FOR THE PRODUCTION OF HYDROGEN
APPARATUS AND METHOD FOR THE PRODUCTION OF HYDROGEN
Also published as: KR20070103072 // WO2006091451 // MX2007010007 // JP2008529955 // EP1871705
Inventor: GRIFFIN LINNARD
EC: C01B3/08; Y02E60/36 ;IPC: C01B3/02; C01B3/08
Abstract -- Disclosed herein is an apparatus, mixture and method for the production of hydrogen comprising a solution with a pH less than 7, at least one colloidal metal suspended in the solution, and a second metal.
Description
TECHNICAL FIELD
[0001] The present invention is directed to a method and apparatus for the production of hydrogen gas from water.
BACKGROUND
[0002] Hydrogen gas is a valuable commodity with many current and potential uses. Hydrogen gas may be produced by a chemical reaction between water and a metal or metallic compound. Very reactive metals react with mineral acids to produce a salt plus hydrogen gas. Equations 1 through 5 are examples of this process, where HX represents any mineral acid. HX can represent, for example HCl, HBr, HI, H2SO4, HNO3, but includes all acids.
2Li+2HX->H2+2LiX (1)
2K+2HX->H2+2KX (2)
2Na+2HX->H2+2NaX (3)
Ca+2HX->H2+CaX2 (4)
Mg+2HX->H2+MgX2 (5)
[0003] Each of these reactions take place at an extremely high rate due to the very high activity of lithium, potassium, sodium, calcium, and magnesium, which are listed in order of their respective reaction rates, with lithium reacting the fastest and magnesium reacting the most slowly of this group of metals. In fact, these reactions take place at such an accelerated rate that they have not been considered to provide a useful method for the synthesis of hydrogen gas in the prior art.
[0004] Metals of intermediate reactivity undergo the same reaction but at a much more controllable reaction rate. Equations 6 and 7 are examples, again where HX represents all mineral acids.
Zn+2HX->H2+ZnX2 (6)
2Al+6HX->3H2+2AlX3 (7)
[0005] Reactions of this type provide a better method for the production of hydrogen gas due to their relatively slower and therefore more controllable reaction rate. Metals like these have not, however, been used in prior art production of diatomic hydrogen because of the expense of these metals.
[0006] Iron reacts with mineral acids by either of the following equations:
Fe+2HX->H2+FeX2 (8)
or
2Fe+6HX->3H2+2FeX3 (9)
[0007] Due to the rather low activity of iron, both of these reactions take place at a rather slow reaction rate. The reaction rates are so slow that these reactions have not been considered to provide a useful method for the production of diatomic hydrogen in the prior art. Thus, while iron does provide the availability and low price needed for the production of elemental hydrogen, it does not react at a rate great enough to make it useful for hydrogen production.
[0008] Metals such as silver, gold, and platinum are not found to undergo reaction with mineral acids under normal conditions in the prior art.
Ag+HX->No Reaction (10)
Au+HX->No Reaction (11)
Pt+HX->No Reaction (12)
[0009] Accordingly, a need exists for a method and apparatus for the efficient production of hydrogen gas using relatively inexpensive metals.
SUMMARY
[0010] It is a general object of the disclosed invention to provide a method and apparatus for the production of hydrogen gas. This and other objects of the present invention are achieved by providing a method, mixture and apparatus:
[0011] An apparatus for the production of hydrogen, comprising a reaction medium with a pH less than 7; a first metal, wherein the first metal is a colloidal metal suspended in the reaction medium; and a second metal, wherein the second metal is in contact with the reaction medium.
[0012] According to one preferred embodiment of the present invention, the second metal is in solid, non-colloidal form
[0013] According to another embodiment, the first metal is less reactive than the second metal.
[0014] According to another embodiment, the apparatus comprises a third metal in contact with the reaction medium.
[0015] According to another embodiment, the third metal is in colloidal form.
[0016] According to another embodiment, the third metal is more reactive than the second metal.
[0017] According to another embodiment, the apparatus comprises a reaction vessel for containing the reaction medium, wherein the reaction vessel is inert to the reaction medium.
[0018] According to another embodiment, the reaction vessel is configured to maintain an internal pressure above atmospheric pressure.
[0019] According to another embodiment, the first metal is silver, gold, platinum, tin, lead, copper, zinc, iron, aluminum, magnesium, beryllium, nickel or cadmium.
[0020] According to another embodiment, the second metal is iron, aluminum, magnesium, beryllium, tin, lead, nickel or copper.
[0021] According to another embodiment, the third metal is aluminum, magnesium, beryllium or lithium.
[0022] According to another embodiment, the reaction medium comprises hydrogen peroxide.
[0023] According to another embodiment, the reaction medium comprises formic acid.
[0024] According to another embodiment, the apparatus comprises an elemental nonmetal in contact with the reaction medium.
[0025] According to another embodiment, the apparatus comprises an energy source.
[0026] According to another embodiment, the energy source is a heater.
[0027] According to another embodiment, the energy source is a light source.
[0028] According to another embodiment, the energy source is an electrical potential applied to the reaction medium.
[0029] According to another embodiment, the apparatus comprises an anode and a cathode, wherein the anode and cathode are in contact with the reaction medium and wherein an electrical potential is applied between the anode and cathode.
[0030] According to another embodiment, the apparatus comprises third and fourth metals, wherein at least one of the second, third or fourth metals is in colloidal form.
BRIEF DESCRIPTION OF THE DRAWINGS
[0031] FIG. 1 is a diagram of a reactor for the production of hydrogen; and
[0032] FIG. 2 is a diagram of a laboratory experimental setup.
DETAILED DESCRIPTION
[0033] FIG. 1 shows a mixture and apparatus that may be used for the production of hydrogen. A reaction vessel 100 contains a reaction medium 102. The reaction medium preferably comprises water and an acid, and preferably has a pH less than 5, although other reaction media may be used including other solvents or non-liquid media such as gelatinous or gaseous media. The acid is preferably sulfuric acid with a variable concentration up to 98% by weight or hydrochloric acid with a variable concentration up to 35% by weight, although other acids may be used. The reaction vessel 100 is inert to the reaction medium 102. The reaction medium 102 contains a first colloidal metal (not shown) suspended in the solution. The first colloidal metal is preferably a metal with low activity such as silver, gold, platinum, tin, lead, copper, zinc or cadmium, although other metals may be used.
[0034] The reaction vessel 100 also preferably contains a second metal 104, at least partially submerged in the reaction medium 102. The second metal 104 may be in any form but is preferably in the form of a solid with a relatively large surface area, such as pellet form. The second metal 104 is preferably a metal with a mid-range activity, such as iron, aluminum, zinc, nickel or tin. The second metal 104 preferably has a higher activity than the first colloidal metal. The second metal 104 is most preferably iron, because of its medium reactivity and low cost. Preferably, the reaction medium 102 also contains a second colloidal metal (not shown). The second colloidal metal preferably has a higher activity than the second metal 104, such as aluminum, magnesium, beryllium, and lithium. Preferably, the reaction vessel 100 also contains another metal (not shown), which is a different metal than the second metal 104, but which is in the same general form. Therefore, in the most preferable case, the reaction vessel 100 contains two metals in solid form in contact with the reaction medium 102, as well as two colloidal metals suspended in the reaction medium 102.
[0035] Alternatively to the above, the reaction medium 102 may contain a metal salt or metal oxide, rather than an acid and the second metal 104, in addition to the one or more colloidal metals. Preferably, the reaction medium 102 contains a solid metal and either an acid or a metal salt or metal oxide of the same metal as the solid metal. It is believed that if the reaction medium 102 initially contains a solid metal and a strong acid, such as HCl or H2SO4, the acid reacts with the solid metal, creating metal ions and releasing hydrogen gas, until the acid or solid metal is substantially consumed. It is also believed that a solution initially containing a metal salt along with a proper colloidal catalyst will become acidic, even if the initial pH is greater than 7. Additionally, the apparatus may comprise a combination of metal salts, oxides and solid metals, in addition to one or more colloidal metal.
[0036] The reaction vessel 100 has an outlet 106 to allow hydrogen gas (not shown) to escape. The reaction vessel may also have an inlet 108 for adding water or other constituents to maintain the proper concentrations. The reaction vessel may also include one or more anode (not shown) and one or more cathode (not shown) which contact the reaction medium. The anodes and cathodes may be used to provide electrical energy to the reaction or to utilize electrical energy created by the reactions for other purposes.
[0037] Because the reactions expected to occur in the reaction vessel are believed to be collectively endothermic, an energy source 112 is also preferably provided to increase the rate of reaction, although the reaction may potentially be powered by ambient heat. While the energy source shown in FIG. 1 is a heater (hot plate), other forms of energy may be used including electric and light energy. There may be other effects of light or other electromagnetic radiation, in addition to the energy effect. Additionally, the reaction temperature is limited to about 100[deg.] C. at atmospheric pressure where an aqueous solution is used as the reaction medium or boiling may occur (neglecting changes in the boiling point due to the addition of solutes). Therefore, it may be advantageous to perform the reactions in a reaction vessel 100 which is configured to maintain an internal pressure above atmospheric pressure so that higher reaction temperatures may be used.
[0038] Most metals can be produced in a colloidal state in an aqueous solution. A colloid is a material composed of very small particles of one substance that are dispersed (suspended), but not dissolved in solution. Thus colloidal particles do not settle out of solution even though they exist in the solid state. A colloid of any particular metal is then a very small particle of that metal suspended in a solution. These suspended particles of metal may exist in the solid (metallic) form or in the ionic form, or as a mixture of the two. The very small size of the particles of these metals results in a very large effective surface area for the metal. This very large effective surface area for the metal can cause the surface reactions of the metal to increase dramatically when it comes into contact with other atoms or molecules. The colloidal metals used in the experiments described below were obtained using a colloidal silver machine sold by CS Prosystems of San Antonio, Tex. The website of CS Prosystems is www.csprosystems.com. Colloidal solutions of metals that are produced using this apparatus result from an electrolytic process and are thought to contain colloidal particles some of which are electrically neutral and some of which are positively charged. Other methods can be employed in the production of colloidal metal solutions where all of the colloidal particles are thought to be electrically neutral. It is believed that the positive charge on the colloidal metal particles used in the experiments described below provides additional rate enhancement effects. It is still believed however that it is to a great extent the size and the resulting surface area of the colloidal particles that causes a significant portion of the rate enhancement effects that are detailed below, regardless of the charge on the colloidal particles. Based on materials from the manufacturer, the particles of a metal in the colloidal solutions used in the experiments described below are believed to range in size between 0.001 and 0.01 microns. In such a solution of colloidal metals, the concentration of the metals is believed to be between about 5 to 20 parts per million.
[0039] Alternative to using a catalyst in colloidal form, it may be possible to use a catalyst in another form that offers a high surface-area to volume ratio, such as a porous solid, nanometals, colloid-polymer nanocomposites and the like. In general, any catalysts may be in any form with an effective surface area of at least 298,000,000 m<2 > per cubic meter of catalyst metal, although smaller surface area ratios may also work.
[0040] Thus when any metal, regardless of its normal reactivity, is used in its colloidal form, the reaction of the metal with mineral acids can take place at an accelerated rate. Equations 13-15 are thus general equations that are believed to occur for any metals in spite of their normal reactivity, where M represents any metal. M, for instance, can represent, but is not limited to, silver, copper, tin, zinc, lead, and cadmium. In fact, it has been found that the reactions shown in equations 13-15 occur at a significant reaction rate even in solutions of 1% aqueous acid.
2M+2HX->2MX+H2 (13)
M+2HX->MX2+H2 (14)
2M+6HX->2MX3+3H2 (15)
[0041] Even though equations 13-15 represent largely endothermic processes for a great many metals, particularly those of traditional low reactivity (for example, but not limited to, silver, gold, copper, tin, lead, and zinc), the rate of the reactions depicted in equations 13-15 is in fact very large due to the surface effects caused by the use of the colloidal metal. While reactions involved with equations 13-15 take place at a highly accelerated reaction rate, these reactions do not result in a useful production of elemental hydrogen since the colloidal metal by definition is present in very low concentrations.
[0042] A useful preparation of hydrogen results, however, by the inclusion of a metal more reactive than the colloidal metal such as, but not limited to, metallic iron, metallic aluminum, or metallic nickel. Thus any colloidal metal in its ionic form would be expected to react with the metal Me as indicated in equation 16, where those metals below Me on the electromotive or activity series of metals would react best.
Me+M<+> ->M+Me<+> (16)
[0043] It is believed that the reaction illustrated by equation 16 in fact takes place quite readily due to the large effective surface area of the colloidal ion, M<+> , and also due to the greater reactivity of the metal Me compared to any metal of lower reactivity which would be of preferable use. In fact, for metals normally lower in reactivity than Me, equation 16 would result in a highly exothermic reaction. The resulting metal, M, would be present in colloidal quantities and thus, it is believed, undergoes a facile reaction with any mineral acid including, but not limited to, sulfuric acid, hydrochloric acid, hydrobromic acid, nitric acid, hydroiodic acid, perchloric acid, and chloric acid. However, the mineral acid is preferably sulfuric acid, H2SO4, or hydrochloric acid, HCl. Equation 17 describes this reaction where the formula HX (or H<+> +X<-> in its ionic form) is a general representation for any mineral acid.
2M+2H<+> +2X<-> ->2M<+> +H2+2X<-> (17)
[0044] While equation 17 represents an endothermic reaction, it is believed the exothermicity of the reactions in equation 16 compensates for this, making the combination of the two reactions energetically obtainable using the thermal energy supplied by ambient conditions. Of course the supply of additional energy would accelerate the process.
[0045] Consequently, it is believed that elemental hydrogen is efficiently and easily produced by the combination of the reactions shown in equations 18 and 19.
4Me+4M<+> ->4M+4Me<+> (18)
4M+4H<+> +4X<-> ->4M<+> +2H2+4X<-> (19)
[0046] Thus the metal Me reacts with the colloidal metal ion in equation 18 to produce a colloidal metal and the ionic form of Me. The colloidal metal will then react with a mineral acid in equation 19 to produce elemental hydrogen and regenerate the colloidal metal ion. The colloidal metal ion will then react again by equation 18, followed again by equation 19, and so on in a chain reaction process to provide an efficient source of elemental hydrogen. In principle, any colloidal metal ion should undergo this process successfully. It is found that the reactions work most efficiently when the colloidal metal ion is lower in reactivity than the metal Me on the electromotive series table. The combining of equations 18 and 19 results in the net equation 20. Equation 20 has as its result the production of elemental hydrogen from the reaction of the metal Me and a mineral acid.
4 Me+4M<+> ->4M+4Me<+> (18)
+
4M+4H<+> +4X<-> ->4M<+> +2H2+4X<-> (19)
=
4 Me+4H<+> ->4 Me<+> +2H2 (20)
[0047] Equation 20 summarizes a process that provides a very efficient production of elemental hydrogen where the metal Me and acid are consumed. It is believed, however, that both the elemental metal Me and the acid are regenerated as a result of a voltaic electrochemical process or thermal process that follows. It is believed that a colloidal metal Mr (which can be the same one used in equation 18 or a different one) can undergo a voltaic oxidation-reduction reaction indicated by equations 21 and 22.
Cathode (reduction)
4Mr<+> +4e<-> ->4Mr (21)
Anode (oxidation)
2H2O->4H<+> +O2+4e<-> (22)
[0050] The colloidal metal Mr can in principle be any metal, but reaction 21 progresses most efficiently when the metal has a higher (more positive) reduction potential. Thus, the reduction of the colloidal metal ion, as indicated in equation 21, takes place most efficiently when the colloidal metal is lower than the metal Me on the electromotive series of metals. Consequently, any colloidal metal will be successful, but reaction 21 works best with colloidal metals such as colloidal silver or lead, due to the high reduction potential of these metals. When lead, for example, is employed as the colloidal metal ion in equations 21 and 22, the pair of reactions is found to take place quite readily. The voltaic reaction produces a positive voltage as the oxidation and reduction reactions indicated take place. This positive voltage can be used to supply the energy required for other chemical processes. In fact, the voltage produced can even be used to supply an over potential for reactions employing equations 21 and 22 taking place in another reaction vessel. Thus, this electrochemical process can be made to take place more quickly without the supply of an external source of energy. The resulting colloidal metal, Mr, can then react with oxidized ionic metal, Me, as indicated in equation 23 which would result in the regeneration of the metal, Me, and the regeneration of the colloidal metal in its oxidized form.
4 Me<+> +4Mr->4Mr<+> +4Me (23)
[0051] The reaction described by equation 23 could in fact occur using as starting material any colloidal metal, but will take place most effectively when the colloidal metal, Mr, appears above the metal, Me, on the electromotive series. The combining of equations 21-23 results in equation 24 which represents the regeneration of the elemental metal, Me, the regeneration of the acid, and the formation of elemental oxygen.
4Mr<+> +4e<-> ->4Mr (21)
+
2H2O->4H<+> +O2+4e<-> (22)
+
4Me<+> +4Mr->4Mr<+> +4Me (23)
=
4Me<+> +2H2O->4H<+> +4Me+O2 (24)
[0052] The reaction shown in equations 21 and 22 seems to occur best when the colloidal metal, Mr, is as low as possible on the electromotive series of metals; however, the reaction depicted by equation 23 takes place most efficiently when the colloidal metal, Mr, is as high as possible on the electromotive series of metals. The net reaction illustrated by equation 24, which is merely the sum of equations 21, 22, and 23, could in fact be maximally facilitated by either colloidal metals of higher activity or by colloidal metals of low activity. The relative importance of the reaction illustrated by equations 21 and 22 compared to the reaction shown in equation 23 would determine the characteristics of the colloidal metal that would best assist the net reaction in equation 24. It has been found that the net reaction indicated in equation 24 proceeds at a maximal rate when the colloidal metal is of maximum activity, that is, when the colloidal metal is as high as possible on the electromotive series of metals. It has been found that the more reactive colloidal metals such as, but not limited to, colloidal magnesium ion or colloidal aluminum ion produce the most facile processes for the reduction of cationic metals.
[0053] The combination of equations 20 and 24 results in a net process indicated in equation 25. As discussed above, the reaction depicted in equation 21 proceeds most efficiently when the colloidal metal is found below the metal, Me, on the electromotive series. However, the reaction represented by equation 23 is most favorable when the colloidal metal is found above the metal, Me, on the electromotive series. Accordingly, it has been observed that the concurrent use of two colloidal metals, one above the metal, Me, and one below it in the electromotive series, for example, but not limited to, colloidal lead and colloidal aluminum, produces optimum results in terms of the efficiency of the net process. Since equation 25 merely depicts the decomposition of water into elemental hydrogen and elemental oxygen, the complete process for the production of elemental hydrogen now has only water as an expendable substance, and the only necessary energy source is supplied by ambient thermal conditions.
4Me+4H<+> ->4Me<+> +2H2 (20)
4 Me<+> +2H2O->4H<+> +4Me+O2 (24)
2H2O->2H2+O2 (25)
[0054] The net result of this process is exactly that which would result from the electrolysis of water. Here, however, no electrical energy needs to be supplied. Although the providing of additional energy would result in an enhanced rate of hydrogen formation, the reaction proceeds efficiently when the only energy supplied is ambient thermal energy. When additional energy is supplied, it can be supplied in the manner of thermal energy, electrical energy or as radiant energy. As discussed above, when the additional energy supplied is in the form of thermal energy, it may be preferable to use a reaction vessel 100 configured to maintain internal pressures greater than the prevailing atmospheric pressure in order increase the boiling point of the solution, and increase the amount of thermal energy that can be supplied. The colloidal metallic ion catalysts, as well as the metal Me, and the acid are regenerated in the process, leaving only water as a consumable material.
[0055] A further means by which the rate of hydrogen production could be increased would involve the inclusion of a nonmetal in the reaction such as, but not limited to, carbon or sulfur. Using the symbol Z to represent the nonmetal, equation 22 would be replaced by equation 22A which would provide a more facile reaction due to the thermodynamic stability of the oxide of the nonmetal.
2H2O+Z->4H<+> +ZO2+4e<-> (22A)
Equation 24 would then be replaced by equation 24A, and equation 25 would be replaced by equation 25A.
4Me<+> +2H2O+Z->4H<+> +4Me+ZO2 (24A)
2H2O+Z->2H2+ZO2 (25A)
Thus, rather than resulting in the formation of elemental oxygen, the reaction would produce an oxide of a nonmetal such as CO2 or SO2, where the thermodynamic stability of the nonmetal oxide would provide an additional driving force for the reaction, and thus result in an even faster rate of hydrogen production.
[0056] An alternative to this process involves the introduction of hydrogen peroxide to react in the place of water. Thus, the reactions illustrated in equations 22 and 23 would be replaced by similar reactions illustrated by equations 26 and 27. The net result of these two reactions would be the reaction represented in equation 28, the production of elemental hydrogen using an elemental metal Me and a mineral acid as reactants.
2Me+2M<+> ->2M+2Me<+> (26)
+
2M+2H<+> +2X<-> ->2M<+1> +H2+2X<-> (27)
=
2Me+2H<+> ->2Me<+> +H2 (28)
[0057] The elemental metal, Me, as well as the mineral acid, would then be regenerated as a result of a different voltaic electrochemical process followed by a thermal reaction. Again a colloidal metal, Mr, reacts with hydrogen peroxide in an oxidation-reduction reaction indicated by equations 29 and 30.
Cathode (reduction)
2Mr<+> +2e<-> ->2Mr(29)
Anode (oxidation)
H2O2->2H<+> +O2+2e<-> (30)
[0060] Due to the fact that hydrogen peroxide has a larger (less negative) oxidation potential than water, as shown in the standard oxidation potentials listed below, the oxidation-reduction reaction resulting from equations 29 and 30 takes place at an enhanced rate compared to the oxidation-reduction reaction indicated by equations 21 and 22.
2H2O->4H<+> +O2+4e<-> [epsilon]<0 > oxidation=-1.229V
H2O2->2H<+> +O2+2e<-> [epsilon]<0 > oxidation=-0.695V
[0061] The colloidal metal can in principle be any metal but works most efficiently when the metal has a higher (more positive) reduction potential. Thus, the regeneration process takes place most efficiently when the colloidal metal is as low as possible on the electromotive series of metals. Consequently, any colloidal metal will be successful, but the reaction works well with colloidal silver ion, for example, due to the high reduction potential of silver. When silver is employed as the colloidal metal ion in equations 29 and 30, the pair of reactions is found to take place quite readily. The voltaic reaction produces a positive voltage as the oxidation and reduction reactions indicated take place. This positive voltage can be used to supply the energy required for other chemical processes. In fact, the voltage produced can even be used to supply an over potential for reactions employing equations 29 and 30 taking place in another reaction vessel. Thus, this electrochemical process can be made to take place more quickly without the supply of an external source of energy. The resulting colloidal metal, Mr, will then react to regenerate the metal, Me (equation 31).
2Me<+> +2Mr->2Mr<+> +2Me (31)
[0062] The reaction illustrated by equation 31 will take place most efficiently when the colloidal metal, Mr, is more reactive than the metal, Me. That is, the reaction in equation 31 will proceed most efficiently when the colloidal metal, Mr, is above the metal, Me, on the electromotive series of metals. The combining of equations 29-31 results in equation 32 which represents the regeneration of the elemental metal, Me, the regeneration of the acid, and the formation of elemental oxygen.
2Mr<+> +2e<-> ->2Mr (29)
+
H2O2->2H<+> +O2+2e<-> (30)
+
2Me<+> +2Mr->2Mr<+> +2Me (31)
=
2Me<+> +H2O2->2H<+> +2Me<+> O2 (32)
[0063] The reaction shown in equations 29 and 30 seems to occur best when the colloidal metal, Mr, is as low as possible on the electromotive series of metals; however, the reaction depicted by equation 31 takes place most efficiently when the colloidal metal, Mr, is as high as possible on the electromotive series of metals. The net reaction illustrated by equation 32, which is merely the sum of equations 29, 30, and 31, could in fact be maximally facilitated by either colloidal metals of higher activity, or by colloidal metals of lower activity. The relative importance of the reaction illustrated by equations 29 and 30 compared to the reaction shown in equation 31 would determine the characteristics of the colloidal metal that would best assist the net reaction in equation 32. It has been found that the net reaction indicated in equation 32 proceeds at a maximal rate when the colloidal metal is of maximum activity, that is, when the colloidal metal is as high as possible on the electromotive series of metals. It has been found that the more reactive colloidal metals such as, but not limited to, colloidal magnesium ion, and colloidal aluminum ion produce the most facile reduction processes for the reduction of cationic metals.
[0064] The combination of equations 28 and 32 results in a. net process indicated in equation 33. Since equation 33 merely depicts the decomposition of hydrogen peroxide into elemental hydrogen and elemental oxygen, the complete process for the production of elemental hydrogen now has only hydrogen peroxide as an expendable substance, and the only necessary energy source is supplied by ambient thermal conditions. Although the providing of additional energy would result in an enhanced rate of hydrogen formation, the reaction proceeds efficiently when the only energy supplied is ambient thermal energy. When additional energy is supplied, it can be supplied in the manner of thermal energy, electrical energy or as radiant energy. When the additional energy supplied is in the form of thermal energy, one is limited by the boiling point of the solvent. In aqueous systems this would provide a maximum temperature of 100[deg.] C. Under pressures higher than one atmosphere, however, temperatures higher than 100[deg.] C. could be obtained, and would provide an even more enhanced rate of hydrogen production.
2Me+2H<+> ->2Me<+> +H2 (28)
+
2Me<+> +H2O2->2H<+> +2Me+O2 (32)
=
H2O2->H2+O2 (33)
[0065] Since the regeneration of the metal, Me, and the mineral acid are significantly lower with respect to reaction rate than the oxidation of the metal, Me, by a mineral acid, it is the regeneration of the metal, Me, and the mineral acid that proves to be rate determining in this process. Since the oxidation of hydrogen peroxide (equation 30) is more favorable than the oxidation of water (equation 22), the rate of hydrogen formation is significantly enhanced when hydrogen peroxide is used in the place of water. This, of course, must be balanced by the fact that hydrogen peroxide is obviously a more costly reagent to supply, and that the ratio of elemental hydrogen to elemental oxygen becomes one part hydrogen to one part oxygen as indicated in equation 33. This would differ from the ratio of two parts hydrogen to one part oxygen as found in equation 25, where water is oxidized. In cases where the rate of hydrogen production is the most critical factor, the use of hydrogen peroxide will offer a significant advantage.
[0066] A further means by which the rate of hydrogen production could be increased would involve the inclusion of a nonmetal in the reaction such as, but not limited to, carbon or sulfur. Using the symbol Z to represent the nonmetal, equation 30 would be replaced by equation 30A which would provide a more facile reaction due to the thermodynamic stability of the oxide of the nonmetal.
H2O2+Z->2H<+> +ZO2+2e<-> (30A)
Equation 32 would then be replaced by equation 32A, and equation 33 would be replaced by equation 33A.
2Me<+> +H2O2+Z->2H<+> +2Me+ZO2 (32A)
H2O2+Z->H2+ZO2 (33A)
[0067] Thus, rather than resulting in the formation of elemental oxygen, the reaction would produce an oxide of a nonmetal such as CO2 or SO2, where the thermodynamic stability of the nonmetal oxide would provide an additional driving force for the reaction, and thus result in an even faster rate of hydrogen production. A further alternative to this process involves the introduction of formic acid to react in the place of water, or hydrogen peroxide. Thus, the reactions illustrated in equations 22 and 23 would be replaced by similar reactions illustrated by equations 26 and 27. The net result of these two reactions would be the reaction represented in equation 28, the production of elemental hydrogen using an elemental metal, Me, and a mineral acid as reactants.
2Me+2M<+> ->2M+2Me<+> (26)
+
2M+2H<+> +2X<-> ->2M<+1> +H2+2X<-> (27)
=
2Me+2H<+> ->2Me<+> +H2 (28)
[0068] The elemental Metal, Me, as well as the mineral acid would then be regenerated as a result of a different voltaic electrochemical process followed by a thermal reaction. In this case, however, the colloidal metal, Mr, reacts with formic acid in an oxidation-reduction reaction indicated by equations 29 and 34.
Cathode (reduction)
2Mr<+> +2e<-> 2Mr (29)
Anode (oxidation)
CH2O2->2H<+> +CO2+2e<-> (34)
[0071] Due to the fact that formic acid has a very favorable positive oxidation potential compared to the negative ones reported for water and for hydrogen peroxide, as shown by the standard oxidation potentials listed below, the oxidation-reduction reaction resulting from equations 29 and 34 takes place at an enhanced rate compared to the oxidation-reduction reaction indicated by equations 21 and 22, or the oxidation-reduction reaction indicated by equations 29 and 30.
2H2O->4H<+> +O2+4e<-> [epsilon]<0> oxidation=-1.229V
H2O2->2H<+> +O2+2e<-> [epsilon]<0> oxidation=-0.695V
CH2O2->2H<+> +CO2+2e<-> [epsilon]<0> oxidation=0.199V
[0072] The colloidal metal can in principle be any metal but works most efficiently when the metal has a higher (more positive) reduction potential. Thus, the regeneration process takes place most efficiently when the colloidal metal is as low as possible on the electromotive series of metals. Consequently, any colloidal metal will be successful, but the reaction works well with colloidal silver ion, for example, due to the high reduction potential of silver. When silver is employed as the colloidal metal ion in equations 29 and 34, the pair of reactions is found to take place quite readily. The voltaic reaction produces a positive voltage as the oxidation and reduction reactions indicated take place. This positive voltage can be used to supply the energy required for other chemical processes. In fact, the voltage produced can even be used to supply an over potential for reactions employing equations 29 and 34 taking place in another reaction vessel. Thus, this electrochemical process can be made to take place more quickly without the supply of an external source of energy. The resulting colloidal metal, Mr, will then react to regenerate the metal, Me (equation 31).
2Me<+> +2Mr->2Mr<+> +2Me (31)
[0073] The reaction illustrated by equation 31 will take place most efficiently when the colloidal metal, Mr, is more reactive than the metal, Me. That is, the reaction in equation 31 will proceed most efficiently when the colloidal metal, Mr, is above the metal, Me, on the electromotive series of metals. The combining of equations 29, 34 and 31 produces the net reaction shown by equation 35. The net reaction represented by equation 35 results in the regeneration of the elemental metal, Me, the regeneration of the acid, and the formation of carbon dioxide.
2Mr<+> +2e<-> ->2Mr (29)
+
CH2O2->2H<+> +CO2+2e<-> (34)
+
2Me<+> +2Mr->2Mr<+> +2Me (31)
=
2Me<+> +CH2O2->2H<+> +2Me+CO2 (35)
[0074] The reaction shown in equations 29 and 34 seems to occur best when the colloidal metal, Mr, is as low as possible on the electromotive series of metals; however, the reaction depicted by equation 31 takes place most efficiently when the colloidal metal, Mr, is as high as possible on the electromotive series of metals. The net reaction illustrated by equation 35, which is merely the sum of equations 29, 34, and 31, could, in fact, be maximally facilitated by either colloidal metals of higher activity or by colloidal metals of lower activity. The relative importance of the reaction illustrated by equations 29 and 34 compared to the reaction shown in equation 31 would determine the characteristics of the colloidal metal that would best assist the net reaction in equation 35. It has been found that the net reaction indicated in equation 35 proceeds at a maximal rate when the colloidal metal is of maximum activity, that is, when the colloidal metal is as high as possible on the electromotive series of metals. It has been found that the more reactive colloidal metals such as, but not limited to, colloidal magnesium ion and colloidal aluminum ion, produce the most facile reduction processes for the reduction of the cationic metals.
[0075] The combination of equations 28 and 35 results in a net process indicated in equation 36. Since equation 33 merely depicts the decomposition of formic acid into elemental hydrogen and carbon dioxide, the complete process for the production of elemental hydrogen now has only formic acid as an expendable substance, and the only necessary energy source is supplied by ambient thermal conditions. Although the providing of additional energy would result in an enhanced rate of hydrogen formation, the reaction proceeds efficiently when the only energy supplied is ambient thermal energy. When additional energy is supplied, it can be supplied in the manner of thermal energy, electrical energy or as radiant energy. When the additional energy supplied is in the form of thermal energy, one is limited by the boiling point of the solvent. In aqueous systems, this would provide a maximum temperature of 100[deg.] C. Under pressures higher than one atmosphere, however, temperatures higher than 100[deg.] C. could be obtained and would provide an even more enhanced rate of hydrogen production.
2Me+2H<+> ->2Me<+> +H2 (28)
+
2Me<+> +CH2O2->2H<+> +2Me+CO2 (35)
=
CH2O2->H2+CO2 (36)
[0076] Since the regeneration of the metal, Me, and the mineral acid are significantly lower with respect to reaction rate than the oxidation of the metal, Me, by a mineral acid, it is the regeneration of the metal, Me, and the mineral acid that proves to be rate determining in this process. Since the oxidation of formic acid (equation 34) is more favorable than the oxidation of water (equation 22), or the oxidation of hydrogen peroxide (equation 30), the rate of hydrogen formation is significantly enhanced when formic acid is used in the place of water or in the place of hydrogen peroxide. This, of course, must be balanced by the facts that formic acid is a more costly reagent than water, but a less costly one than hydrogen peroxide, and that the co-product formed along with hydrogen is carbon dioxide rather than oxygen. Additionally, the ratio of elemental hydrogen to carbon dioxide is one part hydrogen to one part carbon dioxide, as indicated in equation 36. This would differ from the ratio of two parts hydrogen to one part oxygen, as found in equation 25, where water is oxidized. In cases, however, where the rate of hydrogen production is the most critical factor, the use of formic acid will offer a significant advantage.
[0077] Finally, while all equations depicted here involve the use of just a single metal, Me, in addition to the colloidal metal(s), it has been shown that all of the reactions discussed herein can be carried out using a combination of two or more different metals in the place of the single metal, Me, along with one or more colloidal metal(s). It has been shown, in fact, that in some cases the use of multiple metals results in a significant rate enhancement over a rather large period of time. In experiments #7 and #10, for example, a mixture of metallic iron and metallic aluminum is used. The steady state production of hydrogen that results from experiment #10, for example, is approximately 100 mL of hydrogen per minute with the total volume of the reaction vessel being just over 100 mL. In experiments #8 and #9, similar reactions are carried out with just a single metal, aluminum, and it is demonstrated that when the reaction rate decreases, the addition of the second metal, iron, results in an immediate rate increase to a rate similar to those reactions where the two metals were present throughout the reaction. It is not clear at this point what causes this impressive rate enhancement. It is possible that the multiple metals all take part in the reaction mechanism to provide a more complicated mechanism having a greater number of steps, but a lower net activation barrier. Another possibility is that a second metal might provide a surface where the regenerated metal, Me, could reform more efficiently. Whatever the explanation, experiments #9 and #10 very clearly demonstrate that the rate enhancement caused by the use of two different metals is quite obvious and quite significant. Experimental Results: Experiment #1 Summary:
[0078] An initial solution comprising 10 mL of 93% concentration H2SO4 and 30 mL of 35% concentration HCl was reacted with iron pellets (sponge iron) and about 50 mL of colloidal magnesium and 80 mL of colloidal lead each at a concentration believed to be about 20 ppm. A theoretical maximum of 8.06 liters of hydrogen gas could be produced if solely from the consumption of the acids as indicated in Table 1.
TABLE 1
Starting Solution Maximum H2 Yield with Acid Consumption
Effective
Total Grams of Maximum H2
Acid mL Concentration Grams Acid Yield
H2SO4 10 93.0% 18.97 17.64 4.03 liters
HCl 30 35.0% 37.52 13.13 4.03 liters
Maximum H2 Yield: 8.06 liters
1 mole H2SO4 yields 1 mole H2 (22.4 liters)
1 mole H2SO4 = 98 grams
[0079] Therefore, the maximum yield is 0.23 liters of H2 per gram of H2SO4.
[0080] 2 moles of HCl yields 1 mole H2 (22.4 liters)
[0081] 2 moles of HCl=73 grams
[0082] Therefore, a theoretical maximum yield of 0.31 liters of H2 per gram of HCl is expected without the regeneration reaction.
[0083] The experimental setup was as illustrated in FIG. 2. The acid and iron solution was placed in flask 202. A hot plate 204 was used to provide thermal energy for the reaction and maintain the solution at a temperature of about 71[deg.] C. The gas produced by the reaction was fed through tube 206 to a volume-measuring apparatus 208. The volume-measuring apparatus 208 was an inverted reaction vessel 210 filled with water and placed in a water bath 212. The primary purpose of the experiment was to provide evidence that more than the theoretical maximum 8.06 liters of hydrogen was being produced by the closed-loop process of the invention.
[0084] The rate of the reaction initially is very fast with hydrogen generation at ambient temperature. When the acids are temporally consumed, the regeneration process takes into effect and the reaction rate slows. Heat may be added to the process to accelerate the regeneration process.
[0085] At least 15 liters of gas was observed to have been produced, and the reaction was still proceeding in a continuous fashion (about 2 bubbles of gas per second at 71[deg.] C.) when interrupted. It should be noted that the 15 liters of gas observed does not account for hydrogen gas losses likely due to leakage. Based upon previous observations and theoretical projections, the first 8.06 liters of gas produced is likely to be made up of essentially pure hydrogen, and beyond the theoretical threshold of 8.06 liters, 66.7% by volume of the gas produced would be hydrogen and the other 33.3% by volume would be oxygen. It is believed this experiment provides ample evidence of the regeneration process.
[0086] A follow-up experiment was conducted using iron (III) chloride (FeCl3) as the only source of iron in an attempt to qualitatively verify the reverse reaction. Pure iron (III) chloride was chosen because it could be shown to be free of iron in any other oxidation state. While similar experiments had been successfully carried out using iron (III) oxide as the source of iron, the results were clouded by the fact that other oxidation states of iron may have been present. The results are described in Experiment #2, below. Experiment #2 Summary:
[0087] An experiment was conducted using 150 mL of iron (III) chloride in an aqueous solution (commonly used as an etching solution, purchased from Radio Shack) as the starting materials. Ten mL of 93% concentration sulfuric acid (H2SO4) was added to the solution, at which point no reaction occurred. About 50 mL of colloidal magnesium and 80 mL of colloidal lead each at a concentration believed to be about 20 ppm were then added, at which point a chemical reaction began and the bubbling of gases was evident at ambient temperature. The production of gas accelerated when the solution was heated to a temperature of 65[deg.] C. The product gas was captured in soap bubbles and the bubbles were then ignited. The observed ignition of the gaseous product was typical for a mixture of hydrogen and oxygen.
[0088] Since hydrogen gas could only be produced with a concurrent oxidation of iron, it is evident that the iron (III) had to be initially reduced before it could be oxidized, thereby providing strong evidence of the reverse reaction. This experiment has subsequently been repeated with hydrochloric acid (HCl) instead of sulfuric acid, with similar results.
[0089] Two additional follow-up experiments (#3 using aluminum metal and #4 using iron metal) were conducted to determine if more hydrogen is produced compared to the maximum amount expected solely from the consumption of the metal. These results are described below. Experiment #3 Summary:
[0090] The starting solution had a total volume of 250 mL, including water, about 50 mL of colloidal magnesium and 80 mL of colloidal lead, each at a concentration believed to be about 20 ppm, 10 mL of 93% concentration H2SO4 and 30 mL of 35% concentration HCl as in experiment #1 above. Ten grams of aluminum metal was added to the solution which was heated and maintained at 90[deg.] C. The reaction ran for 1.5 hours and yielded 12 liters of gas. The pH was found to have a value under 2.0 at the end of 1.5 hours. The reaction was stopped after 1.5 hours by removing the unused metal and weighing it. The non-consumed aluminum weighed 4.5 grams, indicating a consumption of 5.5 grams of aluminum. The maximum amount of hydrogen gas normally expected by the net consumption of 5.5 grams of aluminum is 6.8 liters, as indicated in the table below.
TABLE 2
Starting Solution Maximum H2 Yield With Aluminum Consumption
Total Grams Total Grams Grams Maximum
Metal Initial Supply Final Consumed Yield* of H2
Aluminum 10 4.5 5.5 6.84 liters
(Al)
*If reacted aluminum has exclusively been used for the production of hydrogen:
2 moles Al yields 3 moles H2 (67.2 liters)
2 moles Al = 54 grams
[0091] Therefore, a theoretical maximum yield of 1.24 liters of H2 per gram of Al is expected without the regeneration reaction described above.
[0092] As in experiment #1, based on the total amount of acid supplied, it is expected that the first 8.06 liters of the gas generated is pure hydrogen with the balance being 50% hydrogen. Alternatively, the theoretical amount of hydrogen based on the amount of aluminum consumed is 6.84 liters. After 6.84 liters (the maximum yield expected from the aluminum consumed), it is expected that the remaining gas is 66.7% hydrogen. Therefore, we estimate that about 10.3 liters of hydrogen (out of about 12 total liters of gas) was produced in this experiment compared to the maximum of 6.84 or 8.06 liters expected based on the amount of aluminum consumed and the amount of acid supplied, respectively, thereby providing additional evidence of the regeneration process. Experiment #4 Summary:
[0093] The starting solution included a total volume of 250 mL, including water, about 50 mL of colloidal magnesium and 80 mL of colloidal lead, each at a concentration believed to be about 20 ppm, 10 mL of 93% concentration H2SO4 and 30 mL of 35% concentration HCl, as in experiment #1 above. One hundred grams of iron pellets (sponge iron) was added to the solution, which was heated and maintained at 90[deg.] C. The reaction ran for 30 hours and yielded 15 liters of gas. The pH was found to have a value of about 5.0 at the end of 30 hours. The reaction was stopped after 30 hours by removing the unused metal and weighing it. The non-consumed iron weighed 94 grams, indicating a consumption of 6 grams of iron. The maximum amount of hydrogen gas normally expected by the net consumption of 6 grams of iron, without the regeneration reaction described above, is 2.41 liters, as indicated in the table below.
TABLE 3
Starting Solution Maximum H2 Yield With Iron Consumption
Total Grams Total Grams Grams Maximum
Metal Initial Supply Final Consumed Yield* of H2
Iron 100 94 6 2.41 liters
(Fe)
*If reacted iron has exclusively been used for the production of hydrogen:
1 mole Fe yields 1 mole H2 (22.4 liters)
1 mole Fe = 55.85 grams
[0094] Therefore, a theoretical maximum yield of 0.40 liters of H2 per gram of Fe is expected without the regeneration reaction described above.
[0095] As in experiment #1, based on the total amount of acid supplied, it is expected that the first 8.06 liters of the gas generated is pure hydrogen with the balance being 66.7% hydrogen. However, the maximum theoretical generation of hydrogen based on the amount of iron consumed is 2.41 liters. After 2.41 liters (the maximum yield expected from the iron consumed), it is expected that the remaining gas is 66.7% hydrogen. Therefore, it is estimated that about 10.8 liters of hydrogen (out of about 15 total liters of gas) was produced in this experiment using colloidal catalyst, well over the maximum of 2.41 liters expected with the amount of iron consumed, thereby providing additional evidence of the regeneration process. Experiment #5 Summary:
[0096] An experiment was conducted using 200 mL of the final solution obtained from experiment #4, which contained oxidized iron plus catalyst and was found to have a pH of about 5. Acid was added to the solution, as in the above reactions (10 mL of 93% concentration H2SO4 and 30 mL of 35% concentration HCl), that brought the pH to a level of about 1. No additional colloidal materials were added, but 20 grams of aluminum metal was added. The solution was heated to a constant 96[deg.] C. The reaction proceeded to produce 32 liters of gas in a span of 18 hours, at which point the rate of the reaction had slowed significantly and the pH of the solution had become approximately 5.
[0097] The metal remaining at the end of the 18-hour experiment was separated and found to have a mass of 9 grams. This metal appeared to be a mixture of Al and Fe. Therefore, neglecting the amount of iron and aluminum remaining in solution, there was net consumption of 11 grams of metal and a net production of 32 liters of gas.
[0098] As indicated above, based on the amount of acid added to the reaction, the maximum amount of hydrogen gas expected solely from the reaction of acid with metal would be 8.06 liters. Depending on the makeup of the recovered metal, which had a mass of 9 grams, two extremes are possible: a) assuming the metal recovered was 100% Al, a maximum of 13.75 liters of hydrogen gas would be expected from the consumption of 11 grams of aluminum; and b) alternatively, assuming the metal recovered was 100% Fe, a maximum of 21.25 liters of hydrogen gas would be expected from the consumption of 17 grams of aluminum (20 grams supplied minus three grams used in the production of iron). For purposes of calculating maximum hydrogen gas generation, we assume the regeneration process does not occur and the Fe metal would have been generated from a conventional single displacement reaction with Al.
[0099] The actual percentage of Al and Fe would be somewhere between the two extremes and, therefore, the maximum amount of hydrogen gas generated solely from the consumption of metal (without regeneration) would be between 13.75 liters and 21.25 liters. The observed generation of 32 liters of gas compared to the maximum amount one would expect from the sole consumption of metal indicates that the regeneration process is taking place. It is believed that the increase in the rate of H2 production resulted from a high concentration of metal ions in the solution prior to the introduction of the elemental iron. Thus, resulting solutions from this family of reactions should not be discarded but rather should be used as the starting point for subsequent reactions. Consequently, this process for the generation of H2 will not produce significant chemical wastes that need to be disposed of. Experiment #6 Summary:
[0100] An experiment was conducted using 20 mL FeCl3, 10 mL colloidal magnesium, and 20 mL colloidal lead at a temperature of about 90[deg.] C. A gas was produced that is believed to be a mixture of hydrogen and oxygen, based upon observing the ignition of the gas. The pH of the mixture decreased during the reaction from a value of about 4.5 to a value of about 3.5. These observations show that it is not necessary to introduce either metallic iron or acid into the solution to produce hydrogen. Since the electrochemical oxidation/reduction reactions (equations 21-23 resulting in the net equation 24) result in the production of metallic iron and acid, these two constituents can be produced in this manner. Presumably, this would eventually attain the same steady state that is reached when metallic iron and acid are supplied initially. Experiment #7 Summary
[0101] An initial solution comprising 10 mL of 93% concentration H2SO4 and 30 mL of 35% concentration HCl was reacted with 20 grams of iron pellets, and 20 grams of aluminum pellets. There were then added 50 mL of colloidal magnesium and 80 mL of colloidal lead each at a concentration believed to be about 20 ppm, producing a total volume of about 215 mL. A theoretical maximum of 8.06 liters of hydrogen gas could be produced if solely from the consumption of the acids as indicated in Table 4.
TABLE 4
Starting Solution Maximum H2 Yield with Acid Consumption
Effective
Total Grams of Maximum H2
Acid mL Concentration Grams Acid Yield
H2SO4 10 93.0% 18.97 17.64 4.03 liters
HCl 30 35.0% 37.52 13.13 4.03 liters
Maximum H2 Yield: 8.06 liters
1 mole H2SO4 yields 1 mole of H2 (22.4 liters @ STP)
1 mole H2SO4 = 98 grams
[0102] Therefore, a theoretical maximum yield of 0.23 liters of H2 per gram of H2SO4 is expected without the regeneration reaction.
[0103] 2 moles of HCl yields 1 mole of H2 (22.4 liters @ STP)
[0104] 2 moles of HCl=73 grams
[0105] Therefore, a theoretical maximum yield of 0.31 liters of H2 per gram of HCl is expected without the regeneration reaction.
[0106] The experimental setup was as illustrated in FIG. 2. The mixture of acids and metals was placed in flask 202. A hot plate 204 was used to provide thermal energy for the reaction and maintain the solution at a temperature of about 71[deg.] C. The gas produced by the reaction was fed through tube 206 to a volume-measuring apparatus 208. The volume-measuring apparatus 208 was an inverted reaction vessel 210 filled with water and placed in a water bath 212. The primary purpose of the experiment was to provide evidence that more than the theoretical maximum 8.06 liters of hydrogen was being produced by the closed-loop process of the invention.
[0107] The rate of the reaction initially is very fast with instantaneous hydrogen generation at a rate of about 20 liters per hour. After about an hour the rate slows to a steady-state value of about 8.4 liters of gas produced per hour. Heat may be added to the process to accelerate the process of regenerating the metals and the acids.
[0108] While some gas was lost due to leakage and diffusion, at least 25 liters of gas was collected over a period of three hours, and the reaction was still proceeding in a continuous fashion at a rate of 8.4 liters of gas produced per hour. At this point the experiment was stopped and the remaining metal, a mixture of aluminum and iron was collected and dried, and was found to have a mass of 35.5 grams. Thus, 4.5 grams of metal was consumed. Since the remaining metal was not analyzed, it is not known in what ratio aluminum and iron reacted; however the simple oxidation of a metal by an acid would produce a maximum of 5.6 liters of hydrogen, well below that observed. Based upon previous observations and theoretical projections, the first 8.06 liters of gas produced is likely to be made up of essentially pure hydrogen, and beyond the theoretical threshold of 8.06 liters, 66.7% by volume of the gas produced would be hydrogen and the other 33.3% by volume would be oxygen. It is believed this experiment provides ample evidence for the regeneration process.
[0109] It is believed that the simultaneous use of two metals does not improve the initial rate of gas formation, but rather produces a reaction rate that is sustained over a much greater period of time. In order to further demonstrate this point, two additional experiments were performed. Experiment #8 Summary:
[0110] An initial solution comprising 10 mL of 93% concentration H2SO4 and 30 mL of 35% concentration HCl was reacted with 20 grams of aluminum pellets. There were then added 50 mL of colloidal magnesium and 80 mL of colloidal lead each at a concentration believed to be about 20 ppm, producing a total volume of about 215 mL. A theoretical maximum of 8.06 liters of hydrogen gas could be produced if solely from the consumption of the acids as indicated in Table 5.
TABLE 5
Starting Solution Maximum H2 Yield with Acid Consumption
Effective
Total Grams of Maximum H2
Acid mL Concentration Grams Acid Yield
H2SO4 10 93.0% 18.97 17.64 4.03 liters
HCl 30 35.0% 37.52 13.13 4.03 liters
Maximum H2 Yield: 8.06 liters
1 mole H2SO4 yields 1 mole of H2 (22.4 liters @ STP)
1 mole H2SO4 = 98 grams
[0111] Therefore, a theoretical maximum yield of 0.23 liters of H2 per gram of H2SO4 is expected without the regeneration reaction.
[0112] 2 moles of HCl yields 1 mole of H2 (22.4 liters @ STP)
[0113] 2 moles of HCl=73 grams
[0114] Therefore, a theoretical maximum yield of 0.31 liters of H2 per gram of HCl is expected without the regeneration reaction.
[0115] The experimental setup was as illustrated in FIG. 2. The mixture of acids and metal was placed in flask 202. A hot plate 204 was used to provide thermal energy for the reaction and maintain the solution at a temperature of about 71[deg.] C. The gas produced by the reaction was fed through tube 206 to a volume-measuring apparatus 208. The volume-measuring apparatus 208 was an inverted reaction vessel 210 filled with water and placed in a water bath 212. The primary purpose of the experiment was to provide evidence that more than the theoretical maximum 8.06 liters of hydrogen was being produced by the closed-loop process of the invention.
[0116] The initial reaction rate was similar to that found in experiment #1, where 9 liters of gas was produced in slightly less than one hour. At this point, however, the reaction rate was found to decrease by a factor of approximately one half. The addition of 20 grams of iron caused an immediate increase in reaction rate to the value that was initially observed at the onset of the experiment. Experiment #9 Summary:
[0117] An initial solution comprising 10 mL of 93% concentration H2SO4 and 30 mL of 35% concentration HCl was reacted with 40 grams of aluminum pellets. There were then added 50 mL of colloidal magnesium and 80 mL of colloidal lead each at a concentration believed to be about 20 ppm, producing a total volume of about 215 mL. A theoretical maximum of 8.06 liters of hydrogen gas could be produced if solely from the consumption of the acids as indicated in Table 6.
TABLE 6
Starting Solution Maximum H2 Yield with Acid Consumption
Effective
Total Grams of Maximum H2
Acid mL Concentration Grams Acid Yield
H2SO4 10 93.0% 18.97 17.64 4.03 liters
HCl 30 35.0% 37.52 13.13 4.03 liters
Maximum H2 Yield: 8.06 liters
1 mole H2SO4 yields 1 mole of H2 (22.4 liters @ STP)
1 mole H2SO4 = 98 grams
[0118] Therefore, a theoretical maximum yield of 0.23 liters of H2 per gram of H2SO4 is expected without the regeneration reaction.
[0119] 2 moles of HCl yields 1 mole of H2 (22.4 liters @ STP)
[0120] 2 moles of HCl=73 grams
[0121] Therefore, a theoretical maximum yield of 0.31 liters of H2 per gram of HCl is expected without the regeneration reaction.
[0122] The experimental setup was as illustrated in FIG. 2. The mixture of acids and metal was placed in flask 202. A hot plate 204 was used to provide thermal energy for the reaction and maintain the solution at a temperature of about 71[deg.] C. The gas produced by the reaction was fed through tube 206 to a volume-measuring apparatus 208. The volume-measuring apparatus 208 was an inverted reaction vessel 210 filled with water and placed in a water bath 212. The primary purpose of the experiment was to provide evidence that more than the theoretical maximum 8.06 liters of hydrogen was being produced by the closed-loop process of the invention.
[0123] The initial reaction rate was similar to that found in experiment #1, where 9 liters of gas was produced in slightly less than one hour. At this point however the reaction rate was found to decrease by a factor of approximately one half. The addition of 20 grams of iron caused an immediate increase in reaction rate to the value that was observed at the onset of the experiment.
[0124] Clearly an interaction is taking place between the two metals that produces a reaction that sustains its high rate of gas production a significant period of time. Experiment #10 Summary:
[0125] An initial solution comprising 10 mL of 93% concentration H2SO4 and 30 mL of 35% concentration HCl was reacted with 20 grams of iron pellets, and 20 grams of aluminum pellets. There were then added 25 mL of colloidal magnesium and 40 mL of colloidal lead each at a concentration believed to be about 20 ppm, producing a total volume of about 110 mL. A theoretical maximum of 8.06 liters of hydrogen gas could be produced if solely from the consumption of the acids as indicated in Table 7.
TABLE 7
Starting Solution Maximum H2 Yield with Acid Consumption
Effective
Total Grams of Maximum H2
Acid mL Concentration Grams Acid Yield
H2SO4 10 93.0% 18.97 17.64 4.03 liters
HCl 30 35.0% 37.52 13.13 4.03 liters
Maximum H2 Yield: 8.06 liters
1 mole H2SO4 yields 1 mole of H2 (22.4 liters @ STP)
1 mole H2SO4 = 98 grams
[0126] Therefore, a theoretical maximum yield of 0.23 liters of H2 per gram of H2SO4 is expected without the regeneration reaction.
[0127] 2 moles of HCl yields 1 mole of H2 (22.4 liters @ STP)
[0128] 2 moles of HCl=73 grams
[0129] Therefore, a theoretical maximum yield of 0.31 liters of H2 per gram of HCl is expected without the regeneration reaction.
[0130] The experimental setup was as illustrated in FIG. 2. The mixture of acids and metals was placed in flask 202. A hot plate 204 was used to provide thermal energy for the reaction and maintain the solution at a temperature of about 90[deg.] C. The gas produced by the reaction was fed through tube 206 to a volume-measuring apparatus 208. The volume-measuring apparatus 208 was an inverted reaction vessel 210 filled with water and placed in a water bath 212. The primary purpose of the experiment was to provide evidence that more than the theoretical maximum 8.06 liters of hydrogen was being produced by the closed-loop process of the invention.
[0131] The rate of the reaction initially is very fast with instantaneous hydrogen generation at a rate of about 20 liters per hour. After about an hour the rate slows to a steady-state value of about 6.0 liters per hour. Additional heat may be added to the process to further accelerate the process of regenerating the metals and the acids.
[0132] While some gas was lost due to leakage and diffusion, at least 32 liters of gas was collected over a period of five hours, and the reaction was still proceeding in a continuous fashion at a rate of 6.0 liters per hour. At this point, the experiment was stopped and the remaining metal, a mixture of aluminum and iron was collected and dried, and was found to have a mass of about 40 grams. Thus, only a negligible amount of metal was consumed. Since the remaining metal was not analyzed, it is not known in what ratio aluminum and iron were present; however, it can be assumed that approximately 20 grams of each metal was present in the remaining metallic sample. Based upon previous observations and theoretical projections, the first 8.06 liters of gas produced is likely to be made up of essentially pure hydrogen, and beyond the theoretical threshold of 8.06 liters, 66.7% by volume of the gas produced would be hydrogen and the other 33.3% by volume would be oxygen. It is believed this experiment provides further evidence for a more efficient regeneration process when smaller volumes are used in the reaction vessel.
[0133] The foregoing experiments were carried out under ambient lighting conditions which included a mixture of artificial and natural light sources. When the reactions described were performed under decreased light conditions, the reaction rates decreased. However, separate formal testing under decreased lighting has not been performed.
[0134] It is believed the experimental results described above demonstrate the potential value of the invention described herein. However, the calculations are based on the reaction mechanisms described above and are believed to accurately characterize the reactions involved in these experiments. However, if it is discovered that the theories of reactions or the calculations based thereon are in error, the invention described herein nevertheless is valid and valuable.
[0135] The embodiments shown and described above are exemplary. Many details are often found in the art and, therefore, many such details are neither shown nor described. It is not claimed that all of the details, parts, elements, or steps described and shown were invented herein. Even though numerous characteristics and advantages of the present invention have been described in the drawings and accompanying text, the description is illustrative only, and changes may be made in the detail, especially in matters of shape, size, and arrangement of the parts within the principles of the inventions to the full extent indicated by the broad meaning of the terms of the attached claims.
[0136] The restrictive description and drawings of the specific examples above do not point out what an infringement of this patent would be, but are to provide at least one explanation of how to use and make the inventions. The limits of the invention and the bounds of the patent protection are measured by and defined in the following claims.
US2006180464 // WO2006113463
Apparatus and method for the controllable production of hydrogen at an accelerated rate
Apparatus and method for the controllable production of hydrogen at an accelerated rate
Inventor: GRIFFIN LINNARD
EC: C25B1/04; C25B9/16B; // IPC: C25B1/02; C25B11/00; H01M8/06;
Abstract -- An apparatus for the production of hydrogen is disclosed, the apparatus comprising some or all of the following features, as well as additional features as described and claimed: a reaction medium; an anode in contact with the reaction medium; a cathode in contact with the reaction medium, wherein the cathode is capable of being in conductive contact with the anode; a catalyst suspended in the reaction medium, wherein the catalyst has a high surface-area-to-volume ratio; a salt dissolved in the reaction medium; a second high surface-area-to-volume ratio catalyst; a conductive path connecting the anode and cathode; a controller in the conductive path; an energy source; a reaction vessel and an electrical power source configured to provide an electrical potential between the cathode and the anode. Also disclosed are a method for producing hydrogen; an electric power generator; and a battery.
Description
[0001] This application claims priority from U.S. provisional application No. 60/671,664, filed Apr. 15, 2005; U.S. provisional application No. 60/678,614, filed May 6, 2005; U.S. provisional application No. 60/712,265, filed Aug. 29, 2005; and U.S. provisional application No. 60/737,981, filed Nov. 18, 2005. This application is also a continuation-in-part of application Ser. No. 11/060,960, filed Feb. 18, 2005, which is a continuation-in-part of application Ser. No. 10/919,755, filed Aug. 17, 2004, which claims priority to provisional application Ser. Nos. 60/496,174, filed Aug. 19, 2003; 60/508,989, filed Oct. 6, 2003; 60/512,663, filed Oct. 20, 2003; 60/524,468, filed Nov. 24, 2003; 60/531,766, filed Dec. 22, 2003; and 60/531,767, filed Dec. 22, 2003. Each of the applications listed above is hereby incorporated by reference for all purposes.
TECHNICAL FIELD
[0002] The present invention is directed to a method and apparatus for the production of hydrogen gas from water.
BACKGROUND
[0003] Dihydrogen gas, H2, also referred to as hydrogen gas, diatomic hydrogen, or elemental hydrogen is a valuable commodity with many current and potential uses. Hydrogen gas may be produced by a chemical reaction between water and a metal or metallic compound. Very reactive metals react with mineral acids to produce a salt plus hydrogen gas. Equations 1 through 5 are examples of this process, where HX represents any mineral acid. HX can represent, for example HCl, HBr, HI, H2SO4, HNO3, but includes all acids.
2Li+2HX->H2+2LiX (1)
2K+2HX->H2+2KX (2)
2Na+2HX->H2+2NaX (3)
Ca+2HX->H2+CaX2 (4)
Mg+2HX->H2+MgX2 (5)
[0004] Each of these reactions take place at an extremely high rate due to the very high activity of lithium, potassium, sodium, calcium, and magnesium, which are listed in order of their respective reaction rates, with lithium reacting the fastest and magnesium reacting the most slowly of this group of metals. In fact, these reactions take place at such an accelerated rate that they have not been considered to provide a useful method for the synthesis of hydrogen gas in the prior art.
[0005] Metals of intermediate reactivity undergo the same reaction but at a much more controllable reaction rate. Equations 6 and 7 are examples, again where HX represents all mineral acids.
Zn+2HX->H2+ZnX2 (6)
2Al+6HX->3H2+2AlX3 (7)
[0006] Reactions of this type provide a better method for the production of hydrogen gas due to their relatively slower and therefore more controllable reaction rate. Metals like these have not, however, been used in prior art production of diatomic hydrogen because of the expense of these metals.
[0007] Iron reacts with mineral acids by either of the following equations:
Fe+2HX->H2+FeX2 (8)
or
2Fe+6HX->3H2+2FeX3 (9)
[0008] Due to the rather low activity of iron, both of these reactions take place at a rather slow reaction rate. The reaction rates are so slow that these reactions have not been considered to provide a useful method for the production of diatomic hydrogen in the prior art. Thus, while iron does provide the availability and low price needed for the production of elemental hydrogen, it does not react at a rate great enough to make it useful for hydrogen production.
[0009] Metals such as silver, gold, and platinum are not found to undergo reaction with mineral acids under normal conditions in the prior art.
Ag+HX->No Reaction (10)
Au+HX->No Reaction (11)
Pt+HX->No Reaction (12)
[0010] In neutral or basic solutions very reactive metals react with water to produce hydrogen gas plus a base. Equations 13-16 are examples of this process.
2Li+2H2O->H2+2LiOH (13)
2K+2H2O->H2+2KOH (14)
2Na+2H2O->H2+2NaOH (15)
Ca+2H2O->H2+Ca(OH)2 (16)
[0011] Each of these reactions take place at an extremely high rate due to the very high activity of lithium, potassium, sodium, and calcium, which are listed in order of their respective reaction rates, with lithium reacting the fastest and calcium reacting the slowest of this group of metals. In fact, these reactions take place at such an accelerated rate that they do not provide a useful method for the synthesis of hydrogen gas.
[0012] Metals of intermediate reactivity undergo the same reaction in neutral or basic solution but heat must be supplied to promote these reactions. Equations 17-21 are examples of such a process.
Mg+2H2O->H2+Mg(OH)2 (17)
2Al+6H2O ->3H2+2Al(OH)3 (18)
Zn+2H2O->H2+Zn(OH)2 (19)
Fe+2H2O->H2+Fe(OH)2 (20)
2Fe+6H2O->3H2+2Fe(OH)3 (21)
[0013] While reactions of this type might seem to provide a better method for the production of hydrogen gas due to their relatively slower and therefore more controllable reaction rate, the high temperatures required for these reactions increase the cost of the process. Metals like these have therefore not been used in the production of diatomic hydrogen.
[0014] Accordingly, a need exists for a method and apparatus for the efficient production of hydrogen gas using relatively inexpensive metals.
SUMMARY
[0015] It is a general object of the disclosed invention to provide a method and apparatus for the controllable production of hydrogen gas at an accelerated rate. This and other objects of the present invention are achieved by providing:
[0016] An apparatus for the production of hydrogen generally comprising a reaction medium; an anode in contact with the reaction medium; a cathode in contact with the reaction medium, wherein the cathode is capable of being in conductive contact with the anode; and a catalyst suspended in the reaction medium, wherein the catalyst has a high surface-area-to-volume ratio.
[0017] In an additional embodiment, the catalyst is a colloidal metal.
[0018] In a further additional embodiment, the catalyst has a surface-area-to-volume ratio of at least 298,000,000 m<2 > per cubic meter.
[0019] In a further additional embodiment, a salt is dissolved in the reaction medium.
[0020] In a further additional embodiment, a cation of the salt is less reactive than a metal composing the anode.
[0021] In a further additional embodiment, a cation of the salt comprises zinc or cobalt.
[0022] In a further additional embodiment, the apparatus further comprises a second catalyst suspended in the reaction medium, wherein the second catalyst is a colloidal metal or has a surface-area-to-volume ratio of at least 298,000,000 m<2 > per cubic meter.
[0023] In a further additional embodiment, the anode and cathode are connected via a conductive path.
[0024] In a further additional embodiment, the conductive path is hardwired to the cathode and the anode.
[0025] In a further additional embodiment, the apparatus further comprises a controller in the conductive path between the cathode and the anode, wherein the controller is configured to selectively allow or hinder the flow of electrical current between the cathode and the anode through the conductive path.
[0026] In a further additional embodiment, the reaction medium is an aqueous solution.
[0027] In a further additional embodiment, the reaction medium comprises an acid or a base.
[0028] In a further additional embodiment, the cathode comprises tungsten carbide or carbonized nickel.
[0029] In a further additional embodiment, the anode comprises aluminum.
[0030] In a further additional embodiment, the cathode comprises surface-area-increasing features.
[0031] In a further additional embodiment, the surface area of the cathode is greater than the surface area of the anode.
[0032] In a further additional embodiment, the apparatus further comprises an energy source configured to provide energy to the reaction medium.
[0033] In a further additional embodiment, a reaction vessel containing the reaction medium is configured to maintain an internal pressure above atmospheric pressure.
[0034] In a further additional embodiment, the apparatus further comprises an electrical power source configured to provide an electrical potential between the cathode and the anode.
[0035] Also disclosed is a battery with many of the above features.
[0036] Also disclosed is a method of producing hydrogen gas comprising the steps of: suspending a colloidal metal in a reaction medium; contacting the reaction medium with a cathode; contacting the reaction medium with an anode; and electrically connecting the cathode and the anode.
[0037] In an additional embodiment, the method further comprises the step of dissolving a salt in the reaction medium.
[0038] In an additional embodiment, the method further comprises the steps of: interrupting the conductive path between the anode and cathode; and providing an electrical potential between the anode and cathode.
[0039] In an additional embodiment, the method further comprises the step of adding energy to the reaction medium.
[0040] Also disclosed is a method of controlling the production of hydrogen generally comprising the steps of: suspending a colloidal metal in a reaction medium; contacting the reaction medium with a cathode; contacting the reaction medium with an anode; connecting the cathode and the anode via a conductive path; and varying the resistance along the conductive path.
[0041] Also disclosed is an electrical power generator generally comprising: a reaction vessel; a reaction medium contained within the reaction vessel; an anode in contact with the reaction medium; a cathode in contact with the reaction medium, wherein the cathode is in conductive contact with the anode; a catalyst metal in contact with the reaction medium, wherein the catalyst metal is in colloidal form or has a surface-area-to-volume ratio of at least 298,000,000 m2 per cubic meter; an outlet in the reaction vessel configured to allow hydrogen gas to escape from the reaction vessel; and a fuel cell configured to accept hydrogen has from the outlet and use the gas to produce an electric potential.
BRIEF DESCRIPTION OF THE DRAWING
[0042] FIG. 1 is a diagram of a reactor for the production of hydrogen.
DETAILED DESCRIPTION
[0043] Most metals can be produced in a colloidal state in an aqueous solution. A colloid is a material composed of very small particles of one substance that are dispersed (suspended), but not dissolved in solution. Thus, colloidal particles do not settle out of solution, even though they exist in the solid state. A colloid of any particular metal is then a very small particle of that metal suspended in a solution. These suspended particles of metal may exist in the solid (metallic) form or in the ionic form, or as a mixture of the two. The very small size of the particles of these metals results in a very large effective surface area for the metal. This very large effective surface area for the metal can cause the surface reactions of the metal to increase dramatically when it comes into contact with other atoms or molecules.
[0044] The catalysts used in the experiments described below are colloidal metals obtained using a colloidal silver machine, model: Hvac-Ultra, serial number: U-03-98-198, sold by CS Prosystems of San Antonio, Tex. The website of CS Prosystems is www.csprosystems.com. Colloidal solutions of metals that are produced using this apparatus result from an electrolytic process and are thought to contain colloidal particles, some of which are electrically neutral and some of which are positively charged. Other methods can be employed in the production of colloidal metal solutions. It is believed that the positive charge on the colloidal metal particles used in the experiments described below provides additional rate enhancement effects. It is still believed, however, that it is to a great extent the size and the resulting surface area of the colloidal particles that causes a significant portion of the rate enhancement effects that are detailed below, regardless of the charge on the colloidal particles. Based upon data provided by the manufacturer of the machine used, the particles of a metal in the colloidal solutions used in the experiments described below are believed to range in size between 0.001 and 0.01 microns. In such a solution of colloidal metals, the concentration of the metals is believed to be between about 5 to 20 parts per million.
[0045] Alternative to using a catalyst in colloidal form, it may be possible to use a catalyst in another form that offers a high surface-area-to-volume ratio, such as a porous solid, nanometals, colloid-polymer nanocomposites and the like. In general, the catalysts may be in any form with an effective surface area that preferably on the order of 298,000,000 m2 per cubic meter of catalyst, although smaller surface area ratios may also work. Reactions In Acidic Media
[0046] Thus, when any metal, regardless of its normal reactivity, is used in its colloidal form, the reaction of the metal with mineral acids can take place at an accelerated rate. Equations 22-24 are thus general equations that are believed to occur for any metals in spite of their normal reactivity, where M represents any metal in colloidal form. M, for instance, can represent, but is not limited to, silver, copper, tin, zinc, lead, and cadmium. In fact, it has been found that the reactions shown in equations 22-24 occur at a significant reaction rate even in solutions of 1% aqueous acid.
2M+2HX->2MX+H2 (22)
M+2HX->MX2+H2 (23)
2M+6HX->2MX3+3H2 (24)
[0047] Even though equations 22-24 represent largely endothermic processes for many metals, particularly those of low reactivity (for example, but not limited to, silver, gold, copper, tin, lead, and zinc), the rate of the reactions depicted in equations 22-24 is in fact very high due to the surface effects caused by the use of the colloidal metal. While the reactions portrayed in equations 22-24 take place at a highly accelerated reaction rate, these reactions do not result in a useful production of elemental hydrogen since the colloidal metal by definition is present in very low concentrations.
[0048] A useful preparation of hydrogen results, however, by the inclusion of a metal more reactive than the colloidal metal such as, but not limited to, metallic iron, metallic aluminum, or metallic nickel. Thus, any colloidal metal in its ionic form, M<+> , would be expected to react with the metal Me as indicated in equation 25, where those metals M<+> below Me on the electromotive or activity series of metals would react best.
Me+M<+> ->M+Me<+> (25)
[0049] It is believed that the reaction illustrated by equation 25 takes place quite readily due to the large effective surface area of the colloidal ion, M<+> , and also due to the greater reactivity of the metal Me compared to M<+> , which is preferably of lower reactivity. In fact, for metals normally lower in reactivity than Me, equation 25 would result in a highly exothermic reaction. The metal, M, resulting from reduction of the colloidal ion, M<+> , would be present in colloidal quantities and thus, it is believed, undergoes a facile reaction with any mineral acid including, but not limited to, sulfuric acid, hydrochloric acid, hydrobromic acid, nitric acid, hydroiodic acid, perchloric acid, and chloric acid. However, the mineral acid is preferably sulfuric acid, H2SO4, or hydrochloric acid, HCl. Equation 26 describes this reaction where the formula HX (or H<+> +X<-> in its ionic form) is a general representation for any mineral acid.
2M+2H<+> +2X<-> ->2M<+> +H2+2X<-> (26)
[0050] While equation 26 represents an endothermic reaction, it is believed the exothermicity of the reactions in equation 25 compensates for this, making the combination of the two reactions energetically obtainable using the thermal energy supplied by ambient conditions. Of course the supply of additional energy accelerates the process.
[0051] Consequently, it is believed that elemental hydrogen is efficiently and easily produced by the combination of the reactions shown in equations 27 and 28.
4Me+4M<+> ->4M+4Me<+> (27)
4M+4H<+> +4X<-> ->4M<+> +2H2+4X<-> (28)
[0052] Thus the metal Me reacts with the colloidal metal ion in equation 27 to produce a colloidal metal and the ionic form of Me. The colloidal metal will then react with a mineral acid in equation 28 to produce elemental hydrogen and regenerate the colloidal metal ion. The colloidal metal ion will then react again by equation 27, followed again by equation 28, and so on in a chain process to provide an efficient source of elemental hydrogen.
[0053] In principle, any colloidal metal ion should undergo this process successfully. It is found that the reactions work most efficiently when the colloidal metal is lower in reactivity than the metal Me on the electromotive series table. The combining of equations 27 and 28 produces a net reaction that is illustrated by equation 29. Equation 29 has as its result the production of elemental hydrogen from the reaction of the metal Me and a mineral acid.
4Me+4M<+> ->4M+4Me<+> (27)
+
4M+4H<+> +4X<-> ->4M<+> +2H2+4X<-> (28)
=
4Me+4H<+> ->4Me<+> +2H2 (29)
[0054] Equation 29 summarizes a process that provides for very efficient production of elemental hydrogen where the metal Me and acid are consumed. It is believed, however, that both the elemental metal Me and the acid are regenerated as a result of a voltaic electrochemical process or thermal process that follows. It is believed that a colloidal metal Mr (which can be the same one used in equation 27 or a different metal) can undergo a voltaic oxidation-reduction reaction indicated by equations 30 and 31.
Cathode (reduction) 4Mr<+> +4e<-> ->4Mr (30)
Anode (oxidation) 2H2O->4H<+> +O2+4e<-> (31)
[0055] The colloidal metal Mr can in principle be any metal, but reaction 30 progresses most efficiently when the metal has a higher (more positive) reduction potential. Thus, the reduction of the colloidal metal ion, as indicated in equation 30, takes place most efficiently when the colloidal metal is lower than the metal Me on the electromotive series of metals. Consequently, any colloidal metal will be successful, but reaction 30 works best with colloidal metals such as colloidal silver or lead, due to the high reduction potential of these metals. When lead, for example, is employed as the colloidal metal ion in equations 30 and 31, the pair of reactions is found to take place quite readily. The voltaic reaction produces a positive voltage as the oxidation and reduction reactions take place. This positive voltage can be used to supply the energy required for other chemical processes. In fact, the voltage produced can even be used to supply an over potential for reactions employing equations 30 and 31 taking place in another reaction vessel. Thus, this electrochemical process can be made to take place more quickly without the supply of an external source of energy. The resulting colloidal metal, Mr, can then react with oxidized ionic metal, Me<+> , as indicated in equation 32, which would result in the regeneration of the metal, Me, and the regeneration of the colloidal metal in its oxidized form.
4Me<+> +4Mr->4Mr<+> +4Me (32)
[0056] The reaction described by equation 32 could in fact occur using as starting material any colloidal metal, but will take place most effectively when the colloidal metal, Mr, appears above the metal, Me, on the electromotive series. The combining of equations 30-32 results in equation 33 which represents the regeneration of the elemental metal, Me, the regeneration of the acid, and the formation of elemental oxygen.
4Mr<+> +4e<-> ->4Mr (30)
+
2H2O->4H<+> +O2+4e<-> (31)
+
4Me<+> +4Mr->4Mr<+> +4Me (32)
=
4Me<+> +2H2O->4H<+> +4Me+O2 (33)
[0057] It is believed that the reaction shown in equations 30 and 31 occur best when the colloidal metal, Mr, is as low as possible on the electromotive series of metals; however, it is believed that the reaction depicted by equation 32 takes place most efficiently when the colloidal metal, Mr, is as high as possible on the electromotive series of metals. The net reaction illustrated by equation 33, which is merely the sum of equations 30, 31, and 32, could in fact be maximally facilitated by either colloidal metals of higher activity or by colloidal metals of lower activity. The relative importance of the reaction illustrated by equations 30 and 31 compared to the reaction shown in equation 32 would determine the characteristics of the colloidal metal that would best assist the net reaction in equation 33. It has been found that the net reaction indicated in equation 33 proceeds at a maximal rate when the colloidal metal is of higher activity, that is, when the colloidal metal is higher on the electromotive series of metals. It has been found that the more reactive colloidal metals such as, but not limited to, colloidal magnesium ion or colloidal aluminum ion produce the most facile processes for the reduction of cationic metals.
[0058] The combination of equations 29 and 33 results in a net process indicated in equation 34. As discussed above, the reaction depicted in equation 30 proceeds most efficiently when the colloidal metal is found below the metal, Me, on the electromotive series. However, the reaction represented by equation 32 is most favorable when the colloidal metal is found above the metal, Me, on the electromotive series. Accordingly, it has been observed that the concurrent use of two colloidal metals, one above the metal, Me, and one below it in the electromotive series-for example, but not limited to, colloidal lead and colloidal aluminum-produces optimum results in terms of the efficiency of the net process. Since equation 34 merely depicts the decomposition of water into elemental hydrogen and elemental oxygen, the complete process for the production of elemental hydrogen now has only water as an expendable substance, and the only necessary energy source is supplied by ambient thermal conditions.
4Me+4H<+> ->4Me<+> +2H2 (29)
+
4Me<+> +2H2O->4H<+> +4Me+O2 (33)
=
2H2O->2H2+O2 (34)
[0059] The net result of this process is exactly that which would result from the electrolysis of water. Here, however, no electrical energy needs to be supplied. Although the providing of additional energy would result in an enhanced rate of hydrogen formation, the reaction proceeds efficiently when the only energy supplied is ambient thermal energy. When additional energy is supplied, it can be supplied in the form of thermal energy, solar energy, electrical energy, radiant energy or other energy forms. When the additional energy supplied is thermal in nature, the maximum temperature achievable at atmospheric pressure is the boiling point of the solution; in aqueous systems this would be approximately a temperature of 100[deg.] C. At pressures greater than one atmosphere, however, temperatures higher than 100[deg.] C. could be obtained, and would provide an even more enhanced rate of hydrogen production. Therefore, when the additional energy supplied is in the form of thermal energy, it may be preferable to use a reaction vessel configured to maintain internal pressures greater than the prevailing atmospheric pressure, in order increase the boiling point of the solution and increase the amount of thermal energy that can be supplied. The colloidal metallic ion catalysts M<+> and/or Mr<+> as well as the metal Me and the acid are regenerated in the process, leaving only water as a consumable material. Elemental Nonmetal
[0060] A further means by which the rate of hydrogen production could be increased involves the inclusion of a nonmetal in the reaction such as, but not limited to, carbon or sulfur. Using the symbol Z to represent the nonmetal, equation 31 would be replaced by equation 35 which portrays a more facile reaction due to the thermodynamic stability of the oxide of the nonmetal.
2H2O+Z->4H<+> +ZO2+4e<-> (35)
[0061] Equation 33 would then be replaced by equation 36, and equation 34 would be replaced by equation 37.
4Me<+> +2H2O+Z->4H<+> +4Me+ZO2 (36)
2H2O+Z->2H2+ZO2 (37)
Thus, rather than resulting in the formation of elemental oxygen, O2, the reaction would produce an oxide such as CO2 or SO2 of a nonmetal, where the thermodynamic stability of the nonmetal oxide would provide an additional driving force for the reaction and thus result in an even faster rate of hydrogen production.
Reducing Agents
[0062] An alternative to the above process involves the introduction of a reducing agent such as hydrogen peroxide to react in the place of water. Thus, the reactions illustrated in equations 31 and 32 would be replaced by similar reactions illustrated by equations 38 and 39. The net result of these two reactions would be the reaction represented in equation 40, the production of elemental hydrogen using an elemental metal Me and a mineral acid as reactants.
2Me+2M<+> ->2M+2Me<+> (38)
+
2M+2H<+> +2X<-> ->2M<+1> +H2+2X<-> (39)
=
2Me+2H<+> ->2Me<+> +H2 (40)
[0063] The elemental metal, Me, as well as the mineral acid, would then be regenerated as a result of a different voltaic electrochemical process followed by a thermal reaction. Again, a colloidal metal, Mr, reacts with hydrogen peroxide in an oxidation-reduction reaction indicated by equations 41 and 42.
Cathode (reduction) 2Mr<+> +2e<-> ->2Mr (41)
Anode (oxidation) H2O2->2H<+> +O2+2e<-> (42)
[0064] Due to the fact that hydrogen peroxide has a larger (less negative) oxidation potential than water, as shown in the standard oxidation potentials listed below, the oxidation-reduction reaction resulting from equations 41 and 42 takes place at an enhanced rate compared to the oxidation-reduction reaction indicated by equations 30 and 31.
2H2O->4H<+> +O2+4e<-> [epsilon]<0 > oxidation=-1.229V
H2O2->2H<+> +O2+2e<-> [epsilon]<0 > oxidation=-0.695V
[0065] The colloidal metal, Mr, can, in principle, be any metal but works most efficiently when the metal has a high (more positive) reduction potential. Thus, the regeneration process takes place most efficiently when the colloidal metal is as low as possible on the electromotive series of metals. Consequently, any colloidal metal will be successful, but the reaction works well with colloidal silver ion, for example, due to the high reduction potential of silver. When silver is employed as the colloidal metal ion in equations 41 and 42, the pair of reactions is found to take place readily. The voltaic reaction produces a positive voltage as the oxidation and reduction reactions indicated take place. This positive voltage can be used to supply the energy required for other chemical processes. In fact, the voltage produced can even be used to supply an over-potential for reactions employing equations 41 and 42 taking place in another reaction vessel. Thus, this electrochemical process can be made to take place more quickly without the supply of an external source of energy. The resulting colloidal metal, Mr, will then react to regenerate the metal, Me (equation 43).
2Me<+> +2Mr->2Mr<+> +2 (43)
[0066] The reaction illustrated by equation 43 will take place most efficiently when the colloidal metal, Mr, is more reactive than the metal, Me. That is, the reaction in equation 43 will proceed most efficiently when the colloidal metal, Mr, is above the metal, Me, on the electromotive series of metals. The combining of equations 41-43 results in equation 44 which represents the regeneration of the elemental metal, Me, the regeneration of the acid, and the formation of elemental oxygen.
2Mr<+> +2e<-> ->2Mr (41)
+
H2O2->2H<+> +O2+2e<-> (42)
+
2Me<+> +2Mr->2Mr<+> +2Me (43)
=
2Me<+> +H2O2->2H<+> +2Me+O2 (44)
[0067] The reactions shown in equations 41 and 42 seem to occur best when the colloidal metal, Mr, is as low as possible on the electromotive series of metals; however, the reaction depicted by equation 43 takes place most efficiently when the colloidal metal, Mr, is as high as possible on the electromotive series of metals. The net reaction illustrated by equation 44, which is merely the sum of equations 41, 42, and 43, could in fact be facilitated by either colloidal metals of higher activity or lower activity than Me. The relative importance of the reaction illustrated by equations 41 and 42 compared to the reaction shown in equation 43 would determine the characteristics of the colloidal metal that would best assist the net reaction in equation 44. It has been found that the net reaction indicated in equation 44 proceeds at a maximal rate when the colloidal metal is of higher activity, that is, when the colloidal metal is as high as possible on the electromotive series of metals. It has been found that the more reactive colloidal metals such as, but not limited to, colloidal magnesium ion and colloidal aluminum ion produce the most facile reduction processes for the reduction of cationic metals.
[0068] The combination of equations 40 and 44 results in the net process indicated in equation 45. Since equation 45 merely depicts the decomposition of hydrogen peroxide into elemental hydrogen and elemental oxygen, the complete process for the production of elemental hydrogen now has only hydrogen peroxide as an expendable substance, and the only necessary energy source is supplied by ambient thermal conditions.
2Me+2H<+> ->2Me<+> +H2 (40)
+
2Me<+> +H2O2->2H<+> +2Me+O2 (44)
=
H2O2->H2+O2 (45)
[0069] Since the rate of regeneration of the metal, Me, and the mineral acid are significantly lower than the rate of oxidation of the metal, Me, by a mineral acid, it is the regeneration of the metal, Me, and the mineral acid that proves to be rate-determining in this process. Since the oxidation of hydrogen peroxide (equation 42) is more favorable than the oxidation of water (equation 31), the rate of hydrogen formation is significantly enhanced when hydrogen peroxide is used in the place of water. This, of course, must be balanced by the fact that hydrogen peroxide is obviously a more costly reagent to supply, and that the ratio of elemental hydrogen to elemental oxygen becomes one part hydrogen to one part oxygen as indicated in equation 45. This would differ from the ratio of two parts hydrogen to one part oxygen as found in equation 34, where water is oxidized. In cases where the rate of hydrogen production is the more critical factor, the use of hydrogen peroxide will offer a significant advantage.
[0070] A further means by which the rate of hydrogen production could be increased would involve the inclusion of a nonmetal in the reaction such as, but not limited to, carbon or sulfur. Using the symbol Z to represent the nonmetal, equation 42 would be replaced by equation 46 which portrays a more facile reaction due to the thermodynamic stability of the oxide of the nonmetal.
H2O2+Z->2H<+> +ZO2+2e<-> (46)
Equation 44 would then be replaced by equation 47, and equation 45 would be replaced by equation 48.
2Me<+> +H2O2+Z->2H<+> +2Me+ZO2 (47)
H2O2+Z->H2+ZO2 (48)
[0071] Thus, rather than resulting in the formation of elemental oxygen, O2, the reaction would produce an oxide such as CO2 or SO2 of a nonmetal, where the thermodynamic stability of the nonmetal oxide would provide an additional driving force for the reaction, and thus result in an even faster rate of hydrogen production.
[0072] A further alternative to this process involves the introduction of other reducing agents, such as formic acid, to react in the place of water or hydrogen peroxide. Thus, the reactions illustrated in equations 31 and 32 would be replaced by similar reactions illustrated by equations 38 and 39. The net result of these two reactions would be the reaction represented in equation 40, the production of elemental hydrogen using an elemental metal, Me, and a mineral acid as reactants.
2Me+2M<+> ->2M+2Me<+> (38)
+
2M+2H<+> +2X<-->2> M<+1> +H2+2X<-> (39)
=
2Me+2H<+> ->2Me<+> +H2 (40)
[0073] The elemental metal, Me, as well as the mineral acid would then be regenerated as a result of a different voltaic electrochemical process followed by a thermal reaction. In this case, however, the colloidal metal, Mr, reacts with formic acid in an oxidation-reduction reaction indicated by equations 41 and 49.
Cathode (reduction) 2Mr<+> +2e<-> ->2Mr (41)
Anode (oxidation) CH2O2->2H<+> +CO2+2e<-> (49)
[0074] Due to the fact that formic acid has a very favorable positive oxidation potential compared to the negative ones reported for water and for hydrogen peroxide, as shown by the standard oxidation potentials listed below, the oxidation-reduction reaction resulting from equations 41 and 49 takes place at an enhanced rate compared to the oxidation-reduction reaction indicated by equations 30 and 31, or the oxidation-reduction reaction indicated by equations 41 and 42.
2H2O->4H<+> +O2+4e<-> [epsilon]<0> oxidation=-1.229V
H2O2->2H<+> +O2+2e<-> [epsilon]<0> oxidation=-0.695V
CH2O2->2H<+> +CO2+2e<-> [epsilon]<0> oxidation=0.199V
[0075] The colloidal metal, Mr, can in principle be any metal but works most efficiently when the metal has a high (more positive) reduction potential. Thus, the regeneration process takes place most efficiently when the colloidal metal is as low as possible on the electromotive series of metals. Consequently, any colloidal metal will be successful, but the reaction works well with colloidal silver ion, for example, due to the high reduction potential of silver. When silver is employed as the colloidal metal ion in equations 41 and 49, the pair of reactions is found to take place quite readily. The voltaic reaction produces a positive voltage as the oxidation and reduction reactions indicated take place. This positive voltage can be used to supply the energy required for other chemical processes. In fact, the voltage produced can even be used to supply an over-potential for reactions employing equations 41 and 49 taking place in another reaction vessel. Thus, this electrochemical process can be made to take place more quickly without the supply of an external source of energy. The resulting colloidal metal, Mr, will then react to regenerate the metal, Me (equation 43).
2Me<+> +2Mr->2Mr<+> +2Me (43)
[0076] The reaction illustrated by equation 43 will take place most efficiently when the colloidal metal, Mr, is more reactive than the metal, Me. That is, the reaction in equation 43 will proceed most efficiently when the colloidal metal, Mr, is above the metal, Me, on the electromotive series of metals. The combining of equations 41, 49 and 43 produces the net reaction shown by equation 50. The net reaction represented by equation 50 results in the regeneration of the elemental metal, Me, the regeneration of the acid, and the formation of carbon dioxide.
2Mr<+> +2e<-> ->2Mr (41)
+
CH2O2->2H<+> +CO2+2e<-> (49)
+
2Me<+> +2Mr->2Mr<+> +2Me (43)
=
2Me<+> +CH2O2->2H<+> +2Me+CO2 (50)
[0077] The reactions shown in equations 41 and 49 seem to occur best when the colloidal metal, Mr, is as low as possible on the electromotive series of metals. However, the reaction depicted by equation 43 takes place most efficiently when the colloidal metal, Mr, is as high as possible on the electromotive series of metals. The net reaction illustrated by equation 50, which is merely the sum of equations 41, 49, and 43, could, in fact, be maximally facilitated by either colloidal metals of higher activity or by colloidal metals of lower activity. The relative importance of the reaction illustrated by equations 41 and 49 compared to the reaction shown in equation 43 would determine the characteristics of the colloidal metal that would best assist the net reaction in equation 50. It has been found that the net reaction indicated in equation 50 proceeds at a maximal rate when the colloidal metal is of maximum activity, that is, when the colloidal metal is as high as possible on the electromotive series of metals. It has been found that the more reactive colloidal metals such as, but not limited to, colloidal magnesium ion and colloidal aluminum ion, produce the most facile reduction processes for the reduction of the cationic metals.
[0078] The combination of equations 40 and 50 results in a net process indicated in equation 51. Since equation 51 merely depicts the decomposition of formic acid into elemental hydrogen and carbon dioxide, the complete process for the production of elemental hydrogen now has only formic acid as an expendable substance, and the only necessary energy source is supplied by ambient thermal conditions. Although the providing of additional energy would result in an enhanced rate of hydrogen formation, the reaction proceeds efficiently when the only energy supplied is ambient thermal energy.
2Me+2H<+> ->2Me<+> +H2 (40)
+
2Me<+> +CH2O2->2H<+> +2Me+CO2 (50)
=
CH2O2->H2+CO2 (51)
[0079] Since the regeneration of the metal, Me, and the mineral acid are significantly lower with respect to reaction rate than the oxidation of the metal, Me, by a mineral acid, it is the regeneration of the metal, Me, and the mineral acid that is believed to be rate determining in this process. Since the oxidation of formic acid (equation 49) is more favorable than the oxidation of water (equation 31), or the oxidation of hydrogen peroxide (equation 42), the rate of hydrogen formation is significantly enhanced when formic acid is used in the place of water or in the place of hydrogen peroxide. This, of course, must be balanced by the facts that formic acid is a more costly reagent than water, but a less costly one than hydrogen peroxide, and that the co-product formed along with hydrogen is carbon dioxide rather than oxygen. Additionally, the ratio of elemental hydrogen to carbon dioxide is one part hydrogen to one part carbon dioxide, as indicated in equation 51. This would differ from the ratio of two parts hydrogen to one part oxygen, as found in equation 34, where water is oxidized. In cases, however, where the rate of hydrogen production is the most critical factor, the use of formic acid will offer a significant advantage. Multiple Metals
[0080] Finally, while all equations depicted here involve the use of just a single metal, Me, in addition to the colloidal metal(s) M and/or Mr, it has been found that all of the reactions discussed herein can be carried out using a combination of two or more different metals in the place of the single metal, Me, along with one or more colloidal metal(s). It has been found, in fact, that in some cases the use of multiple metals results in a significant rate enhancement over a rather large period of time. In experiments #7 and #10, for example, both metallic iron and metallic aluminum are used. The steady state production of hydrogen that results from experiment #10, for example, is approximately 100 mL of hydrogen per minute with the total volume of the reaction vessel being just over 100 mL. In experiments #8 and #9, similar reactions are carried out with just a single metal, aluminum, and it is demonstrated that when the reaction rate decreases, the addition of the second metal, iron, results in an immediate rate increase to a rate similar to those reactions where the two metals were present throughout the reaction. Reactions in Neutral or Basic Media
[0081] When any metal, regardless of its normal reactivity, is used in its colloidal form, the reaction of the metal with water in neutral or basic solutions can take place at an accelerated rate. Equations 52-54 are general equations that can be made to occur for any metals in spite of their normal reactivity, where Mf represents any metal in colloidal form. Mf, for instance, can represent but is not limited to Ag, Cu, Sn, Zn, Pb, Mg, Fe, Al and Cd. In fact, it has been found that the reactions shown in equations 52-54 occur at a significant rate.
2Mf+2H2O->2MfOH+H2 (52)
Mf+2H2O->Mf(OH)2+H2 (53)
2Mf+6H2O->2Mf(OH)3+3H2 (54)
[0082] Even though equations 52-54 would represent largely endothermic processes for a great many metals, particularly those of traditional low reactivity (for example but not limited to silver, gold, copper, tin, lead, nickel, and zinc), the rates of the reactions depicted in equations 52-54 could in fact be very large due to the surface effects caused by the use of the colloidal metal. While reactions represented by equations 52-54 would take place at a highly accelerated rate, they would not result in a useful production of elemental hydrogen since the colloidal metal by definition is present in very low concentrations, and would therefore yield insignificant amounts of hydrogen upon reaction.
[0083] A useful preparation of hydrogen can result by the inclusion of a solid metal, Ms, more reactive than the colloidal metal, Mf, such as but not limited to elemental aluminum, iron, lead, nickel, tin, tungsten, or zinc. Thus any colloidal metal in its ionic form would be expected to react with the solid metal, Ms, as indicated in equation 55, where those metals below Ms on the electromotive or activity series of metals would react best.
Ms+Mf<+> ->Mf+Ms<+> (55)
[0084] The reaction illustrated by equation 55 would in fact take place quite readily due to the large effective surface area of the colloidal ion, Mf<+> , and also perhaps due to the greater reactivity of the solid metal Ms, compared to any metal of lower reactivity. In fact, for colloidal metals normally lower in reactivity than Ms, equation 55 would be an exothermic reaction. The resulting metal, Mf, would be theorized to be present in colloidal form and thus would undergo a facile reaction with water to produce elemental hydrogen and a base, either by equation 52, 53, or 54 depending upon the oxidation state of the resulting colloidal metal ion.
[0085] Although the reaction represented by equations 52, 53, or 54 would most likely be endothermic, it is believed that the exothermicity of the reaction shown in equation 55 compensates for this. Therefore, the combination of the two reactions yields a process that is thermally obtainable.
[0086] Consequently, elemental hydrogen is efficiently and easily produced by the combination of the reactions shown in equations 56 and 57.
4Ms+4Mf<+->4> Mf+4Ms<+> (56)
4Mf+4H2O->4Mf<+1> +2H2+4OH<-> (57)
[0087] As shown, the solid metal, Ms, reacts with the colloidal metal ion (equation 56) to produce a product theorized to be a colloidal metal. It is believed the colloidal metal will then react with water in equation 57 to produce elemental hydrogen and regenerate the colloidal metal ion. The colloidal metal ion will then react again by equation 56, followed again by equation 57, and so on in a chain reaction process to provide an efficient source of elemental hydrogen. In principle, any colloidal metal ion should undergo this process successfully. It is found that the reaction works most efficiently when the colloidal metal ion is lower in reactivity than the metal, Ms, on the electromotive series table. Equations 56 and 57 can be combined, and this would result in the net reaction that is illustrated by equation 58. Equation 58 has as its result the production of elemental hydrogen from the reaction of a metal, Ms, and water.
4Ms+4Mf<+->4> Mf+4Ms<+> (56)
+
4Mf+4H2O->4Mf<+1> +2H2+4OH<-> (57)
=
4Ms+4H2O->4Ms<+> +2H2+4OH<-> (58)
[0088] Equation 58 summarizes a process that can provide an efficient production of elemental hydrogen where an elemental metal, Ms, and water are consumed. It is believed, however, that the elemental metal can be regenerated as a result of a voltaic electrochemical process and a thermal process that follows. A colloidal metal, which can be the same or different from the one represented in equation 56 referred to as Mrs in equation 59, can undergo a voltaic oxidation-reduction reaction indicated by equations 59 and 60.
Cathode (reduction) 4Mrs<+> +4e<-> ->4Mrs (59)
Anode (oxidation) 4OH<-> ->2H2O+O2+4e<-> (60)
[0089] The colloidal metal, Mrs, can in principle be any metal but works most efficiently when the metal has a higher (more positive) reduction potential. Thus, the regeneration process takes place most efficiently when the colloidal metal is as low as possible on the electromotive series of metals. Consequently, any colloidal metal will be successful, but the reaction works best with colloidal silver ion, due to the high reduction potential of silver. When silver is employed as the colloidal metal ion, for example, the reactions portrayed in equations 59 and 60 take place readily. The voltaic reaction produces a positive voltage, as the indicated oxidation and reduction reactions occur. This positive voltage can be used to supply the energy required for other chemical processes. In fact, the voltage produced can even be used to supply an over-potential for reactions employing the conversions portrayed by equations 59 and 60 but taking place in another reaction vessel. Thus, this electrochemical process can be made to take place more quickly without the supply of an external source of energy. It is believed that the resulting colloidal metal, Mrs, may then react to regenerate the elemental metal, Ms (equation 61).
4Ms<+> +4Mrs->4Mrs<+> +4Ms (61)
[0090] The reaction illustrated by equation 61 will take place most efficiently when the colloidal metal, Mrs, is more reactive than the metal, Ms. That is, the reaction in equation 61 will proceed most readily when the colloidal metal, Mrs, is above the metal, Ms, on the electromotive series of metals. Combining equations 59-61 results in the chemical reaction represented by equation 62, which results in the regeneration of the elemental metal Ms, and the formation of elemental oxygen.
4Mrs<+> +4e<-> ->4Mrs (59)
+
4OH<-> ->2H2O+O2+4e<-> (60)
+
4Ms<+> +4Mrs->4Mrs<+> +4Ms (61)
=
4Ms<+> +4OH<-> ->2H2O+4Ms+O2 (62)
[0091] The reactions shown in equations 59 and 60 seem to occur best when the colloidal metal, Mrs, is as low as possible on the electromotive series of metals; however, the reaction depicted by equation 61 takes place most efficiently when the colloidal metal, Mrs, is as high as possible on the electromotive series of metals. The net reaction illustrated by equation 62 is merely the sum of equations 59, 60, and 61 and could be maximally facilitated by either colloidal metals of higher activity or by colloidal metals of lower activity. The relative importance of the reaction illustrated by equations 59 and 60 compared to the reaction shown in equation 61 would determine the characteristics of the colloidal metal that would best assist the net reaction in equation 62.
[0092] It has been found that the net reaction indicated in equation 62 proceeds at a maximal rate when the colloidal metal is of maximum activity, that is, when the colloidal metal is as high as possible on the electromotive series of metals. It has been found that the more reactive colloidal metal ions such as, but not limited to colloidal magnesium ion or colloidal aluminum ion produce the most facile processes for the reduction of cationic metals. In fact, it has been found that the overall reaction proceeds most efficiently when at least two colloidal metals are present, preferably where at least one of the colloidal metal ions is higher than the solid metal Me on the electromotive series, and at least one of the colloidal metal ions is lower than the solid metal Me on the electromotive series. In such case, it is believed that the less reactive colloidal metal performs the Mf functions described above, while the more reactive colloidal metal performs the Mrs functions.
[0093] Combining equations 58 and 62 results in a net process indicated in equation 34. Since equation 34 merely depicts the decomposition of water into elemental hydrogen and elemental oxygen, the complete process for the production of elemental hydrogen now has only water as an expendable substance.
4Ms+4 H2O->4Ms<+> +2H2+4OH<-> (58)
+
4Ms<+> +4OH<-> ->2H2O+4Ms+O2 (62)
=
2H2O->2H2+O2 (34)
[0094] The net result of this process is exactly that which would result from the electrolysis of water. Here, however, no electrical energy needs to be supplied. It is believed that the colloidal metallic ion catalysts, as well as the metal Me, are regenerated during the process, leaving only water as a consumable material. Controllable Reactions
[0095] While all of the processes described above can provide an efficient production of hydrogen gas at a wide range of pH levels, and most operate efficiently even at ambient temperatures, it is rather difficult to control the rate of hydrogen formation; that is, once the process has begun, it cannot conveniently be stopped and restarted as needed. An improvement that addresses this difficulty has been developed that uses two electrodes, an anode and a cathode, along with one or more colloidal metal catalysts. The best results have been found when the anode is a metal of low to intermediate reactivity and the cathode is generally inert, but highly conductive. It has been found, in fact, that even metallic-like materials such as tungsten carbide can be employed as the cathode. Additionally, significant rate enhancement has also been achieved using, as the cathode, nickel which has been melted with an acetylene torch with a carbonizing flame and then re-solidified. This process is believed to result in carbonized nickel.
[0096] While in theory any two metals of different reactivity can be employed along with any colloidal metal catalysts at any level of pH, the process will be illustrated in the form of reactions performed at ambient temperature, under basic conditions using the metal-like material tungsten carbide as the cathode, the metal zinc as the anode, and colloidal silver and colloidal magnesium. Similar results were obtained for reactions carried out in acidic media as described in experiments 19-21.
[0097] Zinc is known to undergo reaction under basic conditions with water according to the reaction represented by equation 19.
Zn+2H2O->H2+Zn(OH)2 (19)
Due to the rather modest reactivity of zinc in alkaline solution, the reaction requires the input of significant thermal energy in order to proceed at a reasonable rate. In fact, if this reaction is performed at room temperature, the observed reaction rate is virtually zero. In theory, the rate of this reaction could be enhanced by the inclusion of a colloidal metal catalyst. If colloidal silver in its ionic form, Agc<+> , is introduced, the colloidal silver ion will react efficiently with the zinc, due to the large effective surface area of the colloidal silver ion, and also perhaps due to the enhanced reactivity of zinc compared to silver, a result of the fact that zinc is above silver in the electromotive series. Thus, the colloidal silver ion will undergo reaction with zinc at an impressive rate according to equation 63.
2Agc<+> +Zn->Zn<+2> +2Agc (63)
The reduced silver, Agc, would be theorized to be present in a colloidal form and would thus undergo a facile reaction with water to produce elemental hydrogen and a base, as illustrated in equation 64.
2Agc+2H2O->H2+2Agc<+> +2OH<-> (64)
[0098] Although the reaction represented by equation 64 would most likely be endothermic, it is believed that the exothermicity of the reaction shown in equation 63 compensates for this. Therefore, the combination of the two reactions yields a process that is thermally obtainable.
[0099] Consequently, elemental hydrogen is efficiently and easily produced by the combination of the reactions shown in equations 65 and 66.
2Zn+4Agc<+> ->4Agc+2Zn<+2 > (65)
4Agc+4H2O->4Agc<+> +2H2+4OH<-> (66)
[0100] As shown, the solid zinc metal reacts with the colloidal silver ion in equation 65 to produce a product theorized to be elemental colloidal silver. It is believed the elemental colloidal silver will then react with water in equation 66 to produce elemental hydrogen and regenerate the colloidal-silver ion. The colloidal-silver ion will then react again by equation 65, followed again by equation 66, and so on in a chain reaction process to provide an efficient source of elemental hydrogen. Equations 65 and 66 can be combined, and this would result in the net reaction that is illustrated by equation 67. Equation 67 has as its result the production of elemental hydrogen from the reaction of zinc and water.
2Zn+4Agc<+> ->4Agc+2Zn<+2 > (65)
4Agc+4H2O->4Agc<+> +2H2+4OH<-> (66)
=
2Zn+4H2O->2Zn<+2> +2H2+4OH<-> (67)
[0101] Equation 67 summarizes a process that can provide an efficient production of elemental hydrogen where elemental zinc and water are consumed. It is believed, however, that the elemental zinc can be regenerated as a result of a voltaic electrochemical process and a thermal process that follows. Thus, colloidal magnesium ion Mgc<+2 > can undergo a voltaic oxidation-reduction reaction indicated by equations 68 and 60.
Cathode (reduction) 2Mgc<+> +4e<-> ->2Mgc (68)
Anode (oxidation) 4OH<-> ->2H2O+O2+4e<-> (60)
[0102] It is believed that the resulting colloidal metal, Mgc, may then react to regenerate the elemental zinc (equation 69).
2Zn<+2> +2Mgc->2Mgc<+2> +2Zn (69)
[0103] The reaction illustrated by equation 69 will take place quite efficiently due to the fact that magnesium is above zinc on the electromotive series of metals. Combining equations 68, 60, and 69 results in the reaction illustrated in equation 70, which represents the regeneration of the elemental zinc, and the formation of elemental oxygen.
2Mgc<+2> +4e<-> ->2Mgc (68)
+
4OH<-> ->2H2O+O2+4e<-> (60)
+
2Zn<+2> +2Mgc->2Mgc<+2> +2Zn (69)
=
2Zn<+2> +4OH<-> ->2H2O+2Zn+O2 (70)
[0104] Combining equations 67 and 70 results in a net process indicated in equation 34. Since equation 34 merely depicts the decomposition of water into elemental hydrogen and elemental oxygen, the complete process for the production of elemental hydrogen now has only water as an expendable substance.
2Zn+4H2O->2Zn<+2> +2H2+4OH<-> (67)
+
2Zn<+2> +4OH<-> ->2H2O+2Zn+O2 (70)
=
2H2O->2H2+O2 (34)
[0105] The net result of this process is exactly that which would result from the electrolysis of water. Here, however, no electrical energy needs to be supplied. It is believed that the colloidal metallic ion catalysts as well as the elemental zinc are regenerated during the process; since the base is not consumed, water is the only material consumed.
[0106] While the net process illustrated by equation 67 is catalyzed by colloidal silver ion in an alkaline solution, the reaction rate is still found to be extremely slow at ambient temperatures presumably due to the low reactivity of zinc in the absence of additional thermal energy. The reaction rate can be significantly enhanced by the introduction of a second material that is inert but highly conductive, such as, but not limited to, tungsten carbide, which will be employed for this discussion. For this rate enhancement to be observable, the tungsten carbide must be conductively connected to the metallic zinc. The required connection can be achieved by having the two materials directly in contact, or they can be connected by a conductive medium, preferably made of a material low in reactivity such as copper. Under these conditions, the reaction represented by equation 65 is followed by an electrochemical voltaic process transpiring as illustrated in equations 71 and 60. The oxidation reaction represented by equation 71 takes place at the surface of the zinc electrode and the reduction reaction represented by equation 60 occurs at the surface of the tungsten carbide electrode.
2Zn+4Agc<+> ->4Agc+2Zn<+2 > (65)
Oxidation-4Agc->4Agc<+> +4e<-> (71)
Reduction-4H2O+4e<-> ->2H2+4OH<-> (60)
[0107] When equations 71 and 60 are combined, the result is a voltaic oxidation-reduction reaction that is represented by equation 66.
Oxidation-4Agc->4Agc<+> +4e<-> (71)
+
Reduction-4H2O+4e<-> ->2H2+4OH<-> (60)
=
4Agc+4H2O-4Agc<+> +2H2+4OH<-> (66)
[0108] Thus, the net reaction illustrated by equation 66 has two significant applications that can be employed individually or simultaneously. Equation 66 results in the generation of elemental hydrogen; however equation 66 also produces a measurable electrical potential that will produce a potentially useful electrical current. Therefore the chemical system described here can provide a voltaic cell that produces energy. Concurrently, there is the production of hydrogen gas which can be used to provide additional energy when employed in a hydrogen cell or engine.
[0109] The favorable potential produced by equation 66 allows the entire process to proceed without the requirement of an outside energy source. It is the favorable energetics of equation 66 that provide the driving force for the entire process. If the connection between the zinc electrode and the tungsten carbide electrode is broken, however, the reaction of equation 66 will not occur, resulting in a decrease or a virtual cessation in the rate of production of hydrogen. Thus one can generate hydrogen gas in a completely controllable manner simply by completing and disconnecting the circuit created by connecting the tungsten carbide and zinc electrodes.
[0110] Combining equations 65, 71 and 60 again yields a net reaction that is illustrated by equation 67 as shown below.
2Zn+4Agc<+> ->4Agc+2Zn<+2 > (65)
+
4Agc->4Agc<+> +4e<-> (71)
+
4H2O+4e<-> ->2H2+4OH<-> (60)
=
2Zn+4H2O->2Zn<+2> +2H2+4OH<-> (67)
With the inclusion of the tungsten carbide electrode however, the net reaction shown by equation 67 will now progress at a significantly enhanced rate. It has been found that the generation of elemental hydrogen takes place at a considerable rate even at usual ambient temperatures.
Cathode Surface Area
[0111] Since the rate of hydrogen production is at least partially dependent upon the surface area of the cathode, the reaction rate can be further enhanced using any means that increases the surface area of the cathode. In fact, it has been shown that if the cathode is present as a thin foil or as a mesh in order to increase its surface area, there is an increase in the rate of hydrogen formation. Alternatively, it has been shown that the use of multiple cathodes, each in electrical contact with the metallic zinc anode, produces an increase in the rate of hydrogen production presumably resulting from the increase in the surface area of the cathode. The combination of these two effects results in an large surface area of the cathode, and a corresponding increase in the rate of hydrogen produced. Regeneration of Metal
[0112] Although elemental zinc is consumed (equation 67), it is believed the zinc can be regenerated as a result of a voltaic electrochemical process and a subsequent thermal process similar to that shown for the regeneration of elemental metal, Ms, in equation 61. Thus, colloidal magnesium ion, Mgc<+2> , can take part in a voltaic oxidation-reduction reaction indicated by equations 68 and 60.
Cathode (reduction)-2Mgc<+2> +4e<-> ->2Mgc (68)
Anode (oxidation)-4OH<-> ->2H2O+O2+4e<-> (60)
[0113] The resulting colloidal magnesium, Mgc, will then react to reproduce elemental zinc (equation 69).
2Mgc+2Zn<+2> ->2Mgc<+2> +2Zn (69)
Combining equations 68, 60, and 69 yields a reaction illustrated by equation 70 which represents the regeneration of the elemental zinc, and the formation of elemental oxygen.
2Mgc<+2> +4e<-> ->2Mgc (68)
+
4OH<-> ->2H2O+O2+4e<-> (60)
+
2Mgc+2Zn<+2> ->2Mgc<+2> +2Zn (69)
=
2Zn<+2> +4OH<-> ->2H2O+2Zn+O2 (70)
[0114] Combining equations 67 and 70 results in equation 34. Since equation 34 merely depicts the decomposition of water into elemental hydrogen and elemental oxygen, the complete process for the production of elemental hydrogen now has only water as an expendable substance.
2Zn+4H2O->2Zn<+2> +2H2+4OH<-> (67)
+
2Zn<+2> +4OH<-> ->2H2O+2Zn+O2 (70)
=
2H2O->2H2+O2 (34)
[0115] The net result of this process is exactly that which would result from the electrolysis of water, but no electrical energy needs to be supplied. It is believed that the colloidal metallic ion catalysts as well as the zinc metal are regenerated during the process, leaving water as the only consumable material. Since the net process shown by reaction 34 is dependent upon the electrical connection of the electrodes, the production of elemental hydrogen can be interrupted and resumed simply by breaking and reforming the electrical contact through a switch that connects and disconnects the two electrodes through a conductive inert wire.
[0116] Thus in the process depicted by the net equation 67 elemental hydrogen is produced along with the concurrent oxidation of elemental zinc to zinc ion. In the process portrayed by the net equation 70, the zinc ion is reduced to elemental zinc with the concurrent formation of elemental oxygen. As stated above, the theoretical net result of equations 67 and 70 is equation 34. It has been found, however, that the net reaction represented by equation 70 does not occur at a rate competitive with the net reaction depicted in equation 67. Thus under normal circumstances, the production of hydrogen is believed to take place at a rate significantly greater than the production of oxygen. In addition, the zinc metal will undergo oxidation more quickly than the zinc ion undergoes reduction so therefore the zinc electrode will eventually become depleted. It is clear, then, that the rate of the process will eventually slow to a point where the production of hydrogen will no longer proceed at a useful rate.
[0117] It has been found however that the reduction of zinc ion to yield elemental zinc can be achieved through an electrolytic process. Thus, a potential can be applied in the direction opposite to the normal flow of electrons to produce a different oxidation-reduction process. As outlined in experiments 15 and 16, the application of an external electrical potential causes the oxidation reaction of equation 60 and the reduction reaction of equation 72 to occur.
Oxidation-4OH<-> ->2H2O+O2+4e<-> (60)
Reduction-2Zn<+2> +4e-2Zn (72)
The addition of equation 60 and equation 72 once again results in equation 70, where the elemental zinc is regenerated on the electrode with the simultaneous production of elemental oxygen.
4OH<-> ->2H2O+O2+4e<-> (60)
+
2Zn<+2> +4e<-> ->2Zn (72)
=
2Zn<+2> +4OH<-> ->2H2O+2Zn+O2 (70)
From the standard oxidation and reduction potentials shown below, it is clear that the reactions represented by equations 60 and 72 will not take place spontaneously, having a standard cell potential of -1.136 volts.
4OH<-> ->2H2O+O2+4e<-> [epsilon]<0> oxidation=-0.401 V
2Zn<+2> +4e<-> ->2Zn[epsilon]<0> reduction=-0.762 V
The application of an external electrical potential will however cause this process to easily occur. Thus, when the production of hydrogen slows to an unacceptable rate, the process may be reversed by electrolysis, and the resulting rate of hydrogen formation will increase to that observed at the beginning of the process. Alternatively, the anode may simply be replaced.
[0118] In the preceding discussion, the colloidal metal ion catalysts, Mf and Mr, are supplied along with the reactants as described in experiments 11 through 16. However, it has been found that the process can still proceed even without supplying colloidal metal catalysts as described in experiment 17. Although the reaction rate is decreased by a factor of approximately one-half, the production of elemental hydrogen is visibly obvious, and the voltaic potential produced is about the same as in the catalyzed reaction. The fact that the reaction can proceed with the apparent lack of catalysis is explained by the fact that metallic zinc and many other metals react very slowly with water in neutral or basic solutions to produce cations, such as the Zn<+2 > ion, in very low concentration, as illustrated in equation 73.
Zn+2H2O->H2+Zn<+2> +2OH<-> (73)
[0119] The cations produced in equation 73 will take part in the reaction in the same manner as the colloidal ions; however, they would catalyze the process with a limited efficiency compared to the colloidal catalysts. Thus when the catalysts are not physically added to the reaction mixture, it is still the catalyzed process discussed previously that occurs. Controllable Reactions at an Enhanced Rate in Acidic Media
[0120] The rate of hydrogen production can also be increased by using as the anode a metal of higher reactivity, such as aluminum, and as the cathode a material that is inert but highly conductive, such as tungsten carbide, in a highly acidic solution that contains one or more dissolved acids, such as, but not limited to, sulfuric acid or hydrochloric acid. Additionally, there are preferably one or more salts or metal oxides (where, in acidic media, a metal oxide is the precursor to a salt) dissolved in the acidic solution, where each salt or metal oxide contains a cation of intermediate reactivity. For example, the salts or metal oxides may be, but are not limited to, zinc sulfide, zinc chloride, cobalt(II) sulfate, cobalt(II) chloride, zinc oxide, or cobalt(II) oxide. The solution preferably also contains one or more colloidal-metal ions.
[0121] While there are numerous ways in which this process may be performed, for the purposes of illustration, the process will be described where the reaction medium is a solution of sulfuric acid that contains colloidal silver ion, colloidal magnesium ion and zinc sulfate. Aluminum will be discussed as the metal of high reactivity, and tungsten carbide will be employed as the highly conductive, inert material.
[0122] At low values of pH, aluminum is known to produce hydrogen at a significant rate by reaction with sulfuric acid as illustrated by equation 74.
4Al+12H<+> +6SO4<-2> ->4Al<+3> +6H2+6SO4<-2 > (74)
[0123] The rate of this reaction is in fact so impressive that the reaction of aluminum and sulfuric acid is often described as being uncontrollable. The rate of this reaction can be even further enhanced by the inclusion of colloidal silver ion, Agc<+> , which is believed to catalyze the reaction. Thus, aluminum will react with the colloidal silver ion in a reaction represented by equation 75. The metallic silver, Agc, that results is presumed to be in a colloidal state and is expected to react with sulfuric acid to produce elemental hydrogen by the reaction described by equation 76. Due to the colloidal nature of the silver, this reaction occurs at an even greater rate than the reaction of aluminum and sulfuric acid represented by equation 74.
4Al+12Agc<+> ->4Al<+3> +12Agc (75)
12Agc+12H<+> +6SO4<-2> ->12Agc<+> +6H2+6SO4<-2 > (76)
[0124] Combining equations 75 and 76 results in the net equation 74. However, the rate of hydrogen production will be enhanced by the presence of the colloidal silver.
4Al+12Agc<+> ->4Al+12Agc (75)
+
12Agc+12H<+> +6SO4<-2> ->12Agc<+> +6H2+6SO4<-2 > (76)
=
4Al+12H<+> +6SO4<-2> ->4Al<+3> +6H2+6SO4<-2 > (74)
[0125] Equation 74 summarizes a process that can provide an extremely efficient production of elemental hydrogen where elemental aluminum and sulfuric acid are consumed. It is believed, however, that the elemental aluminum and the sulfuric acid can both be regenerated as a result of a voltaic electrochemical process and a thermal process described below:
[0126] Colloidal magnesium ion Mgc<+2 > can undergo a voltaic oxidation-reduction reaction indicated by equations 77 and 78.
Cathode (reduction) 6Mgc<+2> +12e<-> ->6Mgc (77)
Anode (oxidation) 6H2O->12H<+> +3O2+12e<-> (78)
[0127] It is believed that the resulting colloidal metal, Mgc, may then react to regenerate the elemental aluminum (equation 79).
4Al<+3> +6SO4-<2> +6Mgc->6Mgc<+2> +4Al+6SO4-<2 > (79)
[0128] The reaction illustrated by equation 79 will take place quite efficiently due to the fact that magnesium is above aluminum on the electromotive series of metals. Combining equations 77, 78 and 79 results in the reaction illustrated in equation 80, which represents the regeneration of the elemental aluminum, the regeneration of the sulfuric acid, and the formation of elemental oxygen.
6Mgc<+2> +12e<-> ->6Mgc (77)
+
6H2O->12H<+> +3O2+12e<-> (78)
+
4Al<+3> +6SO4-<2> +6Mgc->6Mgc<+2> +4Al+6SO4-<2 > (79)
=
4Al<[deg.]3> +6SO4-<2> +6H2O->12H<+> +6SO4-<2> +4Al+3O2 (80)
[0129] Combining equations 74 and 80 results in a net process indicated in equation 81. Since equation 81 merely depicts the decomposition of water into elemental hydrogen and elemental oxygen, the complete process for the production of elemental hydrogen now has only water as an expendable starting material.
4Al+12H<+> +6SO4<-2> ->4Al<+3> +6H2+6SO4<-2 > (74)
+
4Al<+3> +6SO4-<2> +6H2O->12H<+> +6SO4-<2> +4Al+3O2 (80)
=
6H2O->6H2+3O2 (81)
[0130] The net result of this process is exactly that which would result from the electrolysis of water. Here, however, no electrical energy needs to be supplied. It is believed that the colloidal metallic ion catalysts as well as the elemental aluminum are regenerated during the process; since the acid is not consumed, water is the only material consumed.
[0131] It has been found that the reaction illustrated by equation 74, whether catalyzed or uncatalyzed, can be inhibited by the dissolving of zinc sulfate into the sulfuric acid solution. In the absence of the colloidal silver catalyst, the elemental aluminum is thought to react with the zinc cation, thus replacing the reaction illustrated by equation 74 with the reaction depicted by equation 82. While the reaction of aluminum and zinc cation occurs preferentially to the reaction of aluminum and sulfuric acid, it has been found that the reaction proceeds at a rather low rate and, therefore, the aluminum is not appreciably consumed.
4Al+6Zn<+2> ->4Al<+3> +6Zn (82)
[0132] With the inclusion of the colloidal silver cation, the reaction illustrated in equation 76 is replaced by the reaction shown in equation 83. Once again, while the reaction of colloidal silver and zinc cation occurs preferentially to the reaction of colloidal silver and sulfuric acid, it has been found that the reaction proceeds at a rather low rate. Thus, combining equations 75 and 84 results in the net equation 85. The reaction illustrated in equation 85 results in the consumption of aluminum; however, it is found to proceed at a rather low rate, and, thus, will not result in a large consumption of aluminum. The reaction shown in equation 85 will still, however, take place preferentially when in competition with the net reaction that is depicted in equation 74.
4Al+12Agc<+> ->4Al<[deg.]3> +12Agc (75)
+
12Agc+6Zn<+2> +6SO4-<2> ->12Agc<+> +6SO4-<2> +6Zn (84)
=
4Al+6Zn<+2> ->4Al<+3> +6Zn(net) (85)
[0133] Thus, the effect of the introduction of zinc chloride would be to severely limit or completely terminate the production of hydrogen from the net oxidation of aluminum. It has been found, however, that the rate of hydrogen formation can be increased to the point where it competes successfully with the net reaction depicted in equation 85. Specifically, the reaction rate for hydrogen production can be significantly enhanced by the introduction of a second material that is inert but highly conductive, such as, but not limited to, tungsten carbide, which will be employed for this discussion. Alternatively, in place of tungsten carbide, significant rate enhancement has also been achieved using nickel which has been melted with an acetylene torch with a carbonizing flame and then re-solidified. For this rate enhancement to be observable, the tungsten carbide must be conductively connected to the metallic aluminum. The required connection can be achieved by having the two materials directly in contact, or they can be attached by a conductive medium, preferably made of a material low in reactivity such as copper. Under these conditions, the reaction represented by equation 75 is followed by an electrochemical voltaic process transpiring as illustrated in equations 86 and 87. The oxidation reaction represented by equation 86 is believed to take place at the surface of the aluminum electrode and the reduction reaction represented by equation 87 is believed to occur at the surface of the tungsten carbide electrode.
4Al+12Agc<+> ->4Al<+3> +12Agc (75)
Oxidation-12Agc->12Agc<+> +12e<-> (86)
Reduction-12H<+> +12e<-> ->6H2 (87)
[0134] When equations 86 and 87 are combined, the result is a voltaic oxidation-reduction reaction that is represented by equation 88.
12Agc->12Agc<+> +12e<-> (86)
+
12H<+> +12e->6H2 (87)
=
12Agc+12H<+> ->12Agc<+1> +6H2 (88)
[0135] Thus, the net reaction illustrated by equation 88 has two significant applications that can be employed individually or simultaneously. Equation 88 results in the generation of elemental hydrogen. Additionally, equation 88 produces a measurable electrical potential that could produce a potentially useful electrical current. Therefore, the chemical system described here can provide a voltaic cell that produces useful electrical energy. Concurrently, there is the production of hydrogen gas, which can be used to provide additional energy when employed in a hydrogen cell or an engine. The favorable potential produced by equation 88 is believed to allow the entire process to proceed without the requirement of an outside energy source. It is the favorable energetics of equation 88 that is believed provides the driving force for the entire process. If the connection between the aluminum electrode and the tungsten carbide electrode is broken, however, the reaction of equation 88 will not occur, resulting in a decrease or a virtual cessation in the rate of production of hydrogen. Thus, one can generate hydrogen gas in a controllable manner simply by completing and disconnecting the circuit created by connecting the tungsten carbide and aluminum electrodes.
[0136] Combining equations 75, 86 and 87 again yields a net reaction that is illustrated by equation 89 as shown below.
4Al+12Agc<+> ->4Al<+3> +12Agc (75)
+
12Agc->12Agc<+> +12e<-> (86)
+
12H<+> +12e<-> ->6H2 (87)
=
4Al+12H<+> ->4Al<+3> +6H2 (89)
The reaction that is represented by equation 89 occurs at an impressive reaction rate due to the high reactivity of aluminum. With the inclusion of the tungsten carbide electrode, however, the net reaction shown by equation 89 will now progress at an even faster rate. It is believed that this is due at least in part to the increased surface area of the tungsten carbide compared to that of the colloidal elemental silver. It has been found that the generation of elemental hydrogen takes place at a considerable rate even at usual ambient temperatures.
[0137] Since the rate of hydrogen production is believed to be at least partially dependent upon the surface area of the tungsten carbide cathode, the reaction rate can be further enhanced using any means that increases the surface area of the cathode. In fact, it has been shown that if the cathode is present as a thin foil or as a mesh in order to increase its surface area, there is an increase in the rate of hydrogen formation. Alternatively, it has been shown that the use of multiple cathodes, each in electrical contact with the metallic aluminum anode, produces an increase in the rate of hydrogen production, presumably resulting from the increase in the surface area of the cathode. The combination of these two effects, that is, the use of multiple cathodes consisting of a tungsten carbide mesh or foil, results in a large surface area of the cathode and a corresponding increase in the rate of hydrogen produced.
[0138] Employing the chemistry described above, a controllable production of hydrogen at an extremely high rate can be achieved.
[0139] FIG. 1 shows a mixture and apparatus that may be used for the production of hydrogen. A reaction vessel 100 contains a reaction medium 102. The reaction medium preferably comprises water and, most preferably, further comprises either a base or an acid, although the reaction can exist at virtually any level of pH. Alternatively, it is believed that other reaction media may be used, including other solvents, or non-liquid media, such as gelatinous or gaseous media. In basic media, the base is preferably sodium hydroxide with a concentration of about 2.5 Molar, although other bases and other concentrations may be used. In acidic media, the acid is preferably sulfuric acid or hydrochloric acid with a pH of about 1.0, although other acids and other concentrations may be used. The reaction vessel 100 is preferably inert to the reaction medium 102.
[0140] The reaction medium 102 preferably contains a first colloidal metal (not shown) suspended in the solution. Although some of the reactions described above may proceed without a colloidal metal in the reaction medium 102, the colloidal metal significantly enhances the reaction rate. The first colloidal metal is preferably a metal with low activity, such as silver, gold, platinum, tin, lead, copper, zinc, or cadmium, although other metals may be used. Alternatively, as discussed above, other high-surface-area catalysts may be used in place of the colloidal metal.
[0141] Preferably, there is at least one cathode 104 in contact with the reaction medium 102. The cathode 104 may be in any form, but is preferably in the form of a solid with a relatively large surface area. Most preferably, the cathode 104 comprises a plurality of surface-area-enhancing features 105, which increase the surface area of the cathode. The surface-area-enhancing features 105 are preferably arranged to allow the reaction medium 102 or its constituents to move between them and to allow bubbles of produced gas to easily escape from the surface of the cathode 104. The surface-area-enhancing features 105 are preferably vertically-oriented rods projecting upwardly from a base of the cathode 104. However, the surface-area-enhancing features may be any feature, in electrical contact with the cathode 104, which effectively increases the surface area of the cathode 104. Alternatively, the cathode 104 may be in another relatively high-surface-area form, such as a coil, film, wool, nanomaterial, nanocoating, or the like. Further alternatively, a plurality of cathodes 104 may be used which combine to provide a larger surface area. The total surface area of the cathode(s) 104 is preferably greater than the surface area of the anode.
[0142] The cathode 104 preferably comprises a material that is highly conductive but virtually inert to the reaction medium 102, such as nickel, carbonized nickel, tungsten, or tungsten carbide. The cathode 104 most preferably comprises tungsten carbide.
[0143] The reaction vessel 100 also preferably comprises an anode 106 in contact with the reaction medium. The anode 106 preferably comprises a metal of high-range activity, and thus of a higher activity than the cathode. Most preferably, the anode 106 comprises aluminum, or a mixture of aluminum and other, less reactive, metals.
[0144] Preferably, the reaction medium 102 also contains a second colloidal metal (not shown). The second colloidal metal preferably has a higher activity than the metals comprising the cathode 104 and the anode 106, such as aluminum, magnesium, beryllium, and lithium. Alternatively, as discussed above, other high-surface-area catalysts may be used in place of the second colloidal metal.
[0145] Preferably, the reaction medium 102 also contains an ionic salt (not shown) comprising a metal cation that is less reactive than the metal composing the anode 106, and an anion that is largely inert to other constituents in the reaction medium, such as, but not limited to, zinc sulfate, zinc chloride, cobalt(II) sulfate, and cobalt(II) chloride.
[0146] The cathode 104 and the anode 106 are preferably conductively connected through conductive paths 107 and 109, respectively, to a controller 108 which may be manipulated to allow or restrict the flow of electricity between the cathode 104 and the anode 106. The controller 108 may be a switch, a variable resistor, or other device for allowing or resisting electric currents. When electrical current flows freely between the cathode 104 and the anode 106, it is found that the production of hydrogen will be maximized. When the conductive contact between the cathode 104 and the anode 106 is broken, hydrogen production will be minimal or zero. It is believed that a variable resistor between the anode 106 and the cathode 104 would allow a user to select from a wide range of hydrogen production rates.
[0147] The electrical energy produced by the reaction, which flows from the anode 104 to the cathode 106 through the conductive paths 107 and 109 may be used to provide electrical energy for a similar reaction occurring in a similar apparatus, or the system may be used as a battery, and the electrical energy created by the reaction can be used for other purposes. Alternatively, the cathode 104 and anode 105 may be placed in direct contact with one another.
[0148] The reaction vessel 100 preferably comprises an outlet 110 to allow hydrogen gas (not shown) and/or other products to escape. The reaction vessel may also have an inlet 112 for adding water or other constituents to maintain desired concentrations.
[0149] In addition, an electrical power source 114 may be used to intermittedly provide an electrical current through the reaction medium 102. The electrical power source 114 may be a battery, power outlet, generator, transformer, or the like. The electrical power source 114 preferably provides DC electrical power at a potential of at least 12 volts. A first terminal 115 of the electrical power source 114 is electrically connected through conductive paths 116 and 109 to the anode 106. A second terminal 117 of the electrical power source 114 is electrically connected through conductive paths 118 and 107 to the cathode 104. Preferably, the first terminal 115 has a higher electrical potential than the second terminal 117 so that when the controller 108 is configured in an open position (restricting current flow between the anode 106 and cathode 108), the electrical potential source 114 will cause a flow of electrical current in the opposite direction from when the controller 108 is closed and no external potential is applied. Power is applied from the electrical power soure 114 as needed to regenerate the anode and increase the hydrogen production rate. For most of the reaction duration, however, current is not applied. Alternatively, the anode 106 may be replaced by a new anode 106.
[0150] In addition, the apparatus preferably comprises an energy source 122. Although most of the reactions described above are believed to proceed without any energy input, hydrogen will be produced at a greater rate when additional energy is added. The energy source 122 shown in FIG. 1 is an electric heating coil, however, any form of thermal energy may be used including solar heating, combustion heating, hot plates, or the like. Generally, any energy source capable of heating the reaction medium above ambient temperatures may be used, and the particular source will preferably be chosen based on cost considerations. Additionally, it is believed that other energy types may be used, including, without limitation, electric energy, nuclear energy or electromagnetic radiation.
[0151] The hydrogen gas produced may be used in many known ways. Particularly, without limitation, the produced gas may be fed to a fuel cell to produce electric energy. Thus, the hydrogen production apparatus shown in FIG. 1 may be combined with a fuel cell (not shown) to form a compact and efficient source of electrical energy, which could be used to power a wide variety of devices. Experimental Results: Experiment #1 Summary:
[0152] An initial solution comprising 10 mL of 93% concentration H2SO4 and 30 mL of 35% concentration HCl was reacted with iron pellets (sponge iron) and about 50 mL of colloidal magnesium and 80 mL of colloidal lead, each at a concentration believed to be about 20 ppm. A theoretical maximum of 8.06 liters of hydrogen gas could be produced if solely from the consumption of the acids as indicated in Table 1.
TABLE 1
Starting Solution Maximum H2 Yield with Acid Consumption
Total Effective Maximum H2
Acid mL Concentration Grams Grams of Acid Yield
H2SO4 10 93.0% 18.97 17.64 4.03 liters
HCl 30 35.0% 37.52 13.13 4.03 liters
Maximum H2 Yield: 8.06 liters
[0153] 1 mole H2SO4 yields 1 mole H2 (22.4 liters)
[0154] 1 mole H2SO4=98 grams
[0155] Therefore, the maximum yield is 0.23 liters of H2 per gram of H2SO4.
[0156] 2 moles of HCl yields 1 mole H2 (22.4 liters)
[0157] 2 moles of HCl=73 grams
[0158] Therefore, a theoretical maximum yield of 0.31 liters of H2 per gram of HCl is expected without the regeneration reaction.
[0159] At least 15 liters of gas was observed to have been produced, and the reaction was still proceeding in a continuous fashion (about 2 bubbles of gas per second at 71[deg.] C.) when interrupted. It should be noted that the 15 liters of gas observed does not account likely losses of hydrogen gas due to leakage. Based upon previous observations and theoretical projections, the first 8.06 liters of gas produced is likely to be made up of essentially pure hydrogen; beyond the theoretical threshold of 8.06 liters, 66.7% by volume of the gas produced would be hydrogen and the other 33.3% by volume would be oxygen. It is believed this experiment provides ample evidence of the regeneration process.
[0160] A follow-up experiment was conducted using iron (III) chloride (FeCl3) as the only source of iron in an attempt to verify the reverse reaction. Pure iron (III) chloride was chosen because it could be shown to be free of iron in any other oxidation state. While similar experiments had been successfully carried out using iron (III) oxide as the source of iron, the results were clouded by the fact that other oxidation states of iron may have been present. The results are described in Experiment #2, below. Experiment #2 Summary:
[0161] An experiment was conducted using 150 mL of iron (III) chloride in an aqueous solution (commonly used as an etching solution, purchased from Radio Shack) as the starting materials. Ten mL of 93% concentration sulfuric acid (H2SO4) was added to the solution, at which point no reaction occurred. About 50 mL of colloidal magnesium and 80 mL of colloidal lead, each at a concentration believed to be about 20 ppm, were then added, at which point a chemical reaction began and the bubbling of gases was evident at ambient temperature. The production of gas accelerated when the solution was heated to a temperature of 65[deg.] C. The product gas was captured in soap bubbles and the bubbles were then ignited. The observed ignition of the gaseous product was typical for a mixture of hydrogen and oxygen.
[0162] Since hydrogen gas could only be produced with a concurrent oxidation of iron, it is evident that the iron (III) had to be initially reduced before it could be oxidized, thereby providing strong evidence of the reverse reaction. This experiment has subsequently been repeated with hydrochloric acid (HCl) instead of sulfuric acid, with similar results.
[0163] Two additional follow-up experiments (#3 using aluminum metal and #4 using iron metal) were conducted to determine if more hydrogen is produced compared to the maximum amount expected solely from the consumption of the metal. These results are described below. Experiment #3 Summary:
[0164] The starting solution had a total volume of 250 mL, including water, about 50 mL of colloidal magnesium and 80 mL of colloidal lead, each at a concentration believed to be about 20 ppm, 10 mL of 93% concentration H2SO4, and 30 mL of 35% concentration HCl as in experiment #1 above. Ten grams of aluminum metal was added to the solution, which was heated and maintained at 90[deg.] C. The reaction ran for 1.5 hours and yielded 12 liters of gas. The pH was found to have a value under 2.0 at the end of 1.5 hours. The reaction was stopped after 1.5 hours by removing the unused metal and weighing it. The non-consumed aluminum weighed 4.5 grams, indicating a consumption of 5.5 grams of aluminum. The maximum amount of hydrogen gas normally expected by the net consumption of 5.5 grams of aluminum is 6.8 liters, as indicated in the table below.
TABLE 2
Starting Solution Maximum H2 Yield
With Aluminum Consumption
Total Grams Total Grams Grams Maximum
Metal Initial Supply Final Consumed Yield* of H2
Aluminum 10 4.5 5.5 6.84 liters
(Al)
*If reacted aluminum has exclusively been used for the production of hydrogen:
2 moles Al yields 3 moles H2 (67.2 liters)
2 moles Al = 54 grams
[0165] Therefore, a theoretical maximum yield of 1.24 liters of H2 per gram of Al is expected without the regeneration reaction described above.
[0166] As in experiment #1, based on the total amount of acid supplied, it is expected that the first 8.06 liters of the gas generated is pure hydrogen with the balance being 50% hydrogen. Alternatively, the theoretical amount of hydrogen based on the amount of aluminum consumed is 6.84 liters. After 6.84 liters (the maximum yield expected from the aluminum consumed), it is expected that the remaining gas is 66.7% hydrogen. Therefore, it is estimated that about 10.3 liters of hydrogen (out of about 12 total liters of gas) was produced in this experiment, compared to the maximum of 6.84 or 8.06 liters expected, based on the amount of aluminum consumed and the amount of acid supplied, respectively, thereby providing additional evidence of the regeneration process. Experiment #4 Summary:
[0167] The starting solution included a total volume of 250 mL, including water, about 50 mL of colloidal magnesium and 80 mL of colloidal lead, each at a concentration believed to be about 20 ppm, 10 mL of 93% concentration H2SO4 and 30 mL of 35% concentration HCl, as in experiment #1 above. One hundred grams of iron pellets (sponge iron) was added to the solution, which was heated and maintained at 90[deg.] C. The reaction ran for 30 hours and yielded 15 liters of gas. The pH was found to have a value of about 5.0 at the end of 30 hours. The reaction was stopped after 30 hours by removing the unused metal and weighing it. The non-consumed iron weighed 94 grams, indicating a consumption of 6 grams of iron. The maximum amount of hydrogen gas normally expected by the net consumption of 6 grams of iron, without the regeneration reaction described above, is 2.41 liters, as indicated in the table below.
TABLE 3
Starting Solution Maximum H2 Yield With Iron Consumption
Total Grams Total Grams Grams Maximum
Metal Initial Supply Final Consumed Yield* of H2
Iron (Fe) 100 94 6 2.41 liters
*If reacted iron has exclusively been used for the production of hydrogen:
1 mole Fe yields 1 mole H2 (22.4 liters)
1 mole Fe = 55.85 grams
[0168] Therefore, a theoretical maximum yield of 0.40 liters of H2 per gram of Fe is expected without the regeneration reaction described above.
[0169] As in experiment #1, based on the total amount of acid supplied, it is expected that the first 8.06 liters of the gas generated is pure hydrogen with the balance being 66.7% hydrogen. However, the maximum theoretical generation of hydrogen based on the amount of iron consumed is 2.41 liters. After 2.41 liters (the maximum yield expected from the iron consumed), it is expected that the remaining gas is 66.7% hydrogen. Therefore, it is estimated that about 10.8 liters of hydrogen (out of about 15 total liters of gas) was produced in this experiment using colloidal catalyst, well over the maximum of 2.41 liters expected with the amount of iron consumed, thereby providing additional evidence of the regeneration process. Experiment #5 Summary:
[0170] An experiment was conducted using 200 mL of the final solution obtained from experiment #4, which contained oxidized iron plus catalyst and was found to have a pH of about 5. Acid was added to the solution, as in the above reactions, i.e., 10 mL of 93% concentration H2SO4 and 30 mL of 35% concentration HCl that brought the pH to a level of about 1. No additional colloidal materials were added, but 20 grams of aluminum metal was added. The solution was maintained at a constant temperature of 96[deg.] C. The reaction proceeded to produce 32 liters of gas in a span of 18 hours, at which point the rate of the reaction had slowed significantly and the pH of the solution had become approximately 5.
[0171] The metal remaining at the end of the 18-hour experiment was separated and found to have a mass of 9 grams. This metal appeared to be a mixture of Al and Fe. Therefore, neglecting the amount of iron and aluminum remaining in solution, there was net consumption of 11 grams of metal and a net production of 32 liters of gas.
[0172] As indicated above, based on the amount of acid added to the reaction, the maximum amount of hydrogen gas expected solely from the reaction of acid with metal would be 8.06 liters. Depending on the makeup of the recovered metal, which had a mass of 9 grams, two extremes are possible: a) assuming the metal recovered was 100% Al, a maximum of 13.75 liters of hydrogen gas would be expected from the consumption of 11 grams of aluminum; and b) alternatively, assuming the metal recovered was 100% Fe, a maximum of 21.25 liters of hydrogen gas would be expected from the consumption of 17 grams of aluminum (20 grams supplied minus three grams used in the production of iron). For purposes of calculating maximum hydrogen gas generation, we assume the regeneration process does not occur and the Fe metal would have been generated from a conventional single displacement reaction with Al.
[0173] The actual percentage of Al and Fe would be somewhere between the two extremes and, therefore, the maximum amount of hydrogen gas generated solely from the consumption of metal (without regeneration) would be between 13.75 liters and 21.25 liters. The observed generation of 32 liters of gas compared to the maximum amount one would expect from the sole consumption of metal indicates that the regeneration process is taking place. It is believed that the increase in the rate of H2 production resulted from a high concentration of metal ions in the solution prior to the introduction of the elemental iron. Thus, solutions resulting from this family of reactions should not be discarded but rather should be used as the starting point for subsequent reactions. Consequently, this process for the generation of H2 will not produce significant chemical wastes that require disposal. Experiment #6 Summary:
[0174] An experiment was conducted using 20 mL FeCl3, 10 mL colloidal magnesium, and 20 mL colloidal lead at a temperature of about 90[deg.] C. A gas was produced that is believed to be a mixture of hydrogen and oxygen, based upon observing the ignition of the gas. The pH of the mixture decreased during the reaction from a value of about 4.5 to a value of about 3.5. These observations show that it is not necessary to introduce either metallic iron or acid into the solution to produce hydrogen. Since the electrochemical oxidation/reduction reactions (equations 30-32 resulting in the net equation 33) result in the production of metallic iron and acid, these two constituents can be produced in this manner. Presumably, this would eventually attain the same steady state that is reached when metallic iron and acid are supplied initially. Experiment #7 Summary
[0175] An initial solution comprising 10 mL of 93% concentration H2SO4 and 30 mL of 35% concentration HCl was reacted with 20 grams of iron pellets and 20 grams of aluminum pellets. There were then added 50 mL of colloidal magnesium and 80 mL of colloidal lead, each at a concentration believed to be about 20 ppm, producing a total volume of about 215 mL. A theoretical maximum of 8.06 liters of hydrogen gas could be produced if solely from the consumption of the acids as indicated in Table 4.
TABLE 4
Starting Solution Maximum H2 Yield with Acid Consumption
Total Effective Maximum H2
Acid mL Concentration Grams Grams of Acid Yield
H2SO4 10 93.0% 18.97 17.64 4.03 liters
HCl 30 35.0% 37.52 13.13 4.03 liters
Maximum H2 Yield: 8.06 liters
[0176] 1 mole H2SO4 yields 1 mole of H2 (22.4 liters @ STP)
[0177] 1 mole H2SO4=98 grams
[0178] Therefore, a theoretical maximum yield of 0.23 liters of H2 per gram of H2SO4 is expected without the regeneration reaction.
[0179] 2 moles of HCl yields 1 mole of H2 (22.4 liters @ STP)
[0180] 2 moles of HCl=73 grams
[0181] Therefore, a theoretical maximum yield of 0.31 liters of H2 per gram of HCl is expected without the regeneration reaction.
[0182] While some gas was lost due to leakage and diffusion, at least 25 liters of gas was collected over a period of three hours, and the reaction was still proceeding in a continuous fashion at a rate of 8.4 liters of gas produced per hour. At this point the reaction was stopped and the remaining metal, a mixture of aluminum and iron was collected and dried, and was found to have a mass of 35.5 grams. Thus, 4.5 grams of metal was consumed. Since the remaining metal was not analyzed, it is not known in what ratio aluminum and iron reacted; however the simple oxidation of a metal by an acid would produce a maximum of 5.6 liters of hydrogen, well below that observed. Based upon previous observations and theoretical projections, the first 8.06 liters of gas produced is likely to be made up of essentially pure hydrogen, and beyond the theoretical threshold of 8.06 liters, 66.7% by volume of the gas produced would be hydrogen and the other 33.3% by volume would be oxygen. It is believed this experiment provides ample evidence for the regeneration process.
[0183] It is believed that the simultaneous use of two metals does not improve the initial rate of gas formation, but rather produces a reaction whose rate is sustained over a much greater period of time. In order to demonstrate this point, two additional experiments were performed. Experiment #8 Summary:
[0184] An initial solution comprising 10 mL of 93% concentration H2SO4 and 30 mL of 35% concentration HCl was reacted with 20 grams of aluminum pellets. There were then added 50 mL of colloidal magnesium and 80 mL of colloidal lead each at a concentration believed to be about 20 ppm, producing a total volume of about 215 mL. A theoretical maximum of 8.06 liters of hydrogen gas could be produced if solely from the consumption of the acids as indicated in Table 5.
TABLE 5
Starting Solution Maximum H2 Yield with Acid Consumption
Total Effective Maximum H2
Acid mL Concentration Grams Grams of Acid Yield
H2SO4 10 93.0% 18.97 17.64 4.03 liters
HCl 30 35.0% 37.52 13.13 4.03 liters
Maximum H2 Yield: 8.06 liters
[0185] 1 mole H2SO4 yields 1 mole of H2 (22.4 liters @ STP)
[0186] 1 mole H2SO4=98 grams
[0187] Therefore, a theoretical maximum yield of 0.23 liters of H2 per gram of H2SO4 is expected without the regeneration reaction.
[0188] 2 moles of HCl yields 1 mole of H2 (22.4 liters @ STP)
[0189] 2 moles of HCl=73 grams
[0190] Therefore, a theoretical maximum yield of 0.31 liters of H2 per gram of HCl is expected without the regeneration reaction.
[0191] The initial reaction rate was similar to that found in experiment #1, where 9 liters of gas was produced in slightly less than one hour. At this point, however, the reaction rate was found to decrease by a factor of approximately one-half. The addition of 20 grams of iron caused an immediate increase in reaction rate to the value that was initially observed at the onset of the experiment. Experiment #9 Summary:
[0192] An initial solution comprising 10 mL of 93% concentration H2SO4 and 30 mL of 35% concentration HCl was reacted with 40 grams of aluminum pellets. There were then added 50 mL of colloidal magnesium and 80 mL of colloidal lead, each at a concentration believed to be about 20 ppm, producing a total volume of about 215 mL. A theoretical maximum of 8.06 liters of hydrogen gas could be produced if solely from the consumption of the acids as indicated in Table 6.
TABLE 6
Starting Solution Maximum H2 Yield with Acid Consumption
Total Effective Maximum H2
Acid mL Concentration Grams Grams of Acid Yield
H2SO4 10 93.0% 18.97 17.64 4.03 liters
HCl 30 35.0% 37.52 13.13 4.03 liters
Maximum H2 Yield: 8.06 liters
[0193] 1 mole H2SO4 yields 1 mole of H2 (22.4 liters @ STP)
[0194] 1 mole H2SO4=98 grams
[0195] Therefore, a theoretical maximum yield of 0.23 liters of H2 per gram of H2SO4 is expected without the regeneration reaction.
[0196] 2 moles of HCl yields 1 mole of H2 (22.4 liters @ STP)
[0197] 2 moles of HCl=73 grams
[0198] Therefore, a theoretical maximum yield of 0.31 liters of H2 per gram of HCl is expected without the regeneration reaction.
[0199] The initial reaction rate was similar to that found in experiment #1, where 9 liters of gas was produced in slightly less than one hour. At this point, however, the reaction rate was found to decrease by a factor of approximately one-half. The addition of 20 grams of iron caused an immediate increase in reaction rate to the value that was observed at the onset of the experiment.
[0200] Clearly an interaction is taking place between the two metals that produces a reaction that sustains a high rate of gas production a significant period of time. Experiment #10 Summary:
[0201] An initial solution comprising 10 mL of 93% concentration H2SO4 and 30 mL of 35% concentration HCl was reacted with 20 grams of iron pellets and 20 grams of aluminum pellets. There were then added 25 mL of colloidal magnesium and 40 mL of colloidal lead, each at a concentration believed to be about 20 ppm, producing a total volume of about 110 mL. A theoretical maximum of 8.06 liters of hydrogen gas could be produced if solely from the consumption of the acids as indicated in Table 7.
TABLE 7
Starting Solution Maximum H2 Yield with Acid Consumption
Total Effective Maximum H2
Acid mL Concentration Grams Grams of Acid Yield
H2SO4 10 93.0% 18.97 17.64 4.03 liters
HCl 30 35.0% 37.52 13.13 4.03 liters
Maximum H2 Yield: 8.06 liters
[0202] 1 mole H2SO4 yields 1 mole of H2 (22.4 liters @ STP)
[0203] 1 mole H2SO4=98 grams
[0204] Therefore, a theoretical maximum yield of 0.23 liters of H2 per gram of H2SO4 is expected without the regeneration reaction.
[0205] 2 moles of HCl yields 1 mole of H2 (22.4 liters @ STP)
[0206] 2 moles of HCl=73 grams
[0207] Therefore, a theoretical maximum yield of 0.31 liters of H2 per gram of HCl is expected without the regeneration reaction.
[0208] The rate of the reaction initially is very fast with instantaneous hydrogen generation at a rate of about 20 liters per hour. After about an hour the rate slows to a steady-state value of about 6.0 liters per hour. Additional heat may be added to accelerate the process of regenerating the metals and the acids.
[0209] While some gas was lost due to leakage and diffusion, at least 32 liters of gas was collected over a period of five hours, and the reaction was still proceeding in a continuous fashion at a rate of 6.0 liters per hour. At this point, the reaction was stopped and the remaining metal, a mixture of aluminum and iron was collected and dried, and was found to have a mass of about 40 grams. Thus, only a negligible amount of metal was consumed. Since the remaining metal was not analyzed, it is not known in what ratio aluminum and iron were present; however, it can be assumed that approximately 20 grams of each metal was present in the remaining metallic sample. Based upon previous observations and theoretical projections, the first 8.06 liters of gas produced is likely to be made up of essentially pure hydrogen, and beyond the theoretical threshold of 8.06 liters, 66.7% by volume of the gas produced would be hydrogen and the other 33.3% by volume would be oxygen. It is believed this experiment provides further evidence for a more efficient regeneration process when smaller volumes are used in the reaction vessel. Experiment #11 Summary:
[0210] An initial solution comprising 10 g of sodium hydroxide, 20 mL of colloidal silver, and 10 mL of colloidal magnesium, where each of the colloidal solutions had a concentration believed to be about 20 ppm was diluted with 70 mL of distilled water. There was then added to the solution 20 g of metallic zinc and 20 g of metallic nickel. Initially the two metals were not in contact and virtually no reaction and no gas evolution were observed. When the zinc and nickel metals were moved into contact with each other, a vigorous evolution of gas was observed emanating from the surface of the nickel metal. The gaseous product produced at the surface of the metallic nickel was captured in soap bubbles and was ignited. The explosion upon ignition strongly indicated the presence of elemental hydrogen in the product gas. Experiment #12 Summary:
[0211] An initial solution comprising 10 g of sodium hydroxide, 20 mL of colloidal silver, and 10 mL of colloidal magnesium, where each colloidal solution had a concentration believed to be about 20 ppm, was diluted with 70 mL of distilled water. There was then added to the solution a small piece of metallic zinc and a small piece of metallic nickel each connected to a piece of copper wire approximately three inches long. A vigorous evolution of gas was observed emanating from the surface of the nickel metal. The gaseous product produced at the surface of the metallic nickel was captured in soap bubbles and was ignited. The explosion upon ignition strongly indicated the presence of elemental hydrogen in the product gas. Experiment #13 Summary:
[0212] An initial solution comprising 10 g of sodium hydroxide, 20 mL of colloidal silver, and 10 mL of colloidal magnesium, where each colloidal solution had a concentration believed to be about 20 ppm, was diluted with 70 mL of distilled water. There was then added to the solution a small piece of metallic zinc, and a small piece of a tungsten carbide, each connected to a piece of copper wire that extended outside of the solution. When the ends of the copper wire were not in direct contact, virtually no reaction and no gas evolution were observed. When the two ends of the copper wire were placed into contact a vigorous evolution of gas was observed emanating from the surface of the tungsten carbide electrode. The gas evolution could be stopped and restarted repeatedly simply by removing and then replacing the connection at the two ends of the copper wires. When the two copper wires were not in contact, a potential of about 0.3 volts was measured across the two ends of the copper wires. The gaseous product produced at the surface of the tungsten carbide sample was captured in soap bubbles and was ignited. The explosion upon ignition strongly indicated the presence of elemental hydrogen in the product gas. After about 100 hours the rate of gas evolution and the measured potential were unchanged. Experiment #14 Summary:
[0213] An initial solution comprising 9.8 g of sodium hydroxide, 20 mL of colloidal silver, and 10 mL of colloidal magnesium, where each colloidal solution had a concentration believed to be about 20 ppm, was diluted with 70 mL of distilled water. There was then added to the solution 42.2 g of tungsten carbide directly fused to 30.3 g of metallic zinc. A vigorous evolution of gas was observed emanating from the surface of the tungsten carbide electrode. After a period of two hours, approximately 1.5 L of gaseous product had been collected. The reaction was stopped at this point and the solution was found to have a pH of 11, and it was further determined that 2.8 g of metal had been consumed. Experiment #15 Summary:
[0214] An initial solution comprising 10 g of sodium hydroxide, 20 mL of colloidal silver, and 10 mL of colloidal magnesium, where each colloidal solution had a concentration believed to be about 20 ppm, was diluted with 70 mL of distilled water. There was then added to the solution a small piece of metallic zinc and a small piece of a tungsten carbide, each connected to a piece of copper wire that extended outside of the solution. When the ends of the copper wire were not in direct contact, virtually no reaction and no gas evolution were observed. When the two ends of the copper wire placed into contact, a vigorous evolution of gas was observed emanating from the surface of the tungsten carbide electrode. The gas evolution could be stopped and restarted repeatedly simply by removing and then replacing the connection at the two ends of the copper wires. When the two copper wires were not in contact, a potential of about 0.3 volts was measured across the two ends of the copper wires. The gaseous product produced at the surface of the tungsten carbide sample was captured in soap bubbles and was ignited. The explosion upon ignition strongly indicated the presence of elemental hydrogen in the product gas. After about 100 hours the rate of gas evolution and the measured potential were unchanged. An external 12-volt power source was then attached to the electrodes in order to cause a flow of electrical current in the direction opposite to what had been observed. Upon the application of this potential the zinc metal was observed to reform on the electrode with the concurrent production of a gas thought to be elemental oxygen. Experiment #16 Summary:
[0215] An initial solution comprising 10 g of sodium hydroxide, 20 mL of colloidal silver, and 10 mL of colloidal magnesium, where each colloidal solution had a concentration believed to be about 20 ppm, was diluted with 70 mL of distilled water. There was then added to the solution a small piece of metallic zinc and a small piece of a tungsten carbide, each connected to a piece of copper wire that extended outside of the solution. When the ends of the copper wire were not in direct contact, virtually no reaction and no gas evolution were observed. When the two ends of the copper wire placed into contact a vigorous evolution of gas was observed emanating from the surface of the tungsten carbide electrode. The gas evolution could be stopped and restarted repeatedly simply by removing and then replacing the connection at the two ends of the copper wires. When the two copper wires were not in contact a potential of about 0.3 volts was measured across the two ends of the copper wires. The gaseous product produced at the surface of the tungsten carbide sample was captured in soap bubbles and was ignited. The explosion upon ignition strongly indicated the presence of elemental hydrogen in the product gas. After about 100 hours the rate of gas evolution and the measured potential were unchanged. The zinc electrode was then removed and replaced by an electrode consisting of copper wire. There was no observable chemical reaction when the circuit was completed. An external 12-volt power source was then attached to the electrodes in order to cause a flow of electrical current in the direction opposite to what had been observed. Upon application of this potential the zinc metal was observed to reform on the copper electrode with the concurrent production of a gas thought to be elemental oxygen. After 10 minutes, the external 12-volt power source was disconnected and the circuit was once again completed by placing the two ends of copper wire into contact. When the two ends of the copper wire placed into contact, a vigorous evolution of gas was observed emanating from the surface of the tungsten carbide electrode, the rate of which was approximately equal to the rate that had been initially observed. Experiment #17 Summary:
[0216] An initial solution was prepared by dissolving 10 g of sodium hydroxide in 100 mL of distilled water. There was then added to the solution a small piece of metallic zinc and a small piece of a tungsten carbide each connected to a piece of copper wire that extended outside of the solution. When the ends of the copper wire were not in direct contact, virtually no reaction and no gas evolution were observed. When the two ends of the copper wire were placed into contact, the evolution of gas was observed emanating from the surface of the tungsten carbide electrode. The rate of gas evolution was noticeably less than the rate observed with the inclusion of the colloidal catalysts. The gas evolution could be stopped and restarted repeatedly simply by removing and then replacing the connection at the two ends of the copper wires. When the two copper wires were not in contact, a potential of about 0.3 volts was measured across the two ends of the copper wires. The gaseous product produced at the surface of the tungsten carbide sample was captured in soap bubbles and was ignited. The explosion upon ignition strongly indicated the presence of elemental hydrogen in the product gas. Experiment #18 Summary:
[0217] An initial solution comprising 10 g of sodium hydroxide, 20 mL of colloidal silver, and 10 mL of colloidal magnesium, where each colloidal solution had a concentration believed to be about 20 ppm, was diluted with 70 mL of distilled water. There was then added to the solution a small piece of metallic zinc, and a copper plate connected to four pieces of a tungsten carbide. The metallic zinc and the copper plate were each connected to a piece of copper wire that extended outside of the solution. When the ends of the copper wire were not in direct contact, virtually no reaction and no gas evolution were observed. When the two ends of the copper wire were placed into contact, a vigorous evolution of gas was observed emanating from the surface of each of the pieces of the tungsten carbide. The total rate of gas evolution was approximately four times that obtained when a single piece of tungsten carbide was employed, indicating the relationship between the rate of hydrogen production and the surface area of the cathode. The gas evolution could be stopped and restarted repeatedly simply by removing and then replacing the connection at the two ends of the copper wires. When the two copper wires were not in contact, a potential of about 0.3 volts was measured across the two ends of the copper wires. The gaseous product produced at the surface of the tungsten carbide sample was captured in soap bubbles and was ignited. The explosion upon ignition strongly indicated the presence of elemental hydrogen in the product gas. Experiment #19 Summary:
[0218] An initial solution comprising 5 mL of 93% concentration H2SO4, 10 mL of 35% concentration HCl, 25 mL of colloidal silver, and 10 mL of colloidal magnesium, where each colloidal solution had a concentration believed to be about 20 ppm, was diluted with 50 mL of distilled water. There was then added to the solution a small piece of a metal alloy consisting of metallic tin and metallic lead and a small piece of a tungsten carbide, each connected to a piece of copper wire that extended outside of the solution. When the ends of the copper wire were not in direct contact, virtually no reaction and no gas evolution were observed. When the two ends of the copper wire were placed into contact, a rather evolution of gas was observed emanating from the surface of the tungsten carbide electrode. The gas evolution could be stopped and restarted repeatedly simply by removing and then replacing the connection at the two ends of the copper wires. The gaseous product produced at the surface of the tungsten carbide sample was captured in soap bubbles and was ignited. The explosion upon ignition strongly indicated the presence of elemental hydrogen in the product gas. Experiment #20 Summary:
[0219] An initial solution comprising 5 mL of 93% concentration H2SO4, 10 mL of 35% concentration HCl, 25 mL of colloidal silver, and 10 mL of colloidal magnesium, where each colloidal solution had a concentration believed to be about 20 ppm, was diluted with 50 mL of distilled water. There was then added to the solution a small piece of a metal alloy consisting of metallic tin and metallic lead and a small piece of a tungsten carbide, each connected to a piece of copper wire that extended outside of the solution. When the ends of the copper wire were not in direct contact, virtually no reaction and no gas evolution were observed. When the two ends of the copper wire placed into contact, a vigorous evolution of gas was observed emanating from the surface of the tungsten carbide electrode. The gas evolution could be stopped and restarted repeatedly simply by removing and then replacing the connection at the two ends of the copper wires. The gaseous product produced at the surface of the tungsten carbide sample was captured in soap bubbles and was ignited. The explosion upon ignition strongly indicated the presence of elemental hydrogen in the product gas. After about 10 hours the rate of gas evolution was unchanged. The tin-lead electrode was then removed and replaced by an electrode consisting of copper wire. There was no observable chemical reaction when the circuit was completed. An external 12-volt power source was then attached to the electrodes in order to cause a flow of electrical current in the direction opposite to what had been observed. Upon the application of this potential a metal was observed to reform on the copper electrode, with the concurrent production of a gas thought to be elemental oxygen. After 10 minutes, the external 12-volt power source was disconnected and the circuit was once again completed by placing the two ends of copper wire into contact. When the two ends of the copper wire placed into contact, a vigorous evolution of gas was observed emanating from the surface of the tungsten carbide electrode, the rate of which was approximately equal to the rate that had been initially observed. Experiment #21 Summary:
[0220] An initial solution comprising 5 mL of 93% concentration H2SO4, 10 mL of 35% concentration HCl, 25 mL of colloidal silver, and 10 mL of colloidal magnesium, where each colloidal solution had a concentration believed to be about 20 ppm, was diluted with 50 mL of distilled water. There was then added to the solution a small piece of a metal alloy consisting of metallic tin and metallic lead and a copper plate connected to four pieces of a tungsten carbide. The metallic tin-lead alloy and the copper plate were each connected to a piece of copper wire that extended outside of the solution. When the ends of the copper wire were not in direct contact, virtually no reaction and no gas evolution was observed. When the two ends of the copper wire were placed into contact, a vigorous evolution of gas was observed emanating from the surface of each of the pieces of the tungsten carbide. The total rate of gas evolution was approximately four times that obtained when a single piece of tungsten carbide was employed, indicating the relationship between the rate of hydrogen production and the surface area of the cathode. The gas evolution could be stopped and restarted repeatedly simply by removing and then replacing the connection at the two ends of the copper wires. When the two copper wires were not in contact, a potential of about 0.3 volts was measured across the two ends of the copper wires. The gaseous product produced at the surface of the tungsten carbide sample was captured in soap bubbles and was ignited. The explosion upon ignition strongly indicated the presence of elemental hydrogen in the product gas. Experiment #22 Summary:
[0221] An initial solution comprising 8 mL of 93% concentration H2SO4, 24 mL of 35% concentration HCl, 20 mL of colloidal silver, and 20 mL of colloidal magnesium, where each colloidal solution had a concentration believed to be about 20 ppm, was diluted with 75 mL of distilled water. There was then added to the solution 10 g of zinc sulfate heptahydrate. To a 25 mL aliquot of this solution was added a small piece of aluminum mesh and a small piece of tungsten carbide, each connected to one of two copper wires that extended outside of the solution. When the ends of the copper wires were not in direct contact with each other, virtually no reaction and no gas evolution were observed. When the two ends of the copper wires were placed into contact, a very vigorous evolution of gas was observed emanating from the surface of the tungsten carbide electrode. The rate of hydrogen formation was comparable to that obtained by the uncatalyzed reaction of pure aluminum with mineral acid at a similar level of acidity. The gas evolution could be stopped and restarted repeatedly simply by removing and then replacing the connection between the copper wires. The gaseous product produced at the surface of the tungsten carbide sample was captured in soap bubbles and ignited. The explosion upon ignition strongly indicated the presence of elemental hydrogen in the product gas. Experiment #23 Summary:
[0222] An initial solution comprising 8 mL of 93% concentration H2SO4, 24 mL of 35% concentration HCl, 20 mL of colloidal silver, and 20 mL of colloidal magnesium, where each colloidal solution had a concentration believed to be about 20 ppm, was diluted with 75 mL of distilled water. There was then added to the solution 10 g of cobalt (II) sulfate heptahydrate. To a 25 mL aliquot of this solution was added a small piece of aluminum mesh and a small piece of tungsten carbide, each connected to one of two copper wires that extended outside of the solution. When the ends of the copper wires were not in direct contact, virtually no reaction and no gas evolution were observed. When the two ends of the copper wires were placed into contact, a very vigorous evolution of gas was observed emanating from the surface of the tungsten carbide electrode. The rate of hydrogen formation was comparable to that obtained by the uncatalyzed reaction of pure aluminum with mineral acid at a similar level of acidity. The gas evolution could be stopped and restarted repeatedly simply by removing and then replacing the connection at the two ends of the copper wires. The gaseous product produced at the surface of the tungsten carbide sample was captured in soap bubbles and ignited. The explosion upon ignition strongly indicated the presence of elemental hydrogen in the product gas.
[0223] The foregoing experiments were carried out under ambient lighting conditions that included a mixture of artificial and natural light sources. When the reactions described were performed under decreased light conditions, the reaction rates generally decreased. However, separate formal testing under decreased lighting has not been performed.
[0224] It is believed the experimental results described above demonstrate the potential value of the invention described herein. The calculations are based on the reaction mechanisms described above and are believed to characterize the reactions involved in these experiments accurately. However, if it is discovered that the theories of reactions or the calculations based thereon are in error, the inventions described herein nevertheless are valid and valuable.
[0225] The embodiments shown and described above are exemplary. Many details are often found in the art and, therefore, many such details are neither shown nor described. It is not claimed that all of the details, parts, elements, or steps described and shown were invented herein. Even though numerous characteristics and advantages of the present invention have been described in the drawings and accompanying text, the description is illustrative only, and changes may be made in the detail, especially in matters of shape, size, and arrangement of the parts within the principles of the inventions to the full extent indicated by the broad meaning of the terms of the attached claims.
[0226] The restrictive description and drawings of the specific examples above do not point out what an infringement of this patent would be, but are to provide at least one explanation of how to use and make the inventions. The limits of the invention and the bounds of the patent protection are measured by and defined in the following claims.
CN1906133
//
MXPA06001987
APPARATUS AND METHOD FOR THE PRODUCTION OF HYDROGEN
APPARATUS AND METHOD FOR THE PRODUCTION OF HYDROGEN
Inventor: GRIFFIN LINNARD
IPC: B01D5/00; C01B3/08; C02F1/461
US2005217432
Apparatus and method for the reduction of metals
Apparatus and method for the reduction of metals
Inventor: GRIFFIN LINNARD
EC: C21B15/00; C22B21/02; IPC: C21B15/00; C22B21/02; C22B3/02
Abstract -- Described is an apparatus for the production of an elemental metal from a metal-containing compound comprising a reaction medium containing ions of a first metal and a second metal, wherein the second metal is in colloidal form, and a related method.
Description
RELATED APPLICATIONS
[0001] This application is a continuation-in-part of application Ser. No. 10/995,934, filed Nov. 23, 2004, which is hereby incorporated by reference for all purposes and which claims priority to provisional applications Ser. No. 60/524,469, filed Nov. 24, 2003; Ser. No. 60/531,763, filed Dec. 22, 2003; and Ser. No. 60/531,764, filed Dec. 22, 2003.
TECHNICAL FIELD
[
0002] The present invention is directed to a method and apparatus for the production of metals from metal ore.
BACKGROUND
[0003] Most metals are found in nature in their oxidized form. In order to extract these metals from their ores, it is necessary to chemically reduce these metals to their elemental form. The reduction of these metals usually requires stringent reaction conditions and therefore results in a significant cost. For example, iron, as found in nature, is generally in the oxidized form iron(III) oxide, Fe2O3, or iron(II) oxide, FeO, or a combination of the two: magnetite, Fe3O4. The reduction of Fe<+2 > or Fe<+3 > to yield Fe is normally carried out at very high temperatures, generally in excess of 1000[deg.] C. This reduction is commonly accomplished by reaction of the iron(III) oxide with carbon as shown in equation 1.
2Fe2O3+3C->4Fe+3CO2 (1)
[0004] The very high temperature required for the reaction employed in equation 1 causes the generation of metallic iron from its oxide to be difficult to achieve, and very expensive. Likewise, aluminum, as found in nature, is generally in the oxidized form aluminum oxide, Al2O3. The reduction of Al<+3 > to yield Al is normally carried out using a procedure called the Hall-Heroult process. In the Hall-Heroult process, aluminum oxide, Al2O3, is dissolved in a carbon-lined bath of molten cryolite, Na3AlF6. Aluminum fluoride, AlF3, is also present to reduce the melting point of the cryolite. The reactants are then electrolyzed, and liquid aluminum is produced at the cathode. The carbon anode is oxidized and forms gaseous carbon dioxide. The net chemical reaction that describes this process is shown in equation 2. The very high temperature (about 600[deg.] C.) required for the reaction employed in equation 2 causes the generation of metallic aluminum from its oxide to be difficult to achieve, and the need for the electrical energy necessary for electrolysis causes the production of aluminum to be a very expensive process.
2Al2O3+3C->4Al+3CO2 (2)
[0005] Accordingly, there exists a need for a method and apparatus for the production of metals from metal ore that requires less extreme conditions and, accordingly, can be done at a lower cost.
SUMMARY
[0006] The above-described need has been addressed by providing an apparatus for the production of an elemental metal from a metal-containing compound which comprises a solution containing ions of a first metal and a second metal, wherein the second metal is in colloidal form.
[0007] In another embodiment of the apparatus, the second metal is less reactive than the first metal.
[0008] In an additional embodiment, the second metal is more reactive than the first metal.
[0009] In an additional embodiment, the apparatus further comprises a third metal, wherein the third metal is in colloidal form.
[0010] In an additional embodiment, the third metal is more reactive than the first metal.
[0011] In an additional embodiment, the apparatus further comprises a vessel for containing the solution, wherein the vessel is inert to the solution. In an additional embodiment the vessel is configured to maintain an internal pressure greater than atmospheric.
[0012] In an additional embodiment, the second metal is silver, gold, platinum, tin, lead, copper, zinc, iron, aluminum, magnesium, beryllium, nickel or cadmium.
[0013] In an additional embodiment, the apparatus further comprises a solid comprising the first metal in contact with the solution.
[0014] In an additional embodiment, the solid comprising the first metal is a metal oxide.
[0015] In an additional embodiment, the apparatus further comprises an energy source. In an additional embodiment, the energy source supplies electric energy.
[0016] In an additional embodiment, the apparatus further comprises a cathode and an anode in electrical contact with the solution.
[0017] In an additional embodiment, the temperature of the solution is less than 500[deg.] C.
[0018] In an additional embodiment, the apparatus further comprises an elemental non-metal in contact with the solution. In an additional embodiment, the elemental non-metal is carbon.
[0019] In an additional embodiment, the apparatus further comprises ions of a salt dissolved in the solution. In an additional embodiment, a cation of the salt is higher on the electromotive series than the first metal. In an additional embodiment, the salt is aluminum sulfate, magnesium sulfate or potassium aluminum sulfate.
[0020] In an additional embodiment, the solution comprises a reducing agent. In an additional embodiment, the reducing agent is hydrogen peroxide. In an additional embodiment, the reducing agent is formic acid.
BRIEF DESCRIPTION OF THE DRAWINGS
[0021] The drawing FIGURE is a diagram of equipment which may be used in connection with one embodiment of the present invention.
DETAILED DESCRIPTION
[0022] The drawing FIGURE shows equipment which may be used in one embodiment of the present invention. A reaction vessel 102 contains a reaction medium 104. The reaction medium 104 preferably comprises water and an acid, and preferably has a pH less than 7, although other reaction media may be used, including other solvents or non-liquid media such as gelatinous or gaseous media. A cathode 106 and an anode 108 are preferably in electrical contact with reaction medium 104. Cathode 106 is preferably in the form of a disk made of carbon, but metallic materials such as lead and iron may also be used. Cathode 106 is preferably positioned on or near a bottom 107 of vessel 102. However, cathode 106 may generally be any shape and may be positioned anywhere that is in contact with reaction medium 104 and not in direct contact with anode 108. Cathode 106 may be made of any material which is inert or of lower reactivity than the metal being reduced and is electrically conductive. Anode 108 is preferably in the form of a rod made of carbon, but metallic materials such as lead and iron may also be used. Anode 108 is preferably positioned to extend into reaction medium 104 through a top surface of reaction medium 104. However, anode 108 may generally be any shape and may be positioned anywhere in contact with reaction medium 104 and not in direct contact with cathode 106. Anode 108 may be made of any material which is inert or of lower reactivity than the metal being reduced and is electrically conductive.
[0023] Vessel 102 also preferably contains an elemental non-metal 109 in contact with reaction medium 104. The elemental non-metal is preferable in solid form, for the sake of convenience, though a gaseous or liquid form should also work. Most preferably, elemental non-metal 109 is carbon, since carbon is relatively abundant and the byproducts produced in the resulting reactions (described below) are not toxic or environmentally harmful.
[0024] An electrical potential source 110 is electrically connected to cathode 106 and anode 108 and provides an electrical potential between cathode 106 and anode 108. Electrical potential source 110 preferably provides a direct current potential of the approximate order of or greater than 12 volts. The invention described herein has been performed using as little as 1 volt and as great as 12 volts. It has been found that higher voltages increase the overall reaction rate. As an alternative to providing electrical energy, other forms of energy, such as thermal energy, or light or other electromagnetic radiation energy, may be provided to reaction medium 104. Additionally, ambient thermal energy may be used, but the necessary reactions (discussed below) will occur at a slower rate. However, unlike prior art methods of metal production, the necessary reactions do not require that the reaction medium 104 temperature be extremely high. The necessary reactions have been observed to occur at a reasonable rate at temperatures of about 75[deg.] C. when the energy source 110 provides electrical energy.
[0025] Reaction medium 104 contains ions (not shown) of a metal which is to be reduced to its elemental state. Preferably, the ion source is a metal-containing compound 112 in the solid state which is in contact with reaction medium 104. Most preferably, the metal-containing compound 112 is a metal ore found in nature, such as iron(III) oxide or aluminum oxide. Alternatively, the metal-containing compound 112 may be a derivative of a metal ore, such as aluminum hydroxide, Al(OH)3. Additionally, the metal ions may be from a salt, either in a solid or dissolved form, a metal oxide or other ion source.
[0026] Reaction medium 104 also preferably contains a dissolved salt (not shown). The salt preferably comprises a metal cation that is higher on the electromotive series of metals than the metal of the metal-containing compound which is being reduced. The salts that have been found to be most effective are aluminum sulfate, Al2(SO4)3, magnesium sulfate, MgSO4, and potassium aluminum sulfate, KAl(SO4)2. However, in theory, any salt could have a similar effect.
[0027] Reaction medium 104 also contains a suspended colloidal catalyst (not shown). Most metals can be produced in a colloidal state in a liquid. A colloid is a material composed of very small particles of one substance that are dispersed (suspended), but not dissolved, in a liquid. Thus, colloidal particles do not settle out of a liquid even though they exist in the solid state. A colloid of any particular metal is then a very small particle of that metal suspended in a liquid. These suspended particles of metal may exist in the solid (metallic) form or in the ionic form, or as a mixture of the two. The very small size of the particles of these metals results in a very large effective surface area for the metal. This very large effective surface area for the metal can cause the surface reactions of the metal to increase dramatically when it comes into contact with other atoms or molecules. The colloidal metals used in the experiments described below were obtained using an apparatus for producing colloidal silver in water sold by CS Prosystems of San Antonio, Tex. The website of CS Prosystems is www.csprosystems.com. Based on materials provided by the manufacturer, the particles of a metal in the colloidal dispersions used in the experiments described below are believed to range in size between 0.001 and 0.01 microns. In such a solution of colloidal metals, the concentrations of the metals are believed to be between about 5 to 20 parts per million with the remainder being water.
[0028] Alternative to using a catalyst in colloidal form, it may be possible to use a catalyst in another form that offers a high surface-area to volume ratio, such as a porous solid or colloid-polymer nanocomposite. In general, any of the catalysts may be in any form with an effective surface area preferably of at least 298,000,000 m<2 > per cubic meter of catalyst metal, although smaller surface area ratios may also work.
[0029] When a colloidal metal ion is treated with an oxidized metal, a voltaic oxidation-reduction will take place. The oxidized metal can be any compound where the metal is in a cationic form. Preferably, the oxidized metal will be the metal ore as found in nature. For many metals, this is the metal oxide (MexOy). Equations 3 and 4 are believed to represent the oxidation and reduction reactions that occur with respect to the colloidal metal. Equations 5 and 6 are believed to represent the oxidation and reduction reactions that occur with the inclusion of elemental non-metal 109, represented by the letter "Z". The process proceeds most successfully when elemental non-metal 109, Z, is either carbon or sulfur, but any non-metal may theoretically be employed.
[0030] The colloidal metal, M, can in principle be any metal, but it has been found that equations 3 and 4, or equations 5 and 6, work most efficiently when the colloidal metal has a higher (more positive) reduction potential than Me. Thus, equations 3 and 4 and equations 5 and 6 proceed most efficiently when the colloidal metal is as low as possible on the electromotive series of metals. Consequently, any colloidal metal will be successful, but the reactions illustrated in equations 3 and 4 and equations 5 and 6 proceed most quickly with colloidal silver ion, due to the high reduction potential of silver. When silver, for example, is employed as the colloidal metal ion in equations 3 and 4, or in equations 5 and 6, the pair of reactions is found to take place quite readily. The voltaic reaction produces a positive voltage as the oxidation and reduction reactions indicated take place. This positive voltage can be used to supply the energy required for other chemical processes. In fact, the voltage produced can even be used to supply an over potential for reactions employing equations 3 and 4, or equations 5 and 6, taking place in another reaction vessel. Thus, this electrochemical process can theoretically be made to take place more quickly without the supply of an external source of energy, if at least two of these reactions are performed in series. The addition of an external source of energy, such as thermal energy, electrical energy or light, or other electromagnetic radiation, will further enhance the reaction rate.
Cathode (Reduction)
4M<+> +4e<-> ->4M (3)
Anode (Oxidation)
2H2O->4H<+> +O2+4e<-> (4)
Cathode (Reduction)
4M<+> +4e<-> ->4M (5)
Anode (Oxidation)
2H2O+Z->4H<+> +ZO2+4e<-> (6)
[0031] It is believed that the oxidation-reduction reaction represented by reactions 5 and 6 occur faster and more easily than the oxidation-reduction reaction represented by equations 3 and 4 due to the thermodynamic stability of the non-metallic oxide, ZO2.
[0032] The net result of the oxidation and reductions shown in equations 5 and 6 is equation 7, which results in the production of a colloidal metal in its elemental state plus a non-metallic oxide plus acid. [mathematical formula - see original document]
[0033] In the absence of the non-metal, Z, the net result of the oxidation and reductions shown in equations 3 and 4 is equation 7A, which is believed to result in the production of a colloidal metal in its elemental state, plus elemental oxygen, plus acid. [mathematical formula - see original document]
[0034] The colloidal elemental metal that has been produced is believed to undergo reaction with the metal ion of the substance that contains the oxidized form of the metal, which will be represented as Me<+> . Me<+> can represent the oxidized form of any metal, which can be present in any oxidation state. Equation 8 illustrates this reaction where the oxidized form of the metal, Me, is an oxide, but in reality can be any compound that contains the metal, Me, in its oxidized form.
4M+2MeO+2H2O->4M<+> +2Me+4OH<-> (8)
[0035] The reaction illustrated by equation 8 will take place most efficiently when the colloidal metal, M, is more reactive than the metal, Me. That is, the reaction in equation 8 will proceed most efficiently when the colloidal metal, M, is above the metal, Me, on the electromotive series of metals. The hydroxide ion produced in equation 8 will react with the hydrogen ion produced in equation 7, or in equation 7A, to produce water as indicated in equation 9.
4H<+> +4OH<-> ->4H2O (9)
[0036] Since the acid produced in the electrochemical reaction depicted in equations 5 and 6 is neutralized by the base produced in the thermal reaction represented by equation 8, the entire reaction system remains at a pH close to 7 throughout. The combining of equations 5, 6, 8, and 9 results in the net process illustrated by equation 10, which represents the production of the elemental metal, Me, produced by a reduction reaction, and the formation of an oxide of a non-metal, ZO2, produced by an oxidation reaction. [mathematical formula - see original document]
[0037] In the absence of the non-metal, Z, the combining of equations 3, 4, 8, and 9 results in the net process illustrated by equation 10A, which represents the production of the elemental metal, Me, produced by a reduction reaction, and the formation of elemental oxygen, produced by an oxidation reaction. [mathematical formula - see original document]
[0038] The reactions shown in equations 3 and 4, or in equations 5 and 6, seem to occur best when the colloidal metal, M, is as low as possible on the electromotive series of metals (less reactive); however, the reaction depicted by equation 8 takes place most efficiently when the colloidal metal, M, is as high as possible on the electromotive series of metals. The net reaction, which is illustrated by equation 10, or by equation 10A, is merely the sum of equations 3, 4, 8, and 9 or of equations 5, 6, 8, and 9, and could, in fact, be maximally facilitated by either colloidal metals of high activity or by colloidal metals of low activity. The relative importance of the reaction illustrated by equations 3 and 4, or by equations 5 and 6, compared to the reaction shown in equation 8 determines the characteristics of the colloidal metal that would best assist the net reaction in equation 10 or in equation 10A. It has been found that the net reaction indicated in equation 10 or in equation 10A proceeds at a maximal rate when the colloidal metal is of maximum activity; that is, when the colloidal metal is as high as possible on the electromotive series of metals (more reactive). It has been found that the more reactive colloidal metals, such as, but not limited to, colloidal aluminum ion or colloidal magnesium ion, produce the most facile reduction processes for the reduction of cationic metals. It is also believed, although not yet shown analytically, that the overall reaction may proceed even more favorably when two colloidal metals are used, especially where one is higher (more reactive) and one lower (less reactive) on the electromotive series than the metal being reduced.
[0039] In addition, it has also been found that the inclusion of a small amount (such as 10 wt %) of a salt leads to a rate increase in the reaction represented by equation 10, or equation 10A. The salt has its maximal effect when it includes a cation of a metal of higher activity than Me; that is, one that is higher (more reactive) than Me on the electromotive series of metals. The salts that have been found to be most effective are aluminum sulfate, Al2(SO4)3, magnesium sulfate MgSO4, and potassium aluminum sulfate, KAl(SO4)2; however, in theory, any salt could potentially have a similar effect.
[0040] Thus, under ambient thermal conditions, the oxide of any metal can be converted to its metallic elemental state, with the concurrent formation of elemental oxygen or the oxide of a non-metal. It is believed that the thermal stability of the oxide of the non-metal, ZO2, lowers the endothermicity of the process, and allows the reduction of the oxidized metal to proceed at lower temperatures, when the non-metal Z is used. The supplying of additional energy leads to an acceleration of the reaction rate for the process. When additional energy is supplied, it can be supplied in the manner of thermal energy, electrical energy, radiant energy, or the like. When the additional energy supplied is in the form of thermal energy, one is limited by the boiling point of the solvent. In aqueous systems, this would provide a maximum temperature of 100[deg.] C. Under pressures higher than one atmosphere, however, temperatures higher than 100[deg.] C. could be obtained, and would provide an even more enhanced reaction rate. It has been found that the increase in reaction rate is most significant when additional energy is supplied in the form of electrical energy.
[0041] An alternative to the above involves the introduction of a reducing agent into reaction medium 104. Hydrogen peroxide has been found to be an effective reducing agent for this process. With the addition of hydrogen peroxide to reaction medium 104, equations 5 and 6 are replaced by equations 11 and 12, or equations 3 and 4 are replaced by equations 11A and 12A.
Cathode (Reduction)
2M<+> +2e<-> ->2M (11)
[0042] Anode (Oxidation)
H2O2+Z->2H<+> +ZO2+2e<-> (12)
Cathode (Reduction)
2M<+> +2e<-> ->2M (11A)
Anode (Oxidation)
H2O2->2H<+> +O2+2e<-> (12A)
[0043] Due to the fact that hydrogen peroxide has a larger (less negative) oxidation potential than water, as indicated in the comparison of equations 4A and 4B, the oxidation-reduction reaction resulting from equations 11 and 12 takes place at an enhanced rate compared to the oxidation-reduction reaction indicated by equations 5 and 6. Likewise, the oxidation-reduction reaction resulting from equations 11A and 12A takes place at an enhanced rate compared to the oxidation-reduction reaction indicated by equations 3 and 4.
H2O->4H<+> +O2+4e<-> [epsilon]<0> =-1.229 Volts (4A)
H2O2->2H<+> +O2+2e<-> [epsilon]<0> =-0.695 Volts (4B)
[0044] The net result of the oxidation and reductions shown in equations 11 and 12 will be equation 13, which results in the production of a colloidal metal in its elemental state plus a non-metallic oxide plus acid [mathematical formula - see original document]
[0045] Likewise, the net result of the oxidation and reductions shown in equations 11A and 12A will be equation 13A, which results in the production of a colloidal metal in its elemental state, plus elemental oxygen, plus acid. [mathematical formula - see original document]
[0046] The colloidal elemental metal that has been produced is believed to undergo reaction with the metal ion of the substance that contains the metal to be reduced, which will be represented as Me<+> . Me<+> can represent the oxidized form of any metal, which can be present in any oxidation state. Equation 14 illustrates this reaction where the oxidized form of the metal, Me, is an oxide, but in reality can be any compound that contains the metal, Me, in its oxidized form.
2M+MeO+H2O->2M<+> +Me+2OH<-> (14)
[0047] The reaction illustrated by equation 14 will take place most efficiently when the colloidal metal, M, is more reactive than the metal, Me. That is, the reaction in equation 14 will proceed most efficiently when the colloidal metal, M, is above the metal, Me, on the electromotive series of metals. The hydroxide ion produced in equation 14 will react with the hydrogen ion produced in equation 13, or in equation 13A, to produce water as indicated in equation 15.
2H<+> +2OH<-> ->2H2O (15)
[0048] Since the acid produced in the electrochemical reaction depicted in equations 11 and 12, or in equations 11A and 12A, is neutralized by the base produced in the thermal reaction represented by equation 14, the entire reaction system remains at a pH close to 7 throughout. The combining of equations 11, 12, 14, and 15 results in the net process illustrated by equation 16, which represents the production of the elemental metal, Me, produced by a reduction reaction, and the formation of an oxide of a non-metal, ZO2, produced by an oxidation reaction. [mathematical formula - see original document]
[0049] If the non-metal, Z, is not used, the combining of equations 11A, 12A, 14, and 15 results in the net process illustrated by equation 16A, which represents the production of the elemental metal, Me, produced by a reduction reaction, and the formation of elemental oxygen, produced by an oxidation reaction. [mathematical formula - see original document]
[0050] The reactions shown in equations 11 and 12, or in equations 11A and 12A, seem to occur best when the colloidal metal, M, is as low as possible on the electromotive series of metals. However, the reaction depicted by equation 14 takes place most efficiently when the colloidal metal, M, is as high as possible on the electromotive series of metals. The net reaction illustrated by equation 16, which is merely the sum of equations 11, 12, 14, and 15, or by equation 16A, which is merely the sum of equations 11A, 12A, 14, and 15, could, in fact, be maximally facilitated by either colloidal metals of higher activity or by colloidal metals of lower activity than the metal being reduced. The relative importance of the reaction illustrated by equations 11 and 12, or by equations 11A and 12A, compared to the reaction shown in equation 14, determines the characteristics of the colloidal metal that would best assist the net reaction in equation 16, or in equation 16A. It has been found that the net reaction indicated in equation 16, or in equation 16A, proceeds at a maximal rate when the colloidal metal is of maximum activity; that is, when the colloidal metal is as high as possible on the electromotive series of metals. It has been found that the more reactive colloidal metals, such as, but not limited to, colloidal aluminum ion or colloidal magnesium ion, produce the most facile reduction processes for the reduction of cationic metals. It is also believed, although not yet shown analytically, that the overall reaction may proceed even more favorably when two colloidal metals are used, especially where one is higher and one lower on the electromotive series than the metal being reduced.
[0051] In addition, it has also been found that the inclusion of a small amount of a salt leads to a rate increase in the reaction represented by equation 16, or by equation 16A. The salt has been found to have a maximal effect when it includes a cation of a metal of higher activity than the metal being reduced; that is, one that is higher (more reactive) on the electromotive series of metals. The salts that have been found most effective are aluminum sulfate, Al2(SO4)3, magnesium sulfate MgSO4, and potassium aluminum sulfate, KAl(SO4)2; however, in theory, any salt could potentially have a similar effect.
[0052] Thus, under ambient thermal conditions, the oxide of any metal can be treated with hydrogen peroxide and a non-metal, and can be converted to its metallic elemental state, with the concurrent formation of the oxide of a non-metal and water. Since the oxidation of hydrogen peroxide (equation 12 or equation 12A) is more favorable than the oxidation of water (equation 6 or equation 4), the rate of metal reduction should be significantly increased when hydrogen peroxide is used in the place of water. This must be balanced by the fact that hydrogen peroxide is a more costly reagent to supply. In those cases where the rate of the metal reduction is the most critical factor, the use of hydrogen peroxide will offer a significant advantage. It is still believed that the thermal stability of the oxide of the non-metal, ZO2, lowers the endothermicity of the process, and allows the reduction of the oxidized metal to proceed at reasonable temperatures. The supplying of additional energy leads to an acceleration of the reaction rate for the process. When additional energy is supplied, it can be supplied in the manner of thermal energy, electrical energy, radiant energy or the like. When the additional energy supplied is in the form of thermal energy, one is limited by the boiling point of the solvent. In aqueous systems, this would provide a maximum temperature of 100[deg.] C. Under pressures higher than one atmosphere, however, temperatures higher than 100[deg.] C. could be obtained, and would provide an even more enhanced reaction rate. It has been found that the increase in reaction rate is most significant when additional energy is supplied in the form of electrical energy.
[0053] A further alternative to the above involves the introduction of a different reducing agent into reaction medium 104. Formic acid has been found to be an effective reducing agent for this process. With the addition of formic acid to reaction medium 104, equations 3 and 4 are replaced by equations 17 and 18.
Cathode (Reduction)
2M<+> +2e<-> ->2M (17)
Anode (Oxidation)
CH2O2->2H<+> +CO2+2e<-> (18)
[0054] Due to the fact that formic acid has a larger (in fact, positive) oxidation potential than water, or than hydrogen peroxide, as indicated in the comparison of equations 4A, 4B and 4C, the oxidation-reduction reaction resulting from equations 17 and 18 takes place at an enhanced rate compared to the oxidation-reduction reaction indicated by equations 3 and 4, or by equations 11A and 12A.
H2O->4H<+> +O2+4e<-> [epsilon]<0> =-1.229 Volts (4A)
H2O2->2H<+> +O2+2e<-> [epsilon]<0> =-0.695 Volts (4B)
CH2O2->2H<+> +CO2+2e<-> [epsilon]<0> =0.199 Volts (4C)
[0055] The net result of the oxidation and reductions shown in equations 17 and 18 will be equation 19, which results in the production of a colloidal metal in its elemental state plus carbon dioxide plus acid [mathematical formula - see original document]
[0056] The colloidal elemental metal that has been produced is believed to undergo reaction with the metal ion of the substance that contains the metal to be reduced, which will be represented as Me<+> . Me<+> can represent the oxidized form of any metal, which can be present in any oxidation state. Equation 14 illustrates this reaction where the oxidized form of the metal, Me, is an oxide, but in reality can be any compound that contains the metal, Me, in its oxidized form.
2M+MeO+H2O->2M<+> +Me+2OH<-> (14)
[0057] The reaction illustrated by equation 14 will take place most efficiently when the colloidal metal, M, is more reactive than the metal, Me. That is, the reaction in equation 14 will proceed most efficiently when the colloidal metal, M, is above the metal, Me, on the electromotive series of metals. The hydroxide ion produced in equation 14 will react with the hydrogen ion produced in equation to produce water as indicated in equation 15.
2H<+> +2OH<-> ->2H2O (15)
[0058] Since the acid produced in the electrochemical reaction depicted in equations 17 and 18 is neutralized by the base produced in the thermal reaction represented by equation 14, the entire reaction system remains at a pH close to 7 throughout. The combining of equations 17, 18, 14, and 15 results in the net process illustrated by equation 20, which represents the production of the elemental metal, Me, produced by a reduction reaction, and the formation of a carbon dioxide, produced by an oxidation reaction. [mathematical formula - see original document]
[0059] The reactions shown in equations 17 and 18 seem to occur best when the colloidal metal, M, is as low as possible on the electromotive series of metals. However, the reaction depicted by equation 14 takes place most efficiently when the colloidal metal, M, is as high as possible on the electromotive series of metals. The net reaction illustrated by equation 20, which is merely the sum of equations 17, 18, 14, and 15, could, in fact, be maximally facilitated by either colloidal metals of higher activity or by colloidal metals of lower activity than the metal being reduced. The relative importance of the reaction illustrated by equations 17 and 18, compared to the reaction shown in equation 14, determines the characteristics of the colloidal metal that would best assist the net reaction in equation 20. It has been found that the net reaction indicated in equation 20 proceeds at a maximal rate when the colloidal metal is of maximum activity; that is, when the colloidal metal is as high as possible on the electromotive series of metals. It has been found that the more reactive colloidal metals, such as, but not limited to, colloidal aluminum ion or colloidal magnesium ion, produce the most facile reduction processes for the reduction of cationic metals. It is also believed, although not yet shown analytically, that the overall reaction may proceed even more favorably when two colloidal metals are used, especially where one is higher and one lower on the electromotive series than the metal being reduced.
[0060] In addition, it has also been found that the inclusion of a small amount of a salt leads to a rate increase in the reaction represented by equation 20. The salt has been found to have a maximal effect when it includes a cation of a metal of higher activity than the metal being reduced; that is, one that is higher (more reactive) on the electromotive series of metals. The salts that have been found most effective are aluminum sulfate, Al2(SO4)3, magnesium sulfate, MgSO4, and potassium aluminum sulfate, KAl(SO4)2; however, in theory, any salt could potentially have a similar effect.
[0061] Thus, under ambient thermal conditions, the oxide of any metal can be treated with formic acid, and can be converted to its metallic elemental state, with the concurrent formation of carbon dioxide and water. Since the oxidation of formic acid (equation 18) is more favorable than the oxidation of water (equation 4), or the oxidation of hydrogen peroxide (equation 12A), the rate of metal reduction should be significantly increased when formic acid is used in the place of water, or in the place of hydrogen peroxide. This must be balanced by the fact that formic acid, while less costly than hydrogen peroxide, is a more costly reagent to supply than is water. In those cases where the rate of the metal reduction is the most critical factor, the use of formic acid will offer a significant advantage. It is believed that the thermal stability of the oxide of the carbon dioxide that is formed lowers the endothermicity of the process, and allows the reduction of the oxidized metal to proceed at reasonable temperatures. The supplying of additional energy leads to an acceleration of the reaction rate for the process. When additional energy is supplied, it can be supplied in the manner of thermal energy, electrical energy, radiant energy, or the like. When the additional energy supplied is in the form of thermal energy, one is limited by the boiling point of the solvent. In aqueous systems, this would provide a maximum temperature of 100[deg.] C. Under pressures higher than one atmosphere, however, temperatures higher than 100[deg.] C. could be obtained, and would provide an even more enhanced reaction rate. It has been found that the increase in reaction rate is most significant when additional energy is supplied in the form of electrical energy.
[0062] Finally, while all equations depicted here involve the use of just a single metal, Me, it has been shown that all of the reactions discussed herein can be carried out using an elemental metal in addition to the oxidized form of the metal being reduced, Me<+> . It has been shown, in fact, that in some cases the use of multiple metals results in a significant increase in the rate, as well as a significant increase in the yield of metal reduction. In experiment #13, experiment #16 and experiment #17, for example, elemental iron in the form of small iron nuggets is used to aid the reduction of oxidized aluminum, Al<+3> . In each of these cases, a sizable yield of metallic aluminum results from a completely thermal, non-electrolytic process requiring, at most, the input of only a small amount of thermal energy. It is not clear at this point what causes these impressive enhancements in the rate of the process as well as in the yield of reduced metal that results. It is possible that the elemental metal takes part in the reaction mechanism to provide a more complicated mechanism having a greater number of steps, but a lower net activation barrier. Another possibility is that the elemental metal might provide a surface where the reduced metal, Me, could reform more efficiently. Whatever the actual explanation is, the results from experiments #13, #16 and #17 very clearly demonstrate that the effects resulting from the addition of an elemental metal different from Me can be quite significant in its effect upon metal reduction.
[0063] Experiments #13, #16 and #17 each result in a sample of iron that has become plated with elemental aluminum. Since this technology should be valid for pairs of metals other than iron and aluminum, what could result is a general method for the plating of surfaces with a metal without the use of electrolysis. Experimental Results:
[0064] Several experiments have been conducted using combinations of embodiments of the technology described above. The results of those experiments are given below: Experiment #1 Summary:
[0065] An experiment was conducted using 150 mL of iron (III) chloride in an aqueous solution (commonly used as an etching solution, purchased from Radio Shack) as the starting materials. Initially, 10 mL of 93% concentration sulfuric acid (H2SO4) was added to the solution, at which point no reaction occurred. About 50 mL of colloidal magnesium and 80 mL of colloidal lead, each at a concentration believed to be about 20 ppm, were then added, at which point a chemical reaction began and the bubbling of gases was evident at ambient temperature. The production of gas accelerated when the solution was heated to a temperature of about 65[deg.] C. The product gas was captured in soap bubbles and the bubbles were then ignited. The observed ignition of the gaseous product was typical for a mixture of hydrogen and oxygen.
[0066] Since, it is believed, the production of hydrogen gas could only be produced with a concurrent oxidation of iron, it is evident that the iron (III) had to be initially reduced before it could be oxidized, thereby providing strong evidence of the reduction reaction. This experiment has subsequently been repeated with hydrochloric acid (HCl) instead of sulfuric acid, with similar results. Experiment #2 Summary:
[0067] An experiment was conducted using 100 grams of Fe3O4 (this sample was found to contain roughly equal amounts of Fe2O3 and FeO plus a small amount of elemental carbon), 50 mL of 5% H2SO4, plus 40 mL of colloidal magnesium and 40 mL of colloidal lead in water. Immediately a stream of gas was evolved that was identified as carbon dioxide by gas chromatography. The mixture was then heated to a temperature of 90[deg.] C. for a period of about three hours. At this point, the stream of gas being evolved was again analyzed by gas chromatography. This gaseous mixture was found to contain 40% hydrogen and 60% carbon dioxide. Since, it is believed, the production of hydrogen gas could only be produced with a concurrent oxidation of iron, it is evident that the iron had to be initially reduced before it could be oxidized, thereby providing strong evidence of the reduction reaction. Experiment #3 Summary:
[0068] An experiment was conducted using 5 g of Al2(SO4)3.18H2O plus 40 mL of colloidal magnesium and 40 mL of colloidal lead in water. Upon being heated to about 75[deg.] C., a stream of gas, presumed to be elemental oxygen, was produced that did not ignite, and also did not extinguish a flame. After 45 minutes of heating, the gas was found to ignite very slightly when it was exposed to a flame, indicative of the production of a small amount of elemental hydrogen. Since, it is believed, the production of hydrogen gas could only be produced with a concurrent oxidation of aluminum, it is evident that the aluminum had to be initially reduced before it could be oxidized, thereby providing strong evidence of the reduction reaction. Experiment #4 Summary:
[0069] An experiment was conducted using 5 g of Fe2O3 plus 40 mL of colloidal magnesium, 40 mL of colloidal lead and 80 mL of 3% aqueous H2O2. Almost immediately a small amount of a gaseous product was produced. As the temperature was increased, over a period of ten minutes, the yield of gas increased with a maximum yield of gas being realized at the maximum temperature of about 75[deg.] C. The product gas was found to contain a substantial amount of hydrogen, based upon the manner in which it ignited when a flame was applied. Since, it is believed, the production of hydrogen gas could only be produced with a concurrent oxidation of iron, it is evident that the iron had to be initially reduced before it could be oxidized, thereby providing strong evidence of the reduction reaction. Experiment #5 Summary:
[0070] An experiment was conducted using 5 g of Al(OH)3 plus 40 mL of colloidal magnesium, 40 mL of colloidal lead and 80 mL of 3% aqueous H2O2. Almost immediately a small amount of a gaseous product was produced. As the temperature was increased, over a period of ten minutes, the yield of gas increased with a maximum yield of gas being realized at the maximum temperature of about 75[deg.] C. The product gas was found to contain a substantial amount of hydrogen, based upon the manner in which it ignited when a flame was applied. Since, it is believed, the production of hydrogen gas could only be produced with a concurrent oxidation of aluminum, it is evident that the aluminum had to be initially reduced before it could be oxidized, thereby providing strong evidence of the reduction reaction. Experiment #6 Summary:
[0071] An experiment was conducted using 5 g of Al2(SO4)3.18H2O plus 40 mL of colloidal magnesium, 40 mL of colloidal lead and 80 mL of 3% aqueous H2O2. Almost immediately a small amount of a gaseous product was produced. As the temperature was increased, over a period of ten minutes, the yield of gas increased with a maximum yield of gas being realized between the temperatures of 50[deg.] C. and 75[deg.] C. The product gas was found to contain a substantial amount of hydrogen, based upon the manner in which it ignited when a flame was applied. Since, it is believed, the production of hydrogen gas could only be produced with a concurrent oxidation of aluminum, it is evident that the aluminum had to be initially reduced before it could be oxidized, thereby providing strong evidence of the reduction reaction. Experiment #7 Summary:
[0072] An experiment was conducted using 5 g of Fe2O3 plus 40 mL of colloidal magnesium, 40 mL of colloidal lead and 1 gram of elemental carbon in water. The mixture was heated to a temperature of about 90[deg.] C. for a period of 72 hours. A metallic-like material was produced and collected that reacted with sulfuric acid to produce an ignitable gas presumed to be hydrogen gas. The metallic material is believed to be elemental iron. Experiment #8 Summary:
[0073] An experiment was conducted using 5 g of Al(OH)3 plus 40 mL of colloidal magnesium, 40 mL of colloidal lead and 1 gram of elemental carbon in water. The mixture was heated to a temperature of about 90[deg.] C. for a period of 72 hours. A metallic-like material was produced and collected that reacted with sulfuric acid to produce an ignitable gas presumed to be hydrogen gas. The metallic material is believed to be elemental aluminum. Experiment #9 Summary:
[0074] An experiment was conducted using 5 g of Fe2O3, 40 mL of colloidal magnesium and 40 mL of colloidal lead in water. A 12 volt, 10 amp power source was then applied for a period of 5 minutes to a pair of lead electrodes that had been introduced into the solution. A metallic-like material that was produced and found on the bottom of the apparatus was collected. The metallic material reacted with sulfuric acid to produce an ignitable gas presumed to be hydrogen gas. The metallic material has been tentatively identified as elemental iron. Experiment #10 Summary:
[0075] An experiment was conducted using 5 g of Al(OH)3 plus 40 mL of colloidal silver and about 0.1 g sodium hydroxide in water. A 12 volt, 10 amp power source was then applied for a period of about thirty minutes to an iron anode and a carbon cathode that had been introduced into the solution. After about five minutes, the solution was titrated to a pH of about 7 using H2SO4. A metallic-like material that was produced and found attached to the anode was collected. The metallic material reacted with sulfuric acid to produce an ignitable gas presumed to be hydrogen gas. The metallic material has been tentatively identified as elemental aluminum. An X-Ray Photoelectric Spectrum (XPS) was taken of this material that indicates the presence of some elemental aluminum in this material. Experiment #11 Summary:
[0076] An experiment was conducted using an initial solution comprising 10 mL of 93% concentration H2SO4 and 30 mL of 35% concentration HCl that was reacted with 25 g of Al2(SO4)3.18H2O plus 80 mL of colloidal lead. Over a period of thirty minutes, the reaction mixture was heated on a hot plate, and the temperature increased to a value of 75[deg.] C. Over this period, a small amount of an ignitable gas presumed to be hydrogen gas was produced. Since, it is believed, the production of hydrogen gas could only be produced with a concurrent oxidation of aluminum, it is evident that the aluminum had to be initially reduced before it could be oxidized, thereby providing strong evidence of the reduction reaction. Experiment #12 Summary:
[0077] An experiment was conducted using an initial solution comprising 10 mL of 93% concentration H2SO4 and 30 mL of 35% concentration HCl that was reacted with 25 g of Al2(SO4)3.18H2O plus 80 mL of colloidal lead, and 80 mL of 3% aqueous H2O2. Over a period of thirty minutes, the reaction mixture was heated on a hot plate, and the temperature increased to a value of 75[deg.] C. Over this period, an impressive amount of an ignitable gas presumed to be hydrogen gas was produced. The rate of gas formation at this point was measured to be 80 mL per minute, or 4.8 L per hour. Since, it is believed, the production of hydrogen gas could only be produced with a concurrent oxidation of aluminum, it is evident that the aluminum had to be initially reduced before it could be oxidized, thereby providing strong evidence of the reduction reaction. Experiment #13 Summary:
[0078] An experiment was conducted using an initial solution comprising 80 mL of 90% aqueous formic acid, CH2O2, that was reacted with 25 g of Al2(SO4)3.18H2O plus 80 mL of colloidal lead. Over a period of thirty minutes, the reaction mixture was heated on a hot plate, and the temperature increased to a value of 75[deg.] C. Over this period, a small amount of an ignitable gas presumed to be hydrogen gas was produced. There was then added to the reaction mixture 80 g of metallic iron. The rate of gas formation was found to increase drastically to a measured rate of 80 mL per minute or 4.8 L per hour. After an additional hour of gas formation at a temperature of 75[deg.] C., the reaction mixture was allowed to cool to a temperature of 20[deg.] C. The iron metal was examined and was found to have metallic aluminum plated over the iron. The aluminum was identified by its reaction with water in an aqueous solution of NaOH to produce an ignitable gas presumed to be hydrogen. Experiment #14 Summary:
[0079] An experiment was conducted using an initial solution comprising 20 mL of 90% aqueous formic acid, CH2O2, that was reacted with 20 mL of colloidal lead and one gram of metallic aluminum. Over a period of thirty minutes, the reaction mixture was heated on a hot plate, and the temperature increased to a value of 75[deg.] C. No significant amounts of gases were emitted and, in fact, no noticeable chemical reaction of any kind was observed to occur under these conditions. Since metallic aluminum does not react significantly under these reaction conditions, it would be likely that if elemental aluminum were to be produced under similar conditions, the aluminum could be isolated. Experiment #15 Summary:
[0080] An experiment was conducted using an initial solution comprising 40 mL of 90% aqueous formic acid, CH2O2, that was reacted with 40 mL of colloidal lead and 40 g of metallic iron. Over a period of thirty minutes, the reaction mixture was heated on a hot plate, and the temperature increased to a value of 75[deg.] C. Over this period, a moderate amount of gas was produced. There was then added to the reaction mixture log of Al2(SO4)3.18H2O, and the rate of gas formation was found to increase significantly. The reaction mixture was allowed to cool to a temperature of 20[deg.] C. The iron metal was examined and found to have metallic aluminum plated over the iron. The aluminum was identified by its reaction with water in an aqueous solution of NaOH to produce an ignitable gas presumed to be hydrogen.
Experiment #16
Summary:
[0081] An experiment was conducted using an initial solution comprising 80 mL of colloidal lead that was reacted with 30 g of Al2(SO4)3.18H2O plus 1 g of metallic iron. The reaction mixture was heated using a hot plate to a temperature of 75[deg.] C., and this temperature was maintained over a period of 24 hours. The reaction mixture was allowed to cool to a temperature of 20[deg.] C. The iron metal was examined and was found to have metallic aluminum plated over the iron. The aluminum was identified by its reaction with water in an aqueous solution of NaOH to produce an ignitable gas presumed to be hydrogen. A different iron nugget from the same reaction mixture was then studied using Energy Dispersive X-Ray Spectroscopy (EDX), and was found to contain approximately 9% elemental aluminum by mass.
Experiment #17
Summary:
[0082] An experiment was conducted using an initial solution comprising 40 mL of 90% aqueous formic acid, CH2O2, that was reacted with 40 mL colloidal lead plus 25 g of Al2(SO4)318H2O plus 9 g of metallic iron. The reaction mixture was maintained at an ambient temperature of approximately 20[deg.] C. over a period of 48 hours. The iron metal was examined and was found to have metallic aluminum plated over the iron. The aluminum was identified by its reaction with water in an aqueous solution of NaOH to produce an ignitable gas presumed to be hydrogen.
[0083] It is believed the experimental results described above demonstrate the potential value of the inventions described herein. However, the results, calculations and conclusions are based on the theoretical reaction mechanisms that are described above and that are believed to accurately characterize the reactions involved in these experiments. However, if it is discovered that the theoretical reaction mechanisms used to rationalize the experimental findings, or the calculations based thereon are in error, the inventions described herein nevertheless are valid and valuable.
[0084] The embodiments shown and described above are exemplary. Many details are often found in the art and, therefore, many such details are neither shown nor described. It is not claimed that all of the details, parts, elements, or steps described and shown were invented herein. Even though numerous characteristics and advantages of the present invention have been described in the drawings and accompanying text, the description is illustrative only, and changes may be made in the detail, especially in matters of shape, size, and arrangement of the parts within the principles of the invention to the full extent indicated by the broad meaning of the terms of the attached claims.
[0085] The restrictive description and drawings of the specific examples above do not point out what an infringement of this patent would be, but are to provide at least one explanation of how to use and make the invention. The limits of the invention and the bounds of the patent protection are measured by and defined in the following claims.