DIAMINE OXIDASE ACTION
Zeller’s idea of the action of diamine Oxidase, as published in “The Enzymes” edited by Sumner and Myrbaeck, 1951, p 554, may be condensed as follows, using putrescine as substrate.
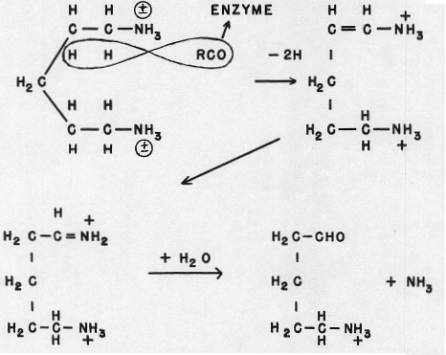
The products are hydrogen peroxide and ammonia, oxygen being the ultimate electron acceptor, and Flavin-Adenine dinucleotide, the electron carrier. The Carbonyl group of the Diamine Oxidase (DO) serves as a dehydrogenator removing the hydrogen atoms from the carbon atoms alpha and beta to the amine group that is to be removed. A double bond is thus produced. The other amine group is not attacked. When the hydrogen atoms so removed are taken up by the dinucleotide, the DO Carbonyl group is ready to start another cycle of detoxication. The steps are oxidation, a reversed Amadori shift, so to speak, and then hydrolysis removing the amine group as ammonia, as shown in the diagrams.
Oxidative Separation of the Integrated Pathogen
Our Postulate proposes oxidation all the way through. Dehydrogenation alpha to a double bond of the pathogen that is integrated with the FCG, by an azomethine condensation. This may take place close to or far from the point of integration. The free radical produced adds molecular oxygen, becomes a peroxide free radical, and splits producing two terminal Carbonyl groups. The double bonds of the Carbonyl groups activate the alpha placed hydrogen atoms facilitating further dehydrogenations by the SSR Carbonyl group. Thus, whether the azomethine double bond yields an oxide of nitrogen in the pathogen and a Carbonyl group, as was originally present in the host cell as its FCG right at the start, or as the end reaction of a stepwise oxidative progression starting at a distance, the results are the same; restoration of the host cell FCG and destruction of the pathogen with the production of energy. The diagrams illustrate.
If oxidation starts at a distant position, more energy is produced and this may be the way viruses are separated with return of the energy to the host cell, which was taken up by the viral colony during its vegetation. The same holds for the separation as in (D) to follow.

Integration of the Pathogen with the Host Cell Energy Producing Mechanism by a Free Radical Addition to the Double Bonds that Activate the F.C.G.
Host Cell Energy Producing Pathogen (P)
System FCG and Double Bonds.

When the Pathogen (P) is dehydrogenated during anoxia a free radical is formed at position (H1) which adds to one pole of the host cell’s ethylene linkage that activates its FCG, thus, —

As the pathogen adds to one pole of the activating double bond of the FCG, the other pole of this double bond becomes a free radical which makes an addition to whatever group it has the most attraction for, is represented by R”, thus, —


+SSR and OXYGEN to form a free radical and then a peroxide free radical at position (2)

Cleavage then takes place leaving the host cell FCG conjugated with a Carbonyl group replacing the former ethylene linkage that activated it.

The residue left by the cleavage at the activating position is a free radical that adds molecular O (2) to undergo further oxidation.
If the H (3) is the most exposed and activated hydrogen atom, it will invite removal by the SSR, and the free radical formed there, in the presence of molecular oxygen, becomes a peroxide free radical and splits the molecule with the production of two terminal Carbonyl groups, as in the former instance. Each Carbonyl group double bond serves as activator of the hydrogen atom in the alpha position to it, and the process is repeated step-by-step until the FCG is reached where a Carbonyl group is formed and the electrons, it can contribute, go to the FCG to activate it as the ethylenic linkage had done previously. The FCG is activated to a higher O/R potential than formerly, as the Carbonyl group is a better electron donor than the ethylene linkage. Also, the Carbonyl group does not add free radicals so readily as does the ethylenic linkage and hence, the FCG system is protected from inactivating additions, therefore, a higher degree of immunity is gained than what existed before. This Hypothesis seems to answer the fact that our cured patients are more resistant to disease than they formerly were and in fact more resistant than others usually are. Since viruses are built up of similar units, as a co-polymerization process, the last monomer added is most exposed and oxidation would start at this position. The energy liberated would then pass on to the host cell and support its reconstruction, so that when the paralysis as in Rabies and Polio has disappeared, one knows that not only the virus is separated out of the way, but the host cell’s functional mechanism has been restored. Since so long as the virus is integrated with the host cell, the function is blocked and the functional mechanism is progressively destroyed to support viral vegetation; the reversal of the process, with reconstruction of the host cell so function has returned, means that the energy for this reconstruction must have come from the oxidative destruction of the virus, as there is no other source for such energy while the FCG is blocked. The facts have been demonstrated. The explanation, of course, is a matter of choice as limited by the facts.
IMPORTANCE OF DIVALENT AND MONOVALENT CATION BALANCE
The parathyroidectomy experiments emphasized another matter of the greatest clinical importance. It is the relation of the balance of divalent and monovalent cations to cell irritability. Ordinarily, the normal cell presents a disposition of the cations, adsorbed and un-adsorbed, by the cell colloids so that a water in lipoid phase is maintained. The lipoids thus diffuse to the periphery of the cell forming a concentration of lipoid there, which serves as a limiting membrane and tends to shut out water-soluble materials, while water and its soluble materials tend to concentrate in the center. The effect is reversed by an excess of monovalent cations and accentuated by divalent cations. The monovalent cations thus tend to cause a lipoid in water phase with the lipoids concentrated at the center and the water-soluble materials and water at the periphery. This tends to increase the entrance of water-soluble substances into the cell. Sodium and potassium thus tend to increase the cell’s exchanges and the entrance of water-soluble toxins. Calcium, magnesium and strontium tend to reverse this situation. Calcium is important with magnesium in lessening the cell irritability, while sodium and potassium increase it. Variations play their roles in functional activities of all cells and will be apparent also in the functions of heart muscle.
After parathyroidectomy, the increase in irritability of the nervous system followed the loss of calcium from the tissues. The entrance of guanidine toxins gave convulsions of greater severity, the more the calcium was lost. Calcium transfusions reduced the convulsions for a time, that is so long as the kidney function was maintained. Other solutions as of potassium and sodium and even distilled water did the same for a time; so diluting the blood and washing the toxic element out via the kidneys showed that calcium metabolism was not the whole problem after parathyroidectomy. However calcium, magnesium and strontium had a greater depressing effect than the monovalent cation solutions, and the suppression was greater in the order of the divalent cations named. Indeed strontium put the cells to “sleep” as it were by its excessive effect. It was such facts as these that convinced the writer that the function of the parathyroids was not just a matter of calcium metabolism as Carlson and his school claimed. As soon as the hemorrhagic glomerular nephritis after parathyroidectomy prevented the washing of the guanidines out of the blood, no amount of calcium or any other solution could prevent or hinder the convulsions and deaths of the animals. Thus it was evident that a toxic element existed, and the writer set out to find it.
A complicating factor is the normal place of calcium in activating ATP-ase for the transfer of energy into the working mechanism of the cell. Here is a chance for energy transfer also to the surfaces of the tissue colloids that aids their dispersion and oxygen transport, in order to get rid of toxic materials and preserve a better colloidal structure in the cell throughout its contents. Normal cell excitability and response to stimulus is thus maintained by this other function of calcium.
When one sees exaggerated reflexes or persistent excitability of a tissue, one therefore thinks of the dispersions of the lipoids to or from the cell surface and also the disposition of the monovalent and divalent cations. In cases of high irritability, one would not give transfusions of solutions that would increase the monovalent salt content. Isotonic salt solutions made with sodium chloride would not be used unless the sodium is balanced by calcium and some sort of a Ringer’s solution should be used. Even the sera of the blood banks could be taken from individuals with high sodium chloride blood content. These matters deserve consideration.
Our diet calls for calcium in plentiful amount, as crude calcium carbonate. We also give the potentized calcium as carbonate to cancer patients. This is to diminish the nutrition of the sodium rich cancer cells and to reduce the pain caused by the ever present incompletely combusted metabolites that enter the nerve endings in the affected areas. Calcium and good intestinal lavage and a well-chosen diet go a long way in abolishing the pain in cancer. Likewise the Carbonyl Reagents tend to burn the incompletely combusted metabolites out of the way so they cause no more pain. The cancer cells also under good calcium supply do not tend to swell up so much and cause so much pressure, for the reasons just mentioned. All of these factors enter the treatment of the neuroses and psychotic states.
An illustration of the parallel run of toxins with failure of the use of calcium is seen in the treatment of dairy cattle that were badly diseased by hemolytic Staph Aureus infections of the mammary glands. After the Carbonyl (SSR) Therapy was used, both the hemolysins disappeared and the calcium content of the blood and milk increased. The lactiferous cells were able to use the calcium for cell building and for milk production, and the germ no longer produced the hemolysins. This work was done at the University of British Columbia and by the scientists of the Ministry of Agriculture. Thus the toxic inhibition of the use of calcium must be removed to obtain its full biological effect.
STERIC ADVANTAGE AND HINDRANCE
There is a problem bound up in the activation of atomic groups by electron shifts as determined by the character of a substituent for hydrogen at a carbon terminal of a double bond. This is the steric arrangement of the groups concerned. It is observed that the groups taking part must lie in the same plane. We took data on this matter in our earliest observations. Comparison of the action of fumaric acid, maleic acid and maleic anhydride were made on the course of glandular tuberculosis where the enlargements were easily measurable — the cervical and supraclavicular glands. It was found that fumaric acid had no action, maleic acid showed some, and maleic anhydride gave a satisfactory response. This response was not continuous longer than a few months and the dose had to be repeated. This is not what we were looking for. We desired a continuous curative action that kept up until the patient was fully cured. Never the less the observations showed the effect of steric influence. Reference to these early experiments was made in our Court Trial in 1943, where comparison was made with Benzoquinone.
In Benzoquinone one finds the Carbonyl groups activated by conjugation with two ethylenic linkages where no hindering substitutions of the hydrogens are had. They all lie in the same plane. High-grade continuous curative action was had in a wide field. In fumaric acid, which is the trans-isomere of maleic acid, only one Carbonyl at a time is coplanar with the ethylenic linkage, while in maleic acid which is the cis-isomere, all are double bonds, Carbonyl and ethylenic both lie in the same plane. Here, however, the hydroxyl in the carboxyl group acts as a substituent of a dampening nature against Carbonyl activity. In maleic anhydride, the two hydroxyls are removed and this offers an advantage, but not one that would equal the advantage given by the presence of hydrogen. Indeed in such an ideal molecule, the energy content is too high to let it exist as such. So as such it was not practical.
The energy content of maleic acid is 7 large calories higher than that of fumaric acid and hence, it is more reactive. The ionizing power of maleic acid is increased by electron shift from the ethylenic linkage and also from the other unsaturated (Carbonyl) group, tending to liberate one hydrogen as a proton. Other properties show this increase in energy content due to all double bonds lying in the same plane so far as the hydroxyl groups allow. This example of steric influence on reactivity must be helpful in understanding the change in reactivity of atomic groups concerned in the mitotic act — that is, the shift of energy that forces cell division. For example observations on fumaric acid by Friedman et al. show that fumaric acid has no influence on mitotic rates while maleic acid and its two methyl derivatives, all showed strong antimitotic effects. Friedman, Marrian, and Simon-Reuss, (Brit. J. Pharmacol., 3 (1948a) pg.263, attempted to learn if the antimitotic action was due to sulphydryl addition, (Biesele p. 34. Mitotic Poisons and the Cancer Problem, Elsevier, 1958). But it was found that the addition products were not active. The chlormaleic acid and chlormaleimide added sulfhydryl in the same way as if non-chlorinated, but were inactive as mitotic inhibitors. Activation of Carbonyl by electron drift from the other double bonds was not considered by these gentlemen, even though the quality of the influence of the chlorine substituent on the distribution of electrons to the Carbonyl groups both in the cis-acid and anhydride forms was to withdraw them and decrease the all around electron density. This shows that the antimitotic effect is due to Carbonyl action, as this writer has interpreted it to depend upon electronic activation throughout his whole Postulate. Due to the presence of a conjugated ethylenic linkage, sulfhydryl can be added where such an ethylenic linkage can contribute electrons to the Carbonyl group, but that the addition of sulfhydryl has nothing to do with the antimitotic act, is right in line with this Postulate. This is just another of the antimitotic agent puzzles this Postulate has solved long before antimitotic work was ever undertaken. The unheeded data in the hands of the investigators showed that the antimitotic effect was due to Carbonyl activity enhanced by electron contributions from the other conjugated systems of double bonds lying in the same plane. Thus the steric effect of a virus or carcinogenic chemical that becomes integrated with the host cell must be viewed, in the light of its effect, on energy distribution and reactivity. This Thesis calls for an oxidative separation of the pathogen from the host cell where a hydrolytic effort could never bring its release, is in line with this Postulate. Indeed, it is a practical application of the idea.