ISOTOPES IN IONIC RECOVERY OF URANIUM
OXIDE
by
( 1958 )
In the following pages we wish to prove that this science of transmutation is not only theoretically possible but it is continually taking place in nature and can be synthesized by man. It is our contention that Transmutation, Activation, and Fission are essentially one in the same reaction depending on the condition of the material receiving the action and the emanation received.
Definition:
Nuclear Reactions ---
Transmutation --- Az + n1 = Bz + P1
Activation --- Az + n1 = Az + 1
Fission --- Az + n1 = Bz + Cy + 2n1
Elementary particles:
Elementary composition elementary charge is – Mass 1
Positron Composition elementary charge is +1 Mass 1
Proton Composition elementary charge is +1 Mass 1830
Neutron composition proton and electron charge is 0 Mass 1830
Deuteron composition is neutron and proton charge is +1 Mass 3660
Alpha particle composition is 2 Neutrons 2 Protons; charge is +2 Mass 7320
(Charge: 1 = 4.803 + 0.003 x 10-10 e.s.u.)
It is the emanation which, by freeing itself and by projecting its disaggregated particles on the surface of other bodies, produces the so-called induced radioactivity. This phenomenon exists in all substances placed in the neighborhood of a radioactive compound, the substance becoming radioactive to a lesser or greater degree for various periods of time. Radioactivity, artificially provoked in any substance, disappears only after a fairly long time:
All gases or metals placed close to a radioactive substance or on which is blown, by means of a long tube, the emanation which it disengages, become momentarily radioactive. It being admitted that this radioactivity is generated by the freeing of electric particles, it must be supposed that these particles are capable of being carried along by the air and of attaching themselves like dust to other bodies, and possess properties singularly different from those of ordinary electricity. It has been demonstrated that the emanation of thorium can pass through water and sulphuric acid without losing its activity. If a metallic wire charged with negative electricity be exposed to the emanation of thorium, it becomes radioactive; if this wire be treated with sulphuric acid and the residuum then evaporated, it will be found that this latter is still radioactive.
The induced radioactivity communicated to an inactive substance may be much more intense than that of the radioactive substance from which it emanated. When, in an enclosed vessel, containing some emanation from a radioactive body (thorium for example), a metal plate charged with negative electricity at a high potential is introduced, all the particles emitted by the thorium concentrate themselves upon it and this plate becomes ten thousand times more active, surface for surface, then the thorium itself. These facts are not, any more than the preceding ones, explainable by the electric current theory.
If a metal (rendered artificially radioactive) is brought to a white heat, it loses its radioactivity, which spreads itself over the bodies in its neighborhood. Here again, we see atoms behaving in a very strange manner.
The phenomenon of induced radioactivity is, then, quite unexplainable with energy ideas as to electric particles. It cannot be admitted that such particles deposited on a metal can remain for weeks in the charged state and be carried along by reagents. It would see, from M. Curie’s experiments, that bismuth, plunged into a solution of bromide or radium and carefully washed immediately, remains radioactive for at least three years. This radioactivity would seem even to persist after energetic chemical treatment or metallic plating.
In respect to induced radioactivity, certain forms of matter or particles can be stored on bodies for a great length of time and expend themselves very slowly. In experiments on phosphorescence it was noted that sulphide of calcium exposed to the sun for a few seconds, radiates invisible light for 18 months, as is proved by the possibility of photographing the isolated object in the dark room or in the most complete darkness. At the end of 18 months it no longer gives any radiation, but still preserves a residual particle charge which persists for an indefinite period, and can be made visible by causing infrared rays to fall on the surface of the isolated body.
A radioactive body was, 50 years ago, compared to a magnet which keeps its magnetism forever and can, without losing its power, magnetize other bodies. There was not foundation for this comparison, for the magnet is not the seat of a constant emission of particles into space. It might, however, be employed to explain roughly the phenomenon of induced radioactivity, which could be reduced to the fact that a radioactive body imparts its particles to a neighboring body, as the lodestone gives magnetization to fragments of iron near it. If the molecules of the air were magnetic (and they are so in a slight degree) a lodestone would magnetize them, and they themselves might magnetize others. If they preserved their magnetism, we should have particle (which we do not) which, like the emanation of radioactive bodies, remain on the surface of a metal without losing is properties.
Finally we come to Gamma radiation which do not remain on any material and which no magnetic attraction can deviate. These are radiations, not particles as the Alpha and Beta particles which were first considered above.
The most striking phenomenon may not attract our attention except when light is thrown upon them by other phenomena, or when some great generalization capable of explaining them forces us to examine them more closely. When, in Lavoisier’s experiments, hydrogen was found to be electrified, it was only because the atoms of this substance had undergone the commencement of dissociation. It is curious to note that the first experiment from which it could be deduced that matter is changeable had for its author the illustrious Lavoisier, whose greatest claim to glory is that of endeavoring to prove that matter is indestructible.
Experiments prove that a large number of chemical reactions, whether accompanied or unaccompanied by the disengagement of gas, produce effluves similar to the cathode rays, and therefore reveal a dissociation or change of matter without return during the reactions.
Among these reactions the decomposition of water by zinc and sulphuric acid or merely by the sodium amalgam, the formation of acetylene by carbide of calcium, the formation of oxygen by the decomposition of oxygenated water by means of dioxide of manganese, and the hydration of quinine sulphate.
As regards quinine sulphate, it presents a highly curious phenomenon. Quinine, it is well known, becomes phosphorescent by the action of heat, but what was not know at first was that after having lost its phosphorescence, if sufficiently heated it becomes highly luminous and radioactive on refrigeration. After seeking the cause of its phosphorescence on cooling, and pricing it to e due to a very light hydration, it was noted that by reason of this hydration the substance became radioactive for a few minutes.
Experiments prove that most substances contain a provision of radioactive matter which can be expelled by a slight heat and spontaneously formed anew; these substances are therefore, like ordinary radioactive substances, subject to spontaneous dissociation. It is, unless “agitated”, extremely slow.
Spontaneous dissociation has been made evident by means of slight heat or bombardment by rays. It is possible, however, by the help of various artifices (for instance, by folding the metal over itself so as to form a closed cylinder) to allow radioactive products to form in the cylinder, the presence of which is verified by the measurement of ray counters. The substance thus experimented on, however, soon ceases to be active. It has not, however, used up all its provisions of radioactivity; it has simply lost all that it can emit at the temperature under which the operation is effected. But, as with phosphorescent substances or radioactive matter, one need but heat it a little for it to produce an increased quantity of active effluves.
These researches prove that all substances in nature are radioactive, and that this radioactivity is no way a property peculiar to a few bodies. All matter, then, tends spontaneously towards dissociation. This latter is most often very small, because it is hindered by the action of opposing forces. It is only exceptionally, and under the influences of light, combustion, chemical action, etc., capable of striving against these forces, that dissociation reaches a certain intensity.
Having proved by experiments that the dissociation of matter is a general phenomenon, it is only reasonable to say that the doctrine of the invariability of the weight of atoms, on which all modern chemistry is based, is only an illusion resulting entirely from lack of out ability to weigh the vibrations. Were our balances sufficiently sensitive, all out chemical laws would be considered as merely approximations. With proper instruments we should note in many circumstances, and especially in chemical reactions, that the atoms loses a part of its weight and that, contrary to the principle laid down as the basis of chemistry, we do not recover in a chemical combination the total weight of the substances employed to bring about this combination.
This discussion which has gone on since the beginning of time in one place to be picked up in another, plays a great part in natural phenomena. It is probably the origin of atmospheric electricity and all energy phenomena.
Time, space, matter and energy form the elements of things and are the fundamental basis of all our knowledge. Time and space are the magnitudes in which we confine the universe, energy is the cause of phenomena, and matter their web. The great French scientist, Dr LeBon, wrote “matter may be reconverted” (please note, he wrote “reconverted”, not just “converted”) into force, not only because it is, as I have proved, a particular form of energy but also because it is only defined in equations of mechanics by the symbols of force”, i.e., energy.
The sun has been giving off energy for hundreds of millions of years according to geological records. If the sun continues to shine at the expense of its mass in accordance with the theory of relativity, it is easy to show that the mass of the sun is diminishing at the rate of 4,200,000 tons per second in order to supply the energy which pours forth from the sun. Less than 200 millionth of its energy is intercepted by the planets and their satellites.
Now the Theory of Relativity is that the radiation of energy is accompanied by a loss of mass. This loss of mass is given as the loss of energy (in ergs) divided by the square of the velocity of light (9 x 10). Could the sun lose mass at this rate for hundreds of millions of years unless its’ mass is millions of times greater than science claims? There is something wrong with this theory of mass energy or we must have a very imperfect understanding of the situation, for at this rate the sun has been squandering its resources of matter vs energy so prodigally that it long since would have ended in bankruptcy. This proves we must doubtless have at present a very imperfect understanding of the situation.
In the far off stellar crucibles we see the same laws being obeyed as in our laboratories and according to Dr M Luckeish’s statements in his book Foundations of the Universe (Dr Luckeish was the Director of Lightning Research Laboratory for General Electric Co.), he states as we trace down infinitesimal constituents of the atom we find that matter apparently does not exist at all as the realistic substance we have supposed it to be. There at its very foundation matter seems to consist of electric charges. As Dr Steinmetz of General Electric said at one time, in the final analysis all things are electricity. That being the case, where can we draw the line between energy and matter? Is it not evolution of energy and an evolution of matter, energy continually changing onto matter and matter into energy?
The atom of matter is known to consist of positive nucleus-protons and negative particles of electricity, i.e., electrons. Usually electrons are considered to revolve around the nucleus, the number varying depending on the element. Thus everywhere from stellar space to atomic space we find matter in motion, and when we think of how small the protons and electrons are we can see how Democritus was not so far from stating the facts when he made his bold statement hundreds of years ago that matter, physical phenomena, reduced itself to a single item --- motion. Again this shows the evolution of energy and matter.
The process of radiation, whether in the form of long heat rays or luminous rays or so-called actinic rays or electrical vibrations all come under one heading, radiant energy. Where does a vibration cease to be energy and become a form of matter or matter a form of energy?
The greatest principle of this theory is in the Kinetic Theory of Matter which can be pressed into further service in explaining the structure of matter which is matter itself. When in 1873 Maxwell propounded the electromagnetic theory of light (all radiation), his achievement was epochal. The exact manner in which this radiant energy traversed space is not known. The quantum theory advanced by Planck in 1900 was equally as epochal. Here we have the atomistic principle applied to energy instead of having it confined only to matter as it has been. In other words, in the quantum theory we have the atomic theory applied to physical processes. The atom of matter and the atom of electricity (the electron) and the atom action (quantum), a product of energy and time, all can be explained in the same category. Elements of energy of equal magnitude analogous to the equality of electrons or atoms of a given element. We use quanta as commonly as we do electrons and atoms and molecules. Matter is built of molecules, the molecules of atoms and the atoms of electrons and protons. Here we see the atomic theory applied to matter, to electricity and finally divided into quanta. With such a picture of the Universe and of matter we may cease to be surprised at anything, but our interest and admiration grow.
The hypothesis of an atomic structure of matter leads of the hypothesis of an atomic structure of electricity and on to the conception of an elementary quantum of elementary charge (electrical charge). Dr Millikan’s work made possible a direct and extremely accurate determination of the elementary quantum of electricity, i.e., energy into matter.
Energy has a definite elastic rigidity and density and is subject to displacement and strain. Dr LeBon, the great French scientist, held he had proven that matter was a particular form of energy. It is a fact that it is impossible to touch matter without causing electricity to come from it. It is also impossible to touch mater without heat coming from it.
In 1903, Edgar Larkin, Director of Lowe Observatory (Echo Mountain, California), wrote: “Energy is defined as a condition of matter in virtue of which any definite portion may affect changes in any other definite portion”. (Prof. Baker first wrote this in 1892 and later discoveries confirm this statement). “Energy then”, Dr Larkin continues, “is a state of matter, a result of a particular condition or state in which matter may be when any observed phase of energy appears”. Dr Larkin continues that these two, matter and energy, are the sum total of all that has been found during three centuries of incessant research in all that portion of the universe which is visible in a 40-inch telescope or the most powerful spectroscope ever made.
Or, in other words, matter and energy are possible one at various rates of vibration.
In the final analysis, all things are rates of frequency or vibration, the evolution of matter into energy and of energy into matter.
Let us examine some of the “accepted” reactions that occur in nature. The most common of these is the Uranium-Radium series in which Uranium fissions or ages to form ordinary lead in a period of a little more than 4-1/2 billion years (See Fig. 1 and Fig. 1a,b,c).
It was the Thorium series that Dr Rutherford must have used in his synthesis of Radium from Thorium.
The last of this decay chain is Actinium.
FIGURE 1

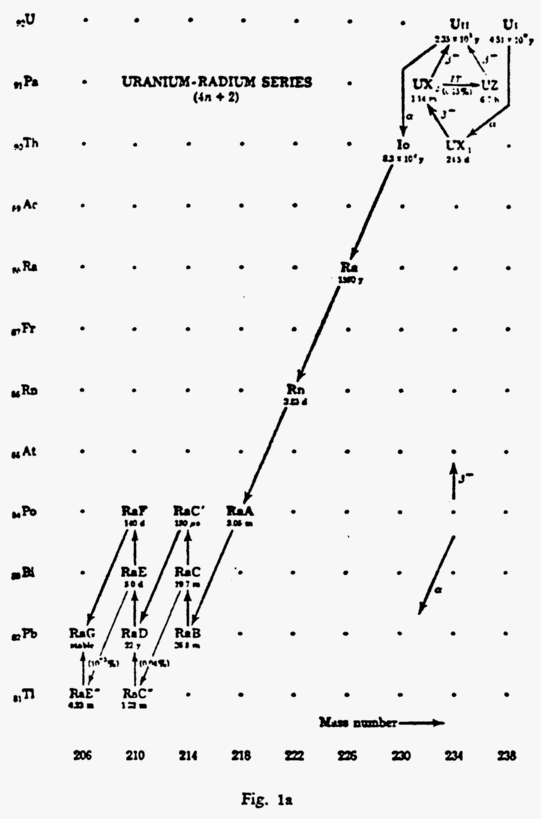


Now with the above we have proven that in nature Uranium becomes Lead. Thorium becomes Lead, and Actinium becomes Lead.
In the non-chain substances we have shown that there are five elements that are known to transmute into seven other elements.
If there are seven elements produced by non-chain reactions, then how many more elements are produced by elements with half-life reactions that have not yet been explored?
In 1919 Lord Rutherford at Cavendish Laboratories, Cambridge, England succeeded in converting nitrogen atoms into oxygen atoms. It was not long after that it was found that radium could be grown or aged at a rapid rate from Ionium.
The net electric charge of the normal hydrogen atom is 0. The positive charge of the nucleus of such an atom is balanced by the negative charges of the electrons on the atom’s orbits. In the atomic complexity after hydrogen is the atom of heavy hydrogen, deuterium. It has a mass of 2, but only a single positive charge on the nucleus and a single orbital electron. Its nucleus is called a deuteron; this contains an additional particle of unit mass, termed a neutron, which has no electric charge.
The next better known elements heavier than deuterium is helium. The normal helium atom has a nucleus consisting of 2 protons and 2 neutrons. It must, therefore, contain 2 orbital electrons. The nucleus of the helium atom is an alpha particle. All other atoms appear to be made up of combinations of protons, neutrons, and alpha particles, with the proper number of orbital electrons to insure electrical neutrality (See Fig. 2).
FIGURE 2

In helium, the 2 electrons occupy similar orbits. In the next substance (lithium), a second group of orbits, or energy level, appears, and successive elements contain an additional electron in this shell or energy level until there are 8, making, with the 2 in the innermost energy level, a total of 10 (neo). With the next substance, sodium (at. no. 11), a third energy level is reached. This development goes on to uranium (at. no. 92, at. wt. 238). One, and only one, element is defined for every number in this series (See Fig. 3).
FIGURE 3

Radiation --- The term radiation has come to include two quite different types of energy propagation through space --- one by means of wave motion, the other by means of particles.
Electromagnetic or wave radiation includes a wide range from radio waves to cosmic rays. Particle radiation, on the other hand, comprises various atomic and subatomic particles set in exceedingly rapid motion by the action either of electric fields or of radioactive sources. Energy content of these particles depends on their mass and speed. When electromagnetic radiation interacts with matter, energy is gained or dissipated in discrete units called photons. The energy of a photon, called a quantum, is proportional to the frequency of the electromagnetic radiation. The higher the frequency or the shorter the wavelength, the greater the energy of the quantum. When electromagnetic radiation interacts with matter in such a manner as to amplify its energy, a wide variety of changes is produced depending on the energy of the quantum absorbed.
[ Part of the following paragraph is illegible in the photocopy from which this was transcribed:]

An example of a change in electronic configuration […] Such changes may result in photochemical action though commonly the energy is degraded into thermal disturbances, thereby affecting only the rate of chemical change, as in vibrational and rotational effects.
FIGURE 4

When a quantum with somewhat higher energy content is absorbed, the action may result in ejection of an electron from an atom or molecule, photoelectric effect, leaving such an atom or molecule positively charged. Thus a pair of ions is produces (radio ionization), one positively charged and the other negatively charged. This process may give rise to actual chemical change.
FIGURE 5

When the quantum of energy absorbed corresponds to that of x-rays or gamma rays, an electron may be ejected from any of the orbital shells of an atom. Such a case of photoelectric effect is illustrated in Fig. 5. Since electrons in the inner shells of atoms are more firmly held than those in outer shells, a large amount of kinetic energy must be imparted t an electron of an inner orbit in causing its liberation.
A high energy quantum of electromagnetic radiation causes electron back-rush to move through matter with initially high kinetic energy, and then this energy is passed along the electron paths in production of a multiplicity of ion pairs. The number of such ion pairs per unit length of path, specific ionization, increases as the kinetic energy of electrons diminishes (Fig. 6).
FIGURE 6

In some cases, however, the full energy of the quantum is not utilized in ejection of an electron from an atom, and the residual energy is transformed into a new photon or quantum of lower potential energy. The new photon, in turn, produces another ion pair and undergoes repeated transformation until all of the energy is completely used up in production of ion pairs or thermal changes. Thus this process resulting from absorption of high energy quanta causes production of vast numbers of ion pairs in the matter acted on by the radiation.
When electrons are removed from the inner orbit of atoms, the vacancies are filled by electrons dropping in from outer energy levels, the consequence being, so far as atomic changes are concerned, a change in the number of valence electrons (Fig. 7). Such an action may change the atom, or molecule, and result in an atomic change such as the change from silver to chromium.
FIGURE 7

Whatever frequency of current used and whatever ray or rays used, the frequency of the current and the wave length of the ray or rays must harmonize so as to blend or synchronize with the natural frequency or wavelength of the substance treated so as to produce the synchronous action that will set up proper vibration. We might coin an expression and call this action a ‘super-ultrasonic reaction” between the frequency of current, the wavelength of the ray, and the vibratory rate of the substance treated to cause the desired catalytic reaction.
By applying combination of certain frequencies generated from specially constructed tubes and applying rays from other especially constructed tubes to the solution, a catalytic reaction is obtained similar to the action of mesons from cosmic rays on fission materials.
In the catalytic recovery of ionic minerals it must be remembered we are dealing with a dispersed material which nature has not yet metallized in the general sense as we know or recognize metals.
The ionic metals may not ionize with the other metal’s atoms to unite in a stable metallic form because the ionic metal formed again as a reaction product presents itself in its original form of ionic metal at the end of the reaction rather than becoming an actual part of the final reaction of becoming a stable metal.
Ionic potential is of the greatest importance. All matter possesses a natural rate of vibration (frequency). Ionic material has elasticity and gives off a natural frequency inherent to the natural rate of vibration of the substances and these vibrations propagate frequencies which take the form of a displacement of oscillations.
When a resilient substance is subject to strain and then set free, one of two things may happen; the substance may slowly recover from the strain and gradually obtain to its natural state, or the oscillating recoil will carry it past its position of equilibrium and cause the medium to execute a series of oscillations. If these oscillations become violent enough, disintegration and transformation will take place because the first flow of oscillations is succeeded by a backrush and the first discharge will over-run itself and severe recoil sets in. this can be proven mathematically.
When oscillations reach a certain frequency and intensity, inertia will assert itself and a stable material is produced. In the uniting of the ionic metallic atoms by the vibrating reaction, they have undergone physical changes by excitation. Disintegration and transformation of the atoms occur to form and unite with other metallic atoms of the same vibration. Molecules of metal which can be recognized as metalized matter result.
In other words, in studying what takes place in the laboratories of the Universe, science has found the uranium and radium series changing from uranium to radium, radium to radon and radium, A, B, C, C1, D (radio lead), radium E, F (polonium), and finally in the series into stable lead after having been isotopic lead (non stable) twice before in the natural process of change, i.e., aging.
By hastening nature’s process the time of change may be shortened from many years to a few days and low-grade ore matured into an ore of higher crystalline content.
In order to fully understand the theories behind Ionic Mining methods, it becomes necessary to state several theories and to accept them as fact. Without these statements to rely upon, any attempt at explaining or understanding the nature of this work must be wasted effort.
[ Illegible sentence, p. 26 ]

… it is necessary to accept the fact that all matter, regardless of its name or weight, is a rate of frequency, of vibration and nothing more. It must also be accepted that all these various types of vibration are electrical in nature and can be duplicated in harmonics and sonics with the proper equipment.
The very fact that in fission materials, such as uranium, a dissociation is taking place, which is indicative of the complexity of the internal structure of the atoms of such materials. It must be remembered that this complexity is also indicative of the entire universe, and that each atom is a miniature of the universe. One other fact must be remembered and accepted, that nature in the laboratories of the universe is constantly evolving and changing matter and energy, one into the other, that the energy of the universe is kinetic in nature and electrical in its action.
Let us consider the following as a representation of a formula in which A,B,C,D, represent the relative number of atoms of an element existing at any time during aging or transformation.
Let us represent by P the number of particles of the atoms of the element initially present both in their ionic and crystalline aging state. The original number of atoms, both ionic and crystalline, of the matter A deposited is taken at 100. the amount of the matter B crystalline is initially zero or zero plus and in this particular material case passes through a…
[ Illegible sentence, p. 27]

… In a similar way the amount of material C passes through a maximum in about three and one half times X after aging starts. After an interval of about 25 times X both B and C increase or decrease approximately to an exponential law with the time falling to half value in both cases or doubling in value and we will refer to this action as Y. Over the intervals considered, the amount of D increases steadily with time, very slowly at first and then with greater rapidity. The maximum is reached when B and C have fully aged. Finally the amount of D, the ionic matter, will increase or decrease exponentially with the time corresponding to a period of 25 times.
It has been found that the uranium atoms ‘age” in many ways and break up into all the elements from zinc (at. no. 30) to gadolinium (at. no. 64). Neutrons are also emitted in the process of aging. In one process, barium (at. no. 56) and krypton (at. no 36) may be formed as a pair (sum of the atomic numbers --- 92, that of uranium). In another process, lanthanum and bromine may be the aged product pair. Since neutrons are also produced in the aging process, these may serve to initiate the aging of other U235 nuclei. One of the reactions is U235 – n – Ba + Kr – xn. [? Possibly division symbol]
The aged products formed in this way are very unstable, having too many neutrons, and therefore a series of transformations proceed, giving off beta particles and gamma radiation until stable isotopes of other elements are produced.
An atom is capable of possessing kinetic energy and is also capable of absorbing internally more of this kinetic energy. When an atom is electrically balanced…
[ Illegible sentence, p. 28]

… because of their violent activity internally, and because of the dissociation observed, are therefore not considered to be stable elements. However, the fact is that no element is ever entirely stable. There is some dissociation in all elements, although in some the rate of change may be so slow as to escape observation.
Fe59 can be produced from Co59 + n = Fe59 – H.
Among the lighter elements, potassium and rubidium are faintly radioactive, emitting beta rays of very low penetrating power (half disintegration periods are respectively 1012 and 1011 years). These rays almost certainly come from the presence in these two alkali metals of traces of unstable isotopes of masses 40 and 86:
K40 -> b = Ca40
Rb86 -> b = 86 [? Illegible text]
In speaking of the transformation of mater, Sir Ernest Rutherford stated in his book Radiations from Radio-Active Substances (1930 edition):
“The bombardment of matter by swift moving alpha particles is a powerful method for the artificial transformation of the nuclei of a number of the ordinary elements. By this method one is able, not only to cause a disintegration of a nucleus with loss of mass but also in some cases to build up a nucleus of greater mass by the capture of the colliding alpha particle”.
Nature is continually evolving matter and forces, one element into another, an eternal process which is more noticeable to us in the heavier elements than in those of less complex structure nevertheless, all matter is in continual vibration or oscillation, and in these oscillations electrical potentials of the nucleus of the atom are lessened or increased until the proper multiplicity of potentials are obtained to allow voluntary dissociation. When this occurs, then the electrical balance of the atom is disturbed to such an extent that certain things must happen. Either the mass of the particle must be replaced by other mass of the same electrical potential, or the internal; structure of the atom must undergo change to compensate for this imbalance. However, this process of rebalancing may take place instantly, or it may stretch over a period of hundreds of millions of years. In the cases of the metals we are concerned with, the process is slow, and atoms which have given up one or more electrons or electrical potential particles may be said to be ionized. Metals in such ionized isotopic form do not respond to conventional methods of recovery or assay. The logical solution to the problem of recovery of these unfinished elements is to copy nature. Observe what nature does and then devise the means to give nature a boost. In the mater of metals, attention must be paid to the crystals of these metals. A crystal of metal must be regarded as a molecule of metal because of the electronic binding of the atoms. If the binding of the atoms into a crystal is accomplished by electrons, and the atoms complete in their electrical potential, then the crystal is metallic in form and responds to conventional methods of treatments or recovery methods. The problem becomes one of obtaining a proper electrical balance…
[Illegible sentence, p. 30]

… respond to conventional methods of treatment.
Now under the process for the recovery of ionic uranium or other metals there are two possibilities of theories as to what actually happens inside the atom under this recovery process. It is unknown whether the atom responds best when it is in the before or in the after stage of electronic excitation. This means that the conversion from isotopic ionic form to metallic form may be caused by generating excitation in the interior of the atom so as to cause the escape of particles or it may be caused by creating such potentials and electrical balances in the atom so as to cause additional particles to become attracted and absorbed by the atom to gain its electrical balance. Whichever may be the case, results are obtained when certain types of frequencies are imposed upon the atom under the influence of specific types of catalysts. In view of the above two points of view as to what happens, the material may become heavier and gain weight, or it may become lighter as it loses particles. However, these gains or losses, as the case may be, taken on an atomic scale are not directly attributable to the addition of any materials to the raw ores during the treatment. The recovery percentages depend entirely upon the amount of isotopic ionized material present in the raw ores. One ton of material does in for treatment, and one tone of material relatively speaking is recovered with the process.
In natural phenomena an alpha particle strikes a nucleus head-on only once in many millions of times. By properly controlled arrangements these head-on collisions can be made to occur artificially and these head-on collisions will cause a complete dissociation of the atom and a complete change or aging of the atomic structure of the ionic mineral. Potentials of many millions of volts may be acquired naturally by these head-on collisions, without the use of the Cyclotron.
Elements are being formed in the stars; then why not artificially by duplicating nature?
In the application of an electrostatic and wave or frequency charge in a chemical catalyst the crystalline structure can be changed to such an extent as to result in almost a complete change of the appearance of individual molecules. It is known that certain liquids under x-rays give evidence of structure resembling crystals. A study of Bragg’s Law may be helpful to grasp the change here. Revision of the change to a definite time of change will result in a change of charge which will result in an alteration of the characteristics of the crystal.
Matter differs in construction only as it differs in rate of frequency, accounting for its atomic weight. The proton, positron, neutron, meson, neutrino and ion are all particles of matter. This is true of alpha and beta particles. Alpha particles are simply helium carrying a double positive charge effected from certain nuclei with very high velocity. Changing the force of any one of the binding forces of the atom changes the crystalline structure.
“There can be no doubt that the stars do serve as reactors in which elements are produced. But not all stars do so in the same manner or with the same efficiency. Those of the Sun’s type are relatively inactive, though even they ‘cook’ the heavy elements on a small scale” (cook, produce, grow, age).
The nature of the chemical bonds which an atom can form is determined by the electronic structure of the atom. By the application of rays and frequencies in an electromagnetic and electrostatic field one element can be changed into another element by such an application of energy. This may more readily be accomplished when the elements are in their ionic stage.
It was pointed out by Shiester in 1903 that every physical property of one element has been found to be shared by all the other elements, in varying degrees (rates of frequency or vibration).
Here is an example of “aging” within nature:
Radioactive elements are widely disseminated throughout the surface of the earth and traces of active matter will be separated with all metals. Take the experiments with lead, the most active of all common materials. With radium present in the ores from which the lead is prepared the product radium “1” will be separated with the lead; the alpha particle activity of such lead will be due to the subsequent product radium F or polonium which has been produced artificially. Now owing to the decay (aging) of radium D (259 years is required) by nature, the activity of the lead will decrease in this aging. Old lead is much less active than new lead. Please note: old lead, new lead, old formations, new formation; ionic uranium is a new formation, metal uranium is old formation. The evolution of matter, aging or call it what you will, all can be done artificially just as it is done by nature.
Capture or loss of electrons by alpha particles is one example of the process of this evolution.
In 1920, would any orthodox scientist dare to have said that it was possible to separate U235 from U238, changing U238 into U239 and U239 into the new element Neptunium, which in turn undergoes beta aging into another new element Plutonium? Aging from isotopic ionic crystals is possible when we acknowledge that aging from one form of uranium to another and finally into lead is taking place all the time in nature. Nature can be copied and her process duplicated in a few weeks, which nature takes hundreds of years to accomplish.
The conclusion reached after years of research in analyzing the nature, character, and commercibility of values existing in these forms of mineral deposits in clays, sands, and various earths proves, beyond a shadow of a doubt, that herein lie the true and greatest mineral values of the world. Although they are not always determinable by commercial assayers, their capture by properly developed frequency and oscillatory action, and recovery in highly commercial quantities is assured.
The greatest source of mineral wealth lies, not in the old method of seeking the fast depleting metallic form of metals but in taking the abundant supplies of the isotopes of the metals to be found practically everywhere in their ionic form and processing this material into their stable metallic form.
This is not a refining method at all. The silicate sands and foreign matter are not removed in this process. This can be done later by regular processes. These experiments are aimed at finding a quick, cheap method of changing ionic isotopes of uranium bearing materials into uranium oxide crystals which will respond to conventional methods. By this process we cut down nature’s time of changing ionic isotopes of minerals into fusible metals from hundreds of thousands of years to a few days, just as wine and beers may now be aged in a fraction of the time which used to be required by the slow, natural oxidation processes.
What practical results can accrue to the uranium industry as a result? An increased yield of several hundred percent has been realized in the laboratory. Samples of very low grade ores, which ran less than 1/2 of one percent before treatment, by the ionic isotope recovery method produced 20 to 30 percent uranium.
The following is a report by the United States Department of the Interior, Bureau of Mines:
“Radioactive Sample Report (September 28, 1956); Sample Reference Number UP 6234-4”
“Radiometric Analysis gave:
No. 1 --- 21.06 % U3O8 equivalent
No. 2 --- 18.82 % “ “
No. 3 --- 27.94 % “ “
No. 4 --- 18.22 % “ “
“Sample No. 1 and sample No. 2 contain a fibrous flat substance which resembled zeolite minerals or volcanic glass. The material is isotopic with a refractive index which varies from 1.50 to 1.52. this substance was not identified and might be an artificial product.”.
The above samples were less than 0.09% U3O8 before processing by the Ionic Isotope process. A sample of 30% U3O8 was processed, and it was reported by the United States Atomic Energy Commission, grand Junction, Colorado, as follows:
“The sample appears to be a fused or reduced uranium dioxide and will probable assay between 75 and 85 % U3O8”.
It has also been reported to us that “Dr Marven (AEC) has said that no ore of the type brought in exists in the United States except by the process of aging --- which you seem to have been able to do”.
A sample of ore was taken at random and the chemical assay was 0.047 % U3O8 and this sample processed with the following results:
Before processing, chemical analysis 0.047 % U3O8
Partially, processed, chemical analysis 4.720 % U3O8
Process completed, chemical analysis 21.947 % U3O8
Although scores of chemical assays relative to ionic mining have been run by commercial assayers and certificates of assays obtained from 1940 to date, it was not until 1956 that it was demonstrated that isotopic ionic recovery could be done commercially and only recently has the data been compiled.
Finally, what has been done? Let us answer this by giving a history of the test and the result. (Only a limited number of tests will be mentioned to save time and space). Variations in the results are due to variations in ores.
March 1956. AEC assay report:
Before treatment // After treatment
1. 2.6% // 67.22%
2. 1.11% // 54.97 %
3. 0.09% // 53.44 %
4. 0.04% // 30.08 %
May 11, 1956. AEC reported sample appeared to contain “75 and 85 % U3O8”.
August 8, 1956. Crisman and Nichols:
Before treatment // After treatment
0.02% // 5.949
Sept. 28, 1956. US Dept. of the Interior. Radiometric Assay after treatment. See Report U.P. 6234-4.
Sept. 9, 1957. Black and Deason:
Before: 0.12% // After: 7.03%
April 29, 1958. Crisman and Nichols
Before treatment: 0.047 %
Step #1. May, 19158 --- 4.72 %
Step #2. May 16, 1958 --- 5.288 %
Step #3. May 16, 1958 --- 21.945 %
Step #4. Dec. 17, 1958 --- 54.48 %
The following are successive runs on ore assayed before treatment by Crisman and Nichols, Dec. 17, 1958 as 4.59 % U2O5 (one pound of ore):
After treatment (Feb. 17, 1959): 23.810 % U3O8
March 10, 1959 --- 19.584 %
March 11, 1959 --- 32.022 %
March 11, 1959 --- 51.408 %
What is now the conclusion we arrive at from the above technical findings? It can be summed down to two points: 1 --- ore of no commercial values can be made of commercial values; 2 --- ore of low commercial values can be made highly valuable. Example: take the ore referred to in a former report and assayed April 29, 1958. This ore, before ionic isotopic processing, had no value, assaying at 0.047 % U3O8; after the first step of treatment this same ore assayed, May 1, 1958, 4.72 %.
Referring to schedule of prices for uranium ore as specified in the U.S. AEC circular #5 revised and circular #6, this ore is worth, including all bonuses paid, about $788 per ton. After the second stage of treatment this same ore assayed at 21.94 % U3O8.
In the AEC schedule of prices referred to above they only list prices on 10 % U3O8. therefore if this 21 % ore is sold at twice the value of 10 % ore, this ore would be worth about $3,300 per ton, and the cost for processing about $230 per ton.
The average upgrading “aging” results of all U3O8 bearing ores we have worked with to date have been well over an increase in values to 10 % U3O8 content.
Remember, this is not a refining process; no silicates or sands are removed by this process. This can be done later by conventional methods now in use. This is strictly an aging process as an aid or hastening of nature’s process as has been explained.
References
1. “Element Formation in the Stars”; Sky and Telescope, July 1956-July 1957
2. See Sky and Telescope, page 241.
3. USA AEC Reactor Handbook, Physics, 1955, Spontaneous Reactions.
4. Linus Pauling: General Chemistry
5. University of Nevada Bulletin, Vol. 254.
6. Readers Digest, June 1955, page 121.
7. Sir Ernest Rutherford: Radiations from Radioactive Substances.
8. Lapp and Andrews: Nuclear Radiation Physics.
9. Horace G. Deming: Fundamental Chemistry.
10. Latimer and Hildebrand: Reference Book of Inorganic Chemistry.
11. T.R. Hogness and Warren C. Johnson: Ionic Equilibrium.
12. Albert Einstein and Leopold Infeld: The Evolution of Physics.
13. Eisenbud and Wigner: Nuclear Structure.
14. Slatter: Quantum Theory of Matter.
15. Halliday: Introductory Nuclear Physics.
