The Observer, 7 January 2012
Hopes rise for new cancer treatment after tests with
electromagnetism
by Robin McKie
by Robin McKie
Scientists have used low-intensity electromagnetic fields to treat cancer patients in trials which they say could lead to the development of a new type of anti-tumour therapy.
Patients hold a spoon-shaped antenna in their mouths to deliver a very low-intensity electromagnetic field in their bodies. In trials of patients with advanced liver cancer, the therapy – given three times a day – resulted in long-term survival for a small number of those monitored, the team has reported in the British Journal of Cancer. Their tumours shrank, while healthy cells in surrounding tissue were unaffected.
However, the scientists – from the US, Brazil, France and Switzerland – also stressed that the technique was still in its infancy and would require several years for further trials to take place. "This is a truly novel technique," said the team's leader, Professor Boris Pasche of the University of Alabama, Birmingham. "It is innocuous, can be tolerated for long periods of time, and could be used in combination with other therapies."
Pasche added that he had obtained permission from the US Food and Drug Administration to carry out trials on large groups of patients and was talking to companies in the US, Asia, South America, Russia and Europe about raising funds for future research.
In 2009, Pasche and his colleagues published results in the Journal of Experimental and Clinical Cancer Research which showed that low-level electromagnetic fields at precise frequencies – ranging from 0.1Hz to 114kHz – halted cancer cell growth in small numbers of patients. Different cancers responded to electromagnetic fields of different frequencies. Cells in surrounding, healthy tissue were unaffected.
The exact mechanism for this process was not explained in the paper. However, results of recent experiments by the team – using cancer cell cultures in the laboratory and published in the British Journal of Cancer – suggest that low-level electromagnetic fields interfere with the activity of genes in cancer cells. In specific cases, this affected the ability of cancer cells to grow and divide. The spread of tumours halted and in some cases they began to shrink.
"This is extremely exciting," said Pasche. "We think the technique could also be used to treat breast tumours and possibly other forms of cancer."
The use of electromagnetic fields was also welcomed, cautiously, by Eleanor Barrie of Cancer Research UK: "This research shows how specific low frequencies of electromagnetic radiation can slow the growth of cancer cells in the lab. It's still unclear why the cancer cells respond in this way, and it's not yet clear if this approach could help patients, but it's an interesting example of how researchers are working to find new ways to home in on cancer cells while leaving healthy cells unharmed."
The use of electromagnetic fields to treat tumours may seem surprising given recent controversy over claims that fields generated by mobile phones and electricity pylons can trigger cancers and leukaemia. However, Pasche stressed that the intensity of the fields used in his team's experiments were between 100 and 1,000 times lower than those from a mobile phone. "In any case, the evidence produced from major studies of users of these phones does not suggest there is a clearly identifiable risk posed by these electromagnetic fields," he said.
EP1974769
MX2009010425
ELECTRONIC SYSTEM FOR INFLUENCING CELLULAR FUNCTIONS IN A WARM-BLOODED MAMMALIAN SUBJECT.
MX2009010425
ELECTRONIC SYSTEM FOR INFLUENCING CELLULAR FUNCTIONS IN A WARM-BLOODED MAMMALIAN SUBJECT.
Inventor: PASCHE BORIS & BARBAULT ALEXANDRE
EC: A61N1/40 // A61N5/02
IPC: A61N1/40 // A61N5/00
Disclosed is an electronic system activatable by electrical power. The system is useful for influencing cellular f unctions or malfunctions in a warm-blooded mammalian subject. The system comprises one or more controllable low energy H F (High Frequency) carrier signal generator circuits, one or more data processors or integrated circuits for receiving control information, one or more amplitude modulation control generators and one or more amplitude modulation frequency control generators. The amplitude modulation frequency control generators are adapted to accurately control the frequency of the amplitude modulations to within an accuracy of at least 1000 ppm, most preferably to within about 1 ppm, relative to one or more determined or predetermined reference amplitude modulation frequencies.
FIELD OF THE INVENTION
[0001] This invention relates to an electronic system for influencing cellular functions in a warm-blooded mammalian subject. More particularly, the invention concerns research findings related to how earlier electronic systems may be modified to achieve both improved and additional therapeutic effects.
BACKGROUND OF THE INVENTION
[0002] Reference is made to European Patent EP 0 592 851 B1 and corresponding Patents and Patent Applications and to the various publications referred to therein. Since the time of the priority Application filed in the USA on 25 September 1992 ( US Serial No 951563 now USP 5,441,528 ), a number of further publications related to effects of electromagnetic fields on patients suffering from insomnia and/or anxiety disorders have taken place:
Koziol JA, Erman M, Pasche B, Hajdukovic R, Mitler MM (1993) Assessing a changepoint in a sequence of repeated measurements with application to a low-energy emission therapy sleep study. J Applied Statistics 20: 393-400
Amato D, Pasche B (1993) An evaluation of the safety of low energy emission therapy. Compr Ther 19: 242-247
Higgs L, Reite M, Barbault A, Lebet JP, Rossel C, Amato D, Dafni U, Pasche B (1994) Subjective and Objective Relaxation Effects of Low Energy Emission Therapy. Stress Medicine 10: 5-13
Reite M, Higgs L, Lebet JP, Barbault A, Rossel C, Kuster N, Dafni U, Amato D, Pasche B (1994) Sleep Inducing Effect of Low Energy Emission Therapy. Bioelectromagnetics 15: 67-75
Lebet JP, Barbault A, Rossel C, Tomic Z, Reite M, Higgs L, Dafni U, Amato D, Pasche B (1996) Electroencephalographic changes following low energy emission therapy. Ann Biomed Eng 24: 424-429
Pasche B, Erman M, Hayduk R, Mitler M, Reite M, Higgs L, Dafni U, Amato D, Rossel C, Kuster N, Barbault A, Lebet J-P (1996) Effects of Low Energy Emission Therapy in chronic psychophysiological insomnia. Sleep 19: 327-336
Kelly TL, Kripke DF, Hayduk R, Ryman D, Pasche B, Barbault A (1997) Bright light and LEET effects on circadian rhythms, sleep and cognitive performance. Stress Medicine 13: 251-258
Pasche B, Barbault A (2003) Low-Energy Emission Therapy: Current Status and Future Directions. In Bioelectromagnetic Medicine, Rosch PJ, Markov MS (eds) pp 321-327. Marcel Dekker, Inc.: New York, New York .
[0003] The above publications are related to an earlier device, system and use thereof described in said EP 0 592 851 B1 . The improved electronic system and control thereof in accordance with the present invention, however, has been determined to find therapeutic application not only for influencing cellular functions (or malfunctions) leading to CNS disorders, but also for influencing other cellular functions (or malfunctions) including particularly directly or indirectly influencing cancerous cell growth or proliferation thereof in warm-blooded mammalian subjects. The direct or indirect influence on cancerous cell growth may involve any of prophylactic avoidance of cancerous cell formation, influencing of cell functions such as influencing leukocyte cell functions which can lead to inhibition of cancerous cell growth or proliferation thereof, or killing of cancerous cells harboured by a warm-blooded mammalian subject.
[0004] Electromagnetic energy generating devices and use of electromagnetic energies for treating living mammalian subjects harbouring cancerous cells described in the literature include: USP 5,908,441 issued June 1, 1999 to Bare; James E. and the references cited therein and so-called "NovoCure technology" involving in vivo implantation of electrodes to either side of tumorous growths.
SUMMARY OF THE INVENTION
[0005] According to invention, an electronic system is provided which is activatable by electrical power. The system is employed to influence cellular functions or malfunctions in a warm-blooded mammalian subject. The system comprises one or more controllable low energy electromagnetic energy generator circuits for generating one or more high frequency carrier signals. One or more microprocessors or integrated circuits comprising or communicating with the one or more generator circuits are provided which are also for receiving control information from a source of control information. The one or more generator circuits include one or more amplitude modulation control signal generators for controlling amplitude modulated variations of the one or more high frequency carrier signals. The one or more generator circuits furthermore include one or more programmable amplitude modulation frequency control signal generators for controlling the frequency at which the amplitude modulations are generated. The one or more amplitude modulation frequency control generators are, in terms of the present invention, adapted to accurately control the frequency of the amplitude modulations to within an accuracy of at least 1000 ppm relative to one or more determined or predetermined reference amplitude modulation frequencies selected from within a range of 0.1 Hz to 50 kHz. The system furthermore comprises a connection or coupling position for connection or coupling to or being connected or coupled to an electrically conductive applicator for applying to the warm-blooded mammalian subject the one or more amplitude-modulated low energy emissions at said accurately controlled modulation frequencies.
[0006] The term, "accurately controlled" means that the modulated low energy electromagnetic emissions should be modulated to within a resolution of at most 1 Hz of an intended higher frequency (greater than about 1000 Hz) determined or predetermined modulation frequencies. For example, if one of the one or more determined or predetermined modulation frequencies to be applied to the warm-blooded mammalian subject is 2000 Hz, the accurate control should lead to such modulated low energy emission being generated at a frequency of between 1999 and 2001 Hz. However, and in terms of what has been determined from experiences in treating human subjects harbouring cancerous cells with the aim of arresting proliferation or killing of such cells, the accurate control should lead to a resolution of 0.5, preferably 0.1, more preferably 0.01 and indeed most preferably 0.001 Hz of the intended determined or predetermined modulation frequency.
[0007] It is furthermore of importance that the stability of the emissions is maintained during emission, and that such stability should be of the order of 10<-5> , preferably 10<-6> , and more preferably 10<-7> , stability being the relative deviation of frequency divided by the desired frequency, e.g. 0,01 Hz (deviation) / 1'000 Hz (desired freq.) = 10<-5> .
[0008] As already described in said EP 0 592 851 B1 , the system includes a microprocessor (which may more recently be replaced by an integrated circuit) into which control information is loaded from an application storage device. The microprocessor (or now alternatively integrated circuit) then controls the function of the system to produce the desired therapeutic emission. Also described is the provision in the system of an impedance transformer connected intermediate the emitter of low energy electromagnetic emissions and a probe (here more broadly described as an electrically conductive applicator) for applying the emissions to the patient. The impedance transformer substantially matches the impedance of the patient seen from the emitter circuit with the impedance of the output of the emitter circuit.
BRIEF DESCRIPTION OF THE DRAWINGS
[0009]
FIG. 1 shows an exemplary casing structure for the electronic circuit shown in Figure 2, an applicator 13 (exemplified as a probe suitable for being placed in the mouth of a patient) and an interface 16 (which may be replaced by a receiver) for receiving information from a source of information 52 such as may be comprised in an information storage device, e.g. of the nature described and illustrated in Figures 12 to 17 of EP 0 592 851 B1 .
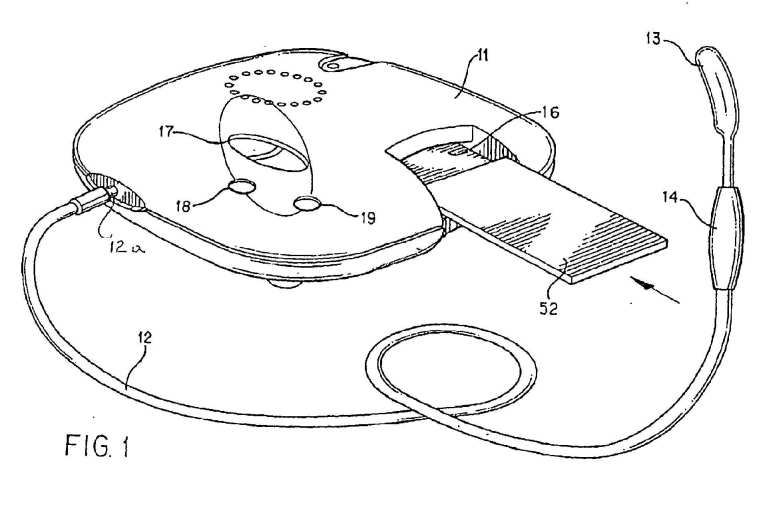
FIG. 2 is a block diagram of exemplary circuitry which may be comprised in the exemplary casing structure of FIG. 1. This Figure 2 differs essentially from Figure 2 of EP 0 592 851 B1 by comprising a highly accurate modulation frequency generator 31 (named a Digital Direct Synthesizer or DDS), which enables accurate control of modulatable oscillator represented by dotted line block 106.

[0010] Reference is made to the various Figures of EP 0 592 851 B1 and the detailed description thereof, a number of which are exemplary of components which may be comprised in the circuit of Figure 2.
[0011] Thus, Figure 3 of EP 0 592 851 B1 is a detailed schematic of a modulation signal generator 31, replaced by a DDS modulation frequency generator 31 comprised in the circuit of present Figure 2;
[0012] Figure 4 of EP 0 592 851 B1 is a detailed schematic of a modulation signal buffer and carrier oscillator circuit which may be employed in the circuit of the present FIG. 2;
[0013] FIG. 5 of EP 0 592 851 B1 is a detailed schematic example of an amplitude modulation (AM) and power generator 34 and output filter 39 which could be comprised in the circuit of the present FIG. 2;
[0014] FIG. 6 of EP 0 592 851 B1 is a detailed schematic example of an impedance transformer 14 which may be comprised in the circuit of the present FIG. 2;
[0015] FIG. 7 of EP 0 592 851 B1 is a detailed schematic example of an emission sensor 53 which may be comprised in the circuit of the present FIG. 2;
[0016] FIG. 8 of EP 0 592 851 B1 is a detailed schematic example of an output power sensor circuit 54 which may be employed in the circuit of the present FIG. 2.
[0017] FIG. 9 of EP 0 592 851 B1 is a detailed schematic example of a display module or information output 17 which may be included in the circuit of the present FIG. 2.
[0018] FIG. 10 of EP 0 592 851 B1 is a detailed schematic example of a power supply control circuit including battery charger 57 which may be comprised in the circuit of the present FIG. 2.
[0019] FIGS. 11 a-d of EP 0 592 851 B1 are exemplary flow charts of the method of operation of the system of FIG's 1 and 2.
DETAILED DESCRIPTION
[0020] Referring to FIG. 1, presented is a modulated low energy electromagnetic emission application system 11, in accordance with the present invention. As described in prior U.S. Pat. Nos. 4,649,935 and 4,765,322 , such a system has proven to be useful in the practice of Low Energy Emission Therapy (LEET, a trademark of Symtonic S.A. or a successor of this Company), which involves application of emissions of low energy radio frequency (RF) electromagnetic waves to a warm-blooded mammalian subject. The application has proven to be an effective mode of treating a warm-blooded mammalian subject suffering from central nervous system (CNS) disorders such as, for example, generalized anxiety disorders, panic disorders, sleep disorders including insomnia, psychiatric disorders such as depression, obsessive compulsive disorders, disorders resulting from substance abuse, sociopathy, post traumatic stress disorders or other disorders of the central nervous system.
[0021] The system includes an electrically conductive applicator 12,13 for applying one or more electromagnetic emissions to the warm-blooded mammalian subject. One form of applicator consists of a probe or mouthpiece 13 which is inserted into the mouth of a subject undergoing treatment. Probe 13 is connected to an electromagnetic energy emitter (see also FIG. 2), through coaxial cable 12 and impedance matching transformer 14.
[0022] It has previously been considered that an efficient connection of an electrically conductive applicator to a subject could only be achieved by means of a probe which is adapted to be applied to any mucosa of the subject, such as by being located within oral, nasal, optical, urethral, anal, and/or vaginal cavities or surfaces. It has however now been determined that in fact satisfactory application of emissions to a patient can be achieved by simpler physical contact of the electrically conductive applicator with the skin of the patient. Emissions to the patient may, for example be achieved by a conductive, inductive, capacitive or radiated coupling to the patient. An example of a coupling found to be effective involving indirect physical contact with the skin of a patient, is an insulated applicator to be placed over or within an ear of the patient. The emissions thus passed to the patient may be both by capacitive and radiated means. An important advantage of a device which does not need to be placed in the mouth of a patient is that the patient is able to speak clearly during a time of treatment. The treatment is accordingly more user-friendly and leads to enhanced patient compliance.
[0023] Electronic system 11 also includes a connector or coupler for connection to a programmable device such as a computer or an interface or receiver 16 which is adapted to receive an application storage device 52 such as, for example, magnetic media, semiconductor media, optical media or mechanically encoded media, or programmed emissions programmed with control information employed to control the operation of system 11 so that the desired type of low energy emission therapy is applied to the patient.
[0024] Application storage device 52 can be provided with a microprocessor which, when applied to interface 16, operates to control the function of system 11 to apply the desired low energy emission therapy. Alternatively, application storage device 52 can be provided with a microprocessor which is used in combination with microprocessor 21 within system 11. In such case, the microprocessor within device 52 could assist in the interfacing of storage device 52 with system 11, or could provide security checking functions.
[0025] System 11 also includes a display 17 which can display various indications of the operation of system 11. In addition, system 11 includes on and off power buttons 18 and 19, optionally replaced by user interface 21A (refer to Figure 2).
[0026] Referring to FIG. 2, presented is a block diagram of exemplary electronic circuitry of system 11, in accordance with the present invention. A data processor, such as for example, microprocessor or integrated circuit 21, operates as the controller for electronic system 11, and is connected to control the various components of the system 11 through address bus 22, data bus 23 and input/output lines 25. The FIG. 2 is modified as compared to FIG.2 of EP 0 592 851 B1 by including what is known as a Digital Direct Synthesizer (DDS) 31 which operates as a highly accurate and stable modulation frequency generator within the system 11. An exemplary DDS device is available from Analog Devices of Norwood, MA 02062-9106, USA, Part No AD9835. The device is a numerically controlled oscillator and modulation capabilities are provided for phase modulation and frequency modulation. As represented by dotted line block 102, entitled "PROCESSOR WITH DAC", the functionality of the DDS may also be combined with microprocessor 21 with digital to analogue converter (DAC).
[0027] Microprocessor 21 preferably includes internal storage for the operation of a coded control program, and temporary data. In addition, microprocessor 21 includes input/output ports and internal timers. Microprocessor 21 may be a microcontroller, for example microcontrollers 8048 or 8051 available from Intel Corporation.
[0028] The timing for microprocessor 21 is provided by system clock oscillator 26A which may be run at any clock frequency suitable for the particular type of microprocessor used. An exemplary clock frequency is 8.0 MHz. Oscillator 26A may be replaced by reference frequency oscillator 26 which secures the stability of the accurate modulation frequency. RF (Radio Frequency) oscillator 32 may also be employed for this purpose. A combination of oscillators is represented by dotted line block 104, entitled "OSCILLATOR".
[0029] An exemplary operating program for microprocessor 21 is presented in flow chart form with reference to FIGS. 11 a-d of EP 0 592 851 B1 . In general, microprocessor 21 functions to control controllable electromagnetic energy generator circuit 29 to produce a desired form of modulated low energy electromagnetic emission for application to a subject through applicator or probe 13.
[0030] Dotted line block 29, entitled CONTROLLABLE GENERATOR, includes DDS modulation frequency generator 31 and carrier signal oscillator 32. Microprocessor 21 operates to activate or deactivate controllable generator circuit 29 through oscillator disable line 33, as described in greater detail in EP 0 592 851 B1 . Controllable generator circuit 29 also includes an AM modulator and power generator 34 which operates to amplitude modulate a carrier signal produced by carrier oscillator 32 on carrier signal line 36, with a modulation signal produced by modulation signal generator circuit 31 on modulation signal line 37. The combination of the functionality of the DDS modulation frequency generator 31, with processor 21 with DAC, represented by dotted line block102, enables output lines 33 and 37 to be combined to produce a single signal. The combination furthermore enables arbitrary or periodic wave forms of any shape to be generated, as similarly described in EP 0 592 851 B1 .
[0031] Modulator 34 produces an amplitude modulated carrier signal on modulated carrier signal line 38, which is then applied to emitter output filter circuit 39. The filter circuit 39 is connected to probe or applicator 13 via power emission sensor 54, coaxial cable 12 and impedance transformer 14.
[0032] Microprocessor 21 controls DDS modulation signal generator circuit 31 of controllable generator circuit 29 via interface lines 25.
[0033] As is illustrated and described in EU 0 592 851 B1 microprocessor 21 may select a desired waveform stored in a modulation waveform storage device 43 and also controls a waveform address generator 41 to produce on waveform address bus 42 a sequence of addresses which are applied to modulation signal storage device 43 in order to retrieve the selected modulation signal. In the embodiment described in EP 0 592 851 B1 , the desired modulation signal is retrieved from modulation signal storage device 43 and applied to modulation signal bus 44 in digital form. Modulation signal bus 44 is applied to wave form generator and Digital to Analog Converter (DAC) 46 which converts the digital modulation signal into analogue form. This analogue modulation signal is then applied to a selective filter 47 which, under control of microprocessor 21, filters the analogue modulation signal by use of a variable filter network including resistor 48 and capacitors 49 and 51 in order to smooth the wave form produced by DAC 46 on modulation signal line 20.
[0034] A further embodiment possibility is a combination of PROCESSOR WITH DAC dotted line block 102 with OSCILLATOR dotted line block 104 or with a combination of oscillators 26 and 26A. With such a combination, the hardware solution described in EP 0 592 851 B1 can be is realized internally in the processor 102 with multiple outputs 33 and 37 or a single output combining these signals.
[0035] The above embodiment from EP 0 592 851 B1 is in part replaced by the functionality of the DDS modulation frequency modulator 31. However, if it is determined that emissions of different wave forms is desirable, it would be desirable to include the modulation signal storage device 43 and wave form generator 46 described in EP 0 592 851 B1 . Various modulation signal wave forms may then be stored in modulation signal storage device 43. Wave forms that have been successfully employed include square wave forms or sinusoidal wave forms. Other possible modulation signal wave forms include rectified sinusoidal, triangular, or other wave forms and combinations of all of the above.
[0036] The particular modulation control information employed by microprocessor 21 to control the operation of controllable generator circuit 29, is stored in application storage device 52. The application storage device is conveniently a computer comprising or being for receiving the information. Alternatively, application storage devices illustrated and described in EP 0 592 851 B1 , with reference to FIGS. 12, 13, 14 and 15, may be selected.
[0037] Interface 16 is configured as appropriate for the particular application storage device 52 in use. Interface 16 translates the control information stored in application storage device 52 into a usable form for storage within the memory of microprocessor 21 to enable microprocessor 21 to control controllable generator circuit 29 to produce the desired modulated low energy emission.
[0038] Interface 16 may directly read the information stored on application storage device 52, or it may read the information through use of various known communications links. For example, radio frequency, microwave, telephone, internet or optical based communications links may be used to transfer information between interface or receiver 16 and application storage device or computer 52.
[0039] The system 11 may comprise a user identification device, included in by block 21a in Figure 2. Conveniently, such a device communicates with the one or more data processors or integrated circuits 21 via interface 16, as shown. The user identification device may be of any type, a finger print reader being an example. Such a reader is for example available from Lenovo, 70563 Stuttgart, Germany, Part No. 73P4774.
[0040] The control information stored in application storage device or computer 52 specifies various controllable parameters of the modulated low energy RF electromagnetic emission which is applied to a subject through applicator or probe 13. Such controllable parameters include, for example, the frequency and amplitude of the carrier, the amplitudes and frequencies and wave forms of the modulation of the carrier, the duration of the emission, the power level of the emission, the duty cycle of the emission (i.e., the ratio of on time to off time of pulsed emissions applied during a treatment), the sequence of application of different modulation frequencies for a particular application, and the total number of treatments and duration of each treatment prescribed for a particular subject.
[0041] For example, the carrier signal and modulation signal may be selected to drive the applicator or probe 13 with an amplitude modulated signal in which the carrier signal includes spectral frequency components below 1 GHz, and preferably between 1 MHz and 900 MHz, and in which the modulation signal comprises spectral frequency components between 0.1 Hz and 10 KHz, and preferably between 1 Hz and 1000 Hz. The one or more modulation frequencies may be simultaneously emitted or sequenced to form the modulation signal.
[0042] As an additional feature, an electromagnetic emission sensor 53 may be provided to detect the presence of electromagnetic emissions at the frequency of the carrier oscillator 32. Emission sensor 53 provides microprocessor 21 with an indication of whether or not electromagnetic emissions at the desired frequency are present. Microprocessor 21 then takes appropriate action, for example, by displaying an error message on display 17, disabling controllable generator circuit 29, or the like.
[0043] A power sensor 54 is preferably included which detects the amount of power applied to the subject through applicator or probe 13 compared to the amount of power returned or reflected from the subject. This ratio is indicative of the proper use of the system during a therapeutic session. Power sensor 54 applies to microprocessor 21 through power sense line 56 an indication of the amount of power applied to patient through applicator or probe 13 relative to the amount of power reflected from the patient.
[0044] The indication provided on power sense line 56 may be digitalized and used by microprocessor 21, for example, to detect and control a level of applied power, and to record on application storage device 52 information related to the actual treatments applied to and received by the patient. Such information may then be used by a physician or other clinician to assess patient treatment compliance and effect. Such treatment information may include, for example: the number of treatments applied for a given time period; the actual time and date of each treatment; the number of attempted treatments; the treatment compliance (i.e., whether the applicator or probe was in place or not during the treatment session); and the cumulative dose of a particular modulation frequency.
[0045] The level of power applied is preferably controlled to cause the specific absorption rate (SAR) of energy absorbed by the patient to be from 1 microWatt per kilogram of tissue to 50 Watts per kilogram of tissue. Preferably, the power level is controlled to cause an SAR of from 100 microwatts per kilogram of tissue to 10 Watts per kilogram of tissue. Most preferably, the power level is controlled to cause an SAR of from 1 milliWatt per kilogram of tissue to 100 milliWatts per kilogram of tissue. These SARs may be in any tissue of the patient, but are preferably in the tissue of the central nervous system.
[0046] System 11 also includes powering circuitry including battery and charger circuit 57 and battery voltage change detector 58.
[0047] The RF carrier oscillator 32 produces a Radio Frequency (RF) carrier frequency of 27 MHz. Other embodiments of the invention contemplate RF carrier frequencies of 48 MHz, 433 MHz or 900 MHz. In general, the RF carrier frequency produced by carrier oscillator 32 has spectral frequency components less than 1 GHz and preferably between 1 MHz and 916 MHz (which is the upper limit of the European 900 MHz band). Although the disclosed embodiment contemplates that once set, the carrier oscillator frequency remains substantially constant, the carrier frequency produced by carrier oscillator 32 may be variable and controllable by microprocessor 21 by use of stored or transmitted control information.
[0048] Carrier oscillator 32 produces on carrier signal line 36 a carrier signal which is then modulated by the modulation signal carried on signal line 37.
[0049] Oscillator disable line 33 enables microprocessor 21 to disable the signal from oscillator 32 by applying an appropriate disable signal to oscillator disable line 33.
[0050] The output of the AM modulator and power generator 34 appears on signal line 38. This modulated signal is applied through emitter output filter 39 which substantially reduces or eliminates the carrier harmonics resulting from side effects of the modulator and power generator circuit 34.
[0051] The output of the AM modulator and power generator 34 and emitter output filter 39 may be designed to possess a 50 Ohm output impedance to match a 50 Ohm impedance of coaxial cable 12.
[0052] It has been determined through impedance measurements that when a probe 13 is applied within the mouth of a subject, the probe/subject combination exhibits a complex impedance of the order of 150+j200 Ohms. Impedance transformer 14 serves to match this complex impedance with the 50 Ohm impedance of coaxial cable 12 and therefore the output impedance of the AM modulator 34 and output filter 39. This promotes power transmission, and minimizes reflections.
[0053] The arrangement described above has been optimized for a contact probe with coupling to the mucosa of the mouth. In a further example, a conductive, isolated probe has been used at a frequency around 433 MHz coupling to the outer ear channel. Due to the different probe design in such a frequency band and with this coupling method, the values of matching elements (79 and 81 described in EP 0592 851 B1 ) would be different or could even be omitted. Applicator or probe 13 may then be regarded as a capacitive coupler or as an antenna matched to the capacitive load.
[0054] As described in EP 0 592 851 B1 , with reference to the flow charts of FIGS. 11 a-d, microprocessor 21 may operate to analyse the signal appearing on power sense line 56 to determine and control the amount of power applied to the patient, and to assess patient treatment compliance, and possibly to record indicia of the patient treatment compliance on application storage device 52 for later analysis and assessment by a physician or other clinician.
[0055] Exemplary of treatments performed on patients have included breast, ovary, pancreas and liver tumour types. The treatments involved applying a 27.12 MHz RF signal, amplitude modulated at specifically defined frequencies ranging from 0.2 to 23,000 Hz at very high precision and stability.
[0056] The following are synopses of abstracts for future publications related to uses of electronic devices of the present invention:
A phase I study of therapeutic amplitude-modulated electromagnetic fields (THERABIONIC) in advanced tumors
[0057] Boris Pasche<1> , Alexandre Barbault <1> , Brad Bottger <2> , Fin Bomholt <3> , Niels Kuster <4> .
<1> Cabinet Médical de l'Avenue de la Gare 6, CH-1003-Lausanne, Switzerland.
<2> Danbury Hospital, Danbury, CT-06810.
<3> SPEAG, Zurich, CH-8004-Zurich, Switzerland
<4> IT'IS Foundation, Swiss Federal Institute of Technology, Zurich, Switzerland.
[0058] Background: In vitrostudies suggest that low levels of amplitude-modulated electromagnetic fields may modify cell growth. We have identified specific frequencies that may block cancer cell growth. We have developed the THERABIONIC device, a portable and programmable device delivering low levels of amplitude-modulated electromagnetic fields. The device emits a 27.12 MHz radiofrequency signal, amplitude-modulated at cancer-specific frequencies ranging from 0.2 to 23,000 Hz with high precision. The device is connected to a spoon-like coupler, which is placed in the patient's mouth during treatment.
[0059] Methods: We conducted a phase I study consisting of three daily 40 min treatments. From March 2004 to September 2006, 24 patients with advanced solid tumors were enrolled. The median age was 57.0 12.2 years. 16 patients were female. As of January 2007, 5 patients are still on therapy, 13 patients died of tumor progression, 2 patients are lost to follow-up and one patient withdrew consent. The most common tumor types were breast (7), ovary (5) and pancreas (3). 22 patients had received prior systemic therapy and 16 had documented tumor progression prior to study entry.
[0060] Results: The median duration of therapy was 15.7 19.9 weeks (range: 0.4-72.0 weeks). There were no NCI grade 2, 3 or 4 toxicities. Three patients experienced grade 1 fatigue during and immediately after treatment. 12 patients reported severe pain prior to study entry. Two of them reported significant pain relief with THERABIONIC treatment. Objective response could be assessed in 13 patients, 6 of whom also had elevated tumor markers. 6 additional patients could only be assessed by tumor markers. Among patients with progressive disease at study entry, one had a partial response for > 14.4 weeks associated with > 50% decrease in CEA, CA 125 and CA 15-3 (previously untreated metastatic breast cancer); one patient had stable disease for 34.6 weeks (add info); one patient had a 50% decrease in CA 19-9 for 12.4 weeks (recurrent pancreatic cancer). Among patients with stable disease at enrollment, four patients maintained stable disease for 17.0, > 19.4, 30.4 and > 63.4 weeks.
[0061] Conclusions: THERABIONIC is a safe and promising novel treatment modality for advanced cancer. A phase II study and molecular studies are ongoing to confirm those results.
A phase II study of therapeutic amplitude-modulated electromagnetic fields (THERABIONIC) in the treatment of advanced hepatocellular carcinoma (HCC)
[0062] Frederico P Costa<1> , Andre Cosme de Oliveira<1> , Roberto Meirelles Jr<1> , Rodrigo Surjan<1> , Tatiana Zanesco<1> , Maria Cristina Chammas<1> , Alexandre Barbault<2> , Boris Pasche<2> .
<1> Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil. <2> Cabinet Médical Avenue de la Gare 6, CH-1003-Lausanne, Switzerland
[0063] Background : Phase I data suggest that low levels of electromagnetic fields amplitude-modulated at specific frequencies administered intrabucally with the THERABIONIC device are a safe and potentially effective treatment for advanced cancer. The device emits a 27.12 MHz RF signal, amplitude-modulated with cancer-specific frequencies ranging from 0.2 to 23,000 Hz with high precision. The device is connected to a spoon-like coupler placed in the patient's mouth during treatment. Patients with advanced HCC and limited therapeutic options were offered treatment with a combination of HCC-specific frequencies.
[0064] Methods: From October 2005 to October 2006, 38 patients with advanced HCC were recruited in a phase II study. The patients received three daily 40 min treatments until disease progression or death. The median age was 64.0 14.2 years. 32 patients were male and 29 patients had documented progression of disease (POD) prior to study entry.
[0065] Results: As of January 2007, 12 patients are still on therapy, 20 patients died of tumor progression, 2 patients are lost to follow-up and 3 patients withdrew consent. 27 patients are eligible for response. The overall objective response rate as defined by partial response (PR) or stable disease (SD) in patients with documented POD at study entry was 31.6%: 3 PR and 9 SD. The median survival was 20.7 weeks with a median duration of therapy of 17.5 weeks. 13 patients have received therapy for more than six months. The median duration of response is 12.9 weeks. 12 patients reported pain at study entry: 8 of them (66%) experienced decreased pain during treatment. There were no NCI grade 2/3/4 toxicities. One patient developed grade 1 mucositis and grade 1 fatigue.
Patient characteristics (n = 38)
Cirrhosis 36
Portal vein thrombosis 9
Elevated AFP 25
Extra-hepatic metastases 12
Previous intrahepatic/systemic therapy 30
Previous hepatic resection/RFA or ethanol 8
CLIP 0/1: 12 2: 22
Okuda I: 14 II/III: 20
Child-Pugh A:15 B: 19
MELD Median: 10
Conclusion: In patients with advanced HCC THERABIONIC treatment is a safe and effective novel therapeutic option, which has antitumor effect and provides pain relief in the majority of patients.
[0066] The electronic device of the present invention, comprising means for the accurate control over the frequencies and stability of amplitude modulations of a high frequency carrier signal, provides a safe and promising novel treatment modality for the treatment of patients suffering from various types of advanced forms of cancer.
EXAMPLES























Method and system for applying low energy emission
therapy
US5441528
US5441528
BACKGROUND OF THE INVENTION
The invention relates to systems and methods for applying low energy emission therapy for the treatment of central nervous system disorders.
Low energy emission therapy involving application of low energy electromagnetic emissions to a patient has been found to be an effective mode of treating a patient suffering from central nervous system (CNS) disorders such as generalized anxiety disorders, panic disorders, sleep disorders including insomnia, circadian rhythm disorders such as delayed sleep, psychiatric disorders such as depression, obsessive compulsive disorders, disorders resulting from substance abuse, sociopathy, post traumatic stress disorders or other disorders of the central nervous system. Apparatus and methods for carrying out such treatment are described in U.S. Pat. Nos. 4,649,935 and 4,765,322, assigned to the same assignee as the present application, the disclosures of which are expressly incorporated herein by reference. Since the time of these earlier disclosures, a substantially greater understanding of the mechanisms of the treatment and how to secure best results has been gained, which has led to important developments being made to the apparatus (herein described as a system).
Although the apparatus and methods described in the above patents have provided satisfactory results in many cases, consistency and significance of results has sometimes been lacking. Also, it was not always possible to properly control or monitor the duration of treatment or the quantities or nature of the low energy emissions being applied to the patient. Furthermore, the efficiency of transfer of the low energy emissions to the patient was limited and was affected by such factors as patient movement, outside interference and the like.
Another limitation of the previously described apparatus is that it is not very amenable to ready marketing by marketing organizations specifically of the nature comprised in the pharmaceutical industry. The apparatus is intended for therapy or treatment of patients and the low energy emissions applied to the patient are akin to pharmaceutical medication. The marketing organization of a pharmaceutical industry should thus be placed in a position to market the therapy in a fashion not widely different from the fashion in which pharmaceutical products are marketed, e.g., through pharmacists, with or without a doctor's prescription.
Research on treatment for insomnia has lagged behind other medical research programs. Current treatment methods for insomnia consist either of hypnotics, behavioral therapies (e.g. biofeedback), or of the use of drug agents, specifically benzodiazepines or imidazopyridines. Tolerance, dependence, memory loss, and lack of efficacy in long-term treatment are among the most common drawbacks of these classes of currently available hypnotics.
Research throughout the past two decades has shown clearly that the brain serves not only as a communication link and thought-processing organ, but also as the source of significant chemical activity, as well as a number of bioactive compounds. Many of these neurotransmitter compounds and ions are secreted following chemical or electrical stimuli. Research has also shown that some of these neuroactive compounds are involved in the regulation of sleep and wake cycles (Koella, "The Organization and Regulation of Sleep," Experientia, 1984; 40(4): 309-408).
During the 1970s, Adey and his group demonstrated that weak electromagnetic fields, modulated at certain well-defined low frequencies, were able to modify the release of ions (calcium) and neurotransmitters (GABA) in the brain (Kaczmarek and Adey, "The Eflux of @45 Ca@2+ and [@3 H]y-aminobutyric Acid from Cat Cerebral Cortex," Brain Research, 1973; 63:331-342; Kaczmarek and Adey, "Weak Electronic Gradients Change Ionic and Transmitter Fluxes in Cortex," Brain Research, 1974; 66:537-540; Bawin et al., "Ionic Factors in Release of @45 Ca@2+ From Chicken Cerebral Tissue by Electromagnetic Fields," Proceedings of the National Academy of Science, 1978; 75(12):6314-6318). In these experiments the cortex of anaesthetized cats was initially incubated with radio-labeled calcium and radio-labeled GABA. When the cortex was exposed to continuous stimulation by weak electric fields modulated at 200 Hz, the researchers found a 1.29-fold increase in Ca++ and a 1.21-fold increase in GABA release (Kaczmarek and Adey, Brain Research, 1973; 63:331-342). Interestingly, the release of GABA happened in parallel with the release of Ca++, suggesting that the two phenomena are closely linked. The findings of increased Ca++ release from brain tissue upon stimulation with modulated electromagnetic fields have been replicated (Dutta et al., "Microwave Radiation Induced Calcium Ions Effused from Human Neuroblastoma Cells in Culture," Bioelectromagnetics, 1984; 5(1):71-78; and Blackman et al., "Influence of Electromagnetic Fields on the Efflux of Calcium Ions from Brain Tissue in Vitro," Bioelectromagnetics, 1988; 9:215-227). It now has become an established fact that weak electric fields modulated at certain low frequencies are able to modulate the release of Ca++ and GABA.
During 1983, it was discovered that weak electromagnetic fields, modulated at low frequencies and delivered by means of an antenna placed in the buccal cavity, caused changes in EEG readings in human volunteers. In agreement with the findings of Adey and Blackman, it was found that only certain well-defined low frequency modulations of a standard carrier frequency (27 MHz), emitted with a well-defined intensity, were capable of eliciting EEG changes.
SUMMARY OF THE INVENTION
The present invention has rendered feasible an entirely new approach to treatment of a patient described in our said earlier patents while avoiding the above-noted drawbacks.
The present invention contemplates provision in the system (apparatus) of an interface for an application storage device, which application storage device can comprise storage media, such as, magnetic storage media, semiconductor memory storage media, optical memory storage media, or mechanical storage media. The selected storage media is programmed to carry various control information. Other information which may be stored in the storage media includes duration control information which would control the duration of the low energy electromagnetic emission and hence the duration of the application of the emission to the patient. Further control information can include duty cycle control information which would control the emissions, for example, in such a fashion that the low energy emission is alternately discontinued and re-initiated for chosen periods of time. Yet further control information which may be programmed into the storage media includes selecting information which would select emissions of various different modulation waveforms and frequencies which emissions can be emitted sequentially, with or without pauses between the emissions. Still further control information that may be programmed into the storage media includes power level control information.
In one embodiment of the invention, the system includes a microprocessor into which is loaded control information from the application storage device. The microprocessor then controls the function of the system to produce the desired therapeutic emission.
Another embodiment of the present invention contemplates that the application storage device would be combined into a single unit, and would be connected to the system through an interface in order to control the system.
In either of these embodiments, the present invention contemplates that the interface may include a communications channel such as, for example, a radio frequency link or telephone line, which connects the application storage device to the rest of the system.
The present invention also contemplates provision in the system of an impedance transformer connected intermediate the emitter of low energy electromagnetic emissions and a probe for applying the emissions to the patient, which impedance transformer substantially matches the impedance of the patient seen from the emitter circuit with the impedance of the output of the emitter circuit.
Another aspect of the present invention is the provision of a power reflectance detector which detects an amount of power applied to a patient and compares that amount to an amount of power emitted by the system. The power detector permits the monitoring of patient compliance with the prescribed treatment. Such patient treatment compliance information may be stored on the application storage device for later retrieval and analysis. For example, the power detector may be used to detect the number of treatments applied to a particular patient, and the elapsed time for each treatment. Further, the actual time of day of each treatment may also be recorded, as may the number of attempted treatments.
These and other features and advantages of the present invention will become apparent to those of skill in this art with reference to the appended drawings-and following details description.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a system for applying modulated low energy electromagnetic emission to a patient, in accordance with the present invention.

FIG. 2 is a block diagram of the circuitry of the system of FIG. 1.

FIG. 3 is a detailed schematic of the modulation signal generator of the circuit of FIG. 2.

FIG. 4 is a detailed schematic of the modulation signal buffer and the carrier oscillator circuit used in the circuit of FIG. 2.

FIG. 5 is a detailed schematic of the AM modulation and power generator and output filter of the circuit of FIG. 2.

FIG. 6 is a detailed schematic of the impedance transformer of the circuit of FIG. 2.

FIG. 7 is a detailed schematic of the emission sensor circuit of the circuit of FIG. 2.

FIG. 8 is a detailed schematic of the output power sensor circuit used in the circuit of FIG. 2.

FIG. 9 is a detailed schematic of the display module used in the circuit of FIG. 2.

FIG. 10 is a detailed schematic of the power supply circuit used in the circuit of FIG. 2.

FIGS. 11 a-e are flow charts of the method of operation of the system of FIG. 1 and 2, in accordance with the present invention.





FIGS. 12, 13, 14, 15, 16 and 17 are examples of an application storage device for use with the present invention.






DETAILED DESCRIPTION
Referring to FIG. 1, presented is a modulated low energy electromagnetic emission application system 11, in accordance with the present invention. As presented in prior U.S. Pat. Nos. 4,649,935 and 4,765,322, such a system has proven useful in the practice of Low Energy Emission Therapy (LEET, a trademark of the assignee of the present application), which involves application of emissions of low energy radio frequency (RF) electromagnetic waves and which has proven an effective mode of treating a patient suffering from central nervous system (CNS) disorders such as, for example, generalized anxiety disorders, panic disorders, sleep disorders including insomnia, psychiatric disorders such as depression, obsessive compulsive disorders, disorders resulting from substance abuse, sociopathy, post traumatic stress disorders or other disorders of the central nervous system. The system includes a probe or mouthpiece 13 which is inserted into the mouth of a patient under treatment. Probe 13 is connected to an electromagnetic energy emitter (see also FIG. 2), through coaxial cable 12 and impedance matching transformer 14. Although probe 13 is illustrated as a mouthpiece, any probe that is adapted to be applied to any mucosa may be used. For example, oral, nasal, optical, urethral, anal, and/or vaginal probes may be used without departing from the scope of the invention. Probes situated closer to the brain, for example endonasal or oral probes, are presently preferred.
Application system 11 also includes an interface 16 which is adapted to receive an application storage device 52 such as, for example, magnetic media, semiconductor media, optical media or mechanically encoded media, which is programmed with control information used to control the operation of system 11 to apply the desired type of low energy emission therapy to the patient under treatment.
As presented in more detail below, application storage device 52 can be provided with a microprocessor which, when applied to interface 16, operates to control the function of system 11 to apply the desired low energy emission therapy. Alternatively, application storage device 52 can be provided with a microprocessor which is used in combination with microprocessor 21 within system 11. In such case, the microprocessor within device 52 could assist in the interfacing of storage device 52 with system 11, or could provide security checking functions.
System 11 also includes a display 17 which can display various indications of the operation of system 11. In addition, system 11 includes on and off power buttons 18 and 19.
It will be understood that configurations of application system 11 other than that presented in FIG. 1, may be used without departing from the spirit and scope of the present invention.
Referring now to FIG. 2, presented is a block diagram of the electronic circuitry of application system 11, in accordance with the present invention. A data processor, such as for example, microprocessor 21, operates as the controller for application system 11, and is connected to control the various components of the system 11 through address bus 22, data bus 23 and I/O lines 25.
Microprocessor 21 preferably includes internal storage for the operation coded control program, and temporary data. In addition, microprocessor 21 includes input/output ports and internal timers. Microprocessor 21 may be, for example, an 8-bit single-chip microcontroller, 8048 or 8051 available from Intel Corporation.
The timing for microprocessor 21 is provided by system clock 24 which includes a clock crystal 26 along with capacitors 27 and 28. System clock 24 may run at any clock frequency suitable for the particular type of microprocessor used. In accordance with one embodiment of the present invention, system clock 24 operates at a clock frequency of 8.0 MHz.
The operating program for microprocessor 21 is presented below in flow chart form with reference to FIGS. 11 a-d. In general, microprocessor 21 functions to control controllable electromagnetic energy generator circuit 29 to produce a desired form of modulated low energy electromagnetic emission for application to a patient through probe 13.
Controllable generator circuit 29 includes modulation frequency generator circuit 31 and carrier signal oscillator 32. Microprocessor 21 operates to activate or de-activate controllable generator circuit 29 through oscillator disable line 33, as described below in more detail. Controllable generator circuit 29 also includes an AM modulator and power generator 34 which operates to amplitude modulate a carrier signal produced by carrier oscillator 32 on carrier signal line 36, with a modulation signal produced by modulation signal generator circuit 31 on modulation signal line 37.
Modulator 34 produces an amplitude moduated carrier signal on modulated carrier signal line 38, which is then applied to the filter circuit 39. The filter circuit 39 is connected to probe 13 via coaxial cable 12 and impedance transformer 14.
Microprocessor 21 controls modulation signal generator circuit 31 of controllable generator circuit 29 through address bus 22, data bus 23 and I/O lines 25. In particular, microprocessor 21 selects the desired waveform stored in modulation waveform storage device 43 via I/O lines 25. Microprocessor 21 also controls waveform address generator 41 to produce on waveform address bus 42 a sequence of addresses which are applied to modulation signal storage device 43 in order to retrieve the selected modulation signal. The desired modulation signal is retrieved from modulation signal storage device 43 and applied to modulation signal bus 44 in digital form. Modulation signal bus 44 is applied to digital to analog converter (DAC) 46 which converts the digital modulation signal into analog form. This analog modulation signal is then applied to selective filter 47 which, under control of microprocessor 21, filters the analog modulation signal by use of a variable filter network including resistor 48 and capacitors 49 and 51 in order to smooth the wave form produced by DAC 46 on modulation signal line 20.
In the present embodiment, the various modulation signal wave forms are stored in modulation signal storage device 43. With a 2 kilobyte memory, storage device 43 can contain up to 8 different modulation signal wave forms. Wave forms that have been successfully employed include square wave forms or sinusoidal wave forms. Other possible modulation signal wave forms include rectified sinusoidal, triangular, and combinations of all of the above.
In the present embodiment, each modulation signal wave form uses 256 bytes of memory and is retrieved from modulation signal storage device 43 by running through the 256 consecutive addresses. The frequency of the modulation signal is controlled by how fast the wave form is retrieved from modulation signal storage device 43. In accordance with the present embodiment, this is accomplished by downloading a control code from microprocessor 21 into programmable counters contained within wave form address generator 41. The output of the programmable counters then drives a ripple counter that generates the sequence of 8-bit addresses on the wave form address bus 42.
Wave form address generator 41 may be, for example, a programmable timer/counter uPD65042C, available from NEC. Modulation signal storage device 43 may be, for example, a type 28C16 Electrical Erasable Programmable Read Only Memory (EEPROM) programmed with the desired wave form table. Digital to analog converter 46 may be, for example, a DAC port, AD557JN available from Analog Devices, and selective filter 47 may be a type 4052 multiplexer, available from National Semiconductor or Harris Semiconductor.
The particular modulation control information used by microprocessor 21 to control the operation of controllable generator circuit 29, in accordance with the present invention, is stored in application storage device 52. As presented below in more detail with reference to FIGS. 12, 13, 14 and 15, application storage device 52 may be any storage device capable of storing information for later retrieval. For example, application storage device 52 may be, for example, a magnetic media based storage device such as a card, tape, disk, or drum. Alternatively, application storage device 52 may be a semiconductor memory-based storage device such as an erasable programmable read only memory (EPROM), an electrical erasable programmable read only memory (EEPROM) or a non-volatile random access memory (RAM). Another alternative for application storage device 52 is a mechanical information storage device such as a punched card, cam, or the like. Yet another alternative for application storage device 52 is an optical storage device such as a compact disk read only memory (CD ROM).
It should be emphasized that although the figures illustrate microprocessor 21 separate from .application storage device 52, microprocessor 21 and application storage device 52 may both be incorporated into a single device, which is loaded into system 11 to control the operation of system 11 as described herein. In this case, interface 16 would exist between the combination of microprocessor 21 and application storage device 52 and the rest of system 11.
Interface 16 is configured as appropriate for the particular application storage device 52 in use. Interface 16 translates the control information stored in application storage device 52 into a usable form for storage within the memory of microprocessor 21 to enable microprocessor 21 to control controllable generator circuit 29 to produce the desired modulated low energy emission.
Interface 16 may directly read the information stored on application storage device 52, or it may read the information through use of various known communications links. For example, radio frequency, microwave, telephone or optical based communications links may be used to transfer information between interface 16 and application storage device 52.
When application storage device 52 and microprocessor 21 are incorporated in the same device, interface 16 is configured to connect microprocessor 21 to the rest of system 11.
The control information stored in application storage device 52 specifies various controllable parameters of the modulated low energy RF electromagnetic emission which is applied to a patient through probe 13. Such controllable parameters include, for example, the frequency and amplitude of the carrier, the amplitudes and frequencies of the modulation of the carrier, the duration of the emission, the power level of the emission, the duty cycle of the emission (i.e., the ratio of on time to off time of pulsed emissions applied during an application), the sequence of application of different modulation frequencies for a particular application, and the total number of treatments and duration of each treatment prescribed for a particular patient.
For example, the carrier signal and modulation signal may be selected to drive the probe 13 with an amplitude modulated signal in which the carrier signal includes spectral frequency components below 1 GHz, and preferably between 1 MHz and 900 Mhz, and in which the modulation signal comprises spectral frequency components between 0.1 Hz and 10 KHz, and preferably between 1 Hz and 1000 Hz. In accordance with the present invention, one or more modulation frequencies may be sequenced to form the modulation signal.
As an additional feature, an electromagnetic emission sensor 53 may be provided to detect the presence of electromagnetic emissions at the frequency of the carrier oscillator 32. Emission sensor 53 provides to microprocessor 21 an indication of whether or not electromagnetic emission at the desired frequency are present. As described below in more detail, microprocessor 21 then takes appropriate action, for example, displaying an error message on display 17, disabling controllable generator circuit 29, or the like.
The invention also includes a power sensor 54 which detects the amount of power applied to the patient through probe 13 compared to the amount of power returned or reflected from the patient. This ratio is indicative of the proper use of the system during a therapeutic session. Power sensor 54 applies to microprocessor 21 through power sense line 56 an indication of the amount of power applied to patient through probe 13 relative to the amount of power reflected from the patient.
The indication provided on power sense line 56 may be digitized and used by microprocessor 21, for example, to detect and control a level of applied power, and to record on application storage device 52 information related to the actual treatments applied. Such information may then be used by a physician or other clinician to assess patient treatment compliance and effect. Such treatment information may include, for example: the number of treatments applied for a given time period; the actual time and date of each treatment; the number of attempted treatments; the treatment compliance (i.e., whether the probe was in place or not in place during the treatment session); and the cumulative dose of a particular modulation frequency.
The level of power applied is preferably controlled to cause the specific absorption rate (SAR) of energy absorbed by the patient to be from 1 microWatt per kilogram of tissue to 50 Watts per kilogram of tissue. Preferably, the power level is controlled to cause an SAR of from 100 microWatts per kilogram of tissue to 10 Watts per kilogram of tissue. Most preferably, the power level is controlled to cause an SAR of from 1 milliWatt per kilogram of tissue to 100 milliWatts per kilogram of tissue. These SARs may be in any tissue of the patient, but are preferably in the tissue of the central nervous system.
System 11 also includes powering circuitry including battery and charger circuit 57 and battery voltage change detector 58.
FIGS. 3-10 present in more detail various components of the system of FIG. 2.
Referring first to FIG. 3, presented is a detailed schematic of controllable modulation frequency generator 31. Modulation frequency generator 31 includes wave form address generator 41, modulation signal storage device 43, digital to analog converter 46 and a selective filter network 47.
Microprocessor 21 controls extended I/O lines 45 and selects the desired wave form from wave form storage device 43. Microprocessor 21 then downloads the control information to the wave form address generator 41 which in turn generates a sequence of the wave form addresses. The sequence of addresses are then applied to the modulation signal storage device 43 through address bus 42. The desired modulation signal is then retrieved from the storage device 43 and appears on signal bus 44 in digital form. After a digital to analog conversion by the digital to analog converter 46, the modulation signal is filtered and is output onto the modulation signal line 20.
The frequency of the modulation signal is determined by the rate at which the sequence of wave form addresses is generated. The type of modulation signal is selected by microprocessor 21 via extended I/O lines 45 and the filtering network is selected via I/O line 50.
Referring now to FIG. 4, presented is a detailed schematic of the modulation signal buffer amplifier 35 and the carrier frequency oscillator circuit 32.
The modulation signal buffer amplifier 35 is basically a non-inverting amplifier in discrete form. The amplifier buffers the modulation signal 20 from the selective filter 47 and provides necessary modulation signal amplitude and current drive to the AM modulator and power generator circuit 34. The output stage is designed in such a way that the output signal 37 achieves a rail-to-rail voltage swing. The output of the modulation signal buffer appears on signal line 37.
It should be noted that although the disclosed embodiment contemplates that the gain of modulation signal buffer amplifier 35 is substantially constant, the invention also contemplates use of a variable gain amplifier that is controlled by microprocessor 21 in order to vary the magnitude of the modulation signal on line 37, thus permitting programmable control of the level of power applied.
The carrier oscillator 32 is constructed around carrier oscillator crystal 59. In one embodiment, carrier oscillator 32 produces a Radio Frequency (RF) carrier frequency of 27 MHz. Other embodiments of the invention contemplate RF carrier frequencies of 48 MHz, 450 MHz or 900 MHz. In general, the RF carrier frequency produced by carrier oscillator 32 has spectral frequency components less than 1 GHz and preferably between 1 MHz and 900 MHz. It should also be noted that while the disclosed embodiment contemplates that once set, the carrier oscillator frequency remains substantially constant, the present invention also contemplates that carrier frequency produced by carrier oscillator 32 is variable and controllable by microprocessor 21 by use of control information stored on application storage device 52. This would be accomplished, for example, by use of high frequency oscillator, the output of which is conditioned by a controllable clock divider circuit to produce a controlled carrier frequency signal.
Carrier oscillator 32 produces on carrier signal line 36 a carrier signal which is to be modulated by the modulation signal carried on signal line 37.
Oscillator disable line 33 is applied to NAND gate 61, the output of which is applied to NAND gate 62. This configuration allows microprocessor 21 to disable both modulation signal buffer 35 and carrier oscillator 32 by applying an appropriate disable signal to oscillator disable line 33.
FIG. 5 presents a detailed schematic of the AM modulator and power generator 34 and the output filter 39. The AM modulator is made up of two transistors 66 and 67 connected in parallel and operated in zero-crossing switching mode. The carrier signal 36 is applied at the bases of the transistors 66 and 67 through NAND gates 63 and 64, and the modulation signal 37 is applied to the collectors of transistors 66 and 67 through inductors 68 and 69. The net result is the modulated carrier that appears at the collectors of the transistors 66 and 67.
The output power is generated by a single-ended tuned resonant converters configured by three pairs of inductors and capacitors, 70, 71 and 72. LC resonant circuits 70, 71 and 72 are tuned to provide the required output power and are optimized to the maximum efficiency of the converter.
The output of the AM modulator and power generator 34 appears on signal line 38. This modulated signal is applied through output filter network 39 to output connector 78. Output filter 39 included three LC filtering stages, 73, 74 and 76.
The first LC filtering stage, 73 is a band-pass and band-notch filter with pass band centered at 27 MHz and band notch centered at 54 MHz. The band-notch filter provides additional suppression to the second harmonic of the carrier. The second and third LC filtering stages 74 and 76 are both band pass filters which have pass band centered at 27 MHz. The three stage output filter serves to substantially eliminate the carrier harmonics that result from zero-crossing switching of the AM modulator circuit 34.
The output series resistor 77 is used to adjust the output impedance of the modulator. It is found from measurement that the output impedance of the AM modulator is considerably lower than 50 ohm. The series resistor 77 adjusts the output impedance of the circuit is approximately 50 ohms.
FIG. 6 presents the details of the impedance transformer 14. Referring also to FIGS. 1, 2, and 5, the output of the AM modulator and power generator 34 and filter stage 39 is designed to have a 50 Ohm output impedance which is chosen to match the 50 Ohm impedance of coaxial cable 12. Impedance transformer 14 includes inductor 79 connected between probe 1 and the middle conductor of coaxial cable 12, and a capacitor 81 connected between probe 13 and the ground conductor of coaxial cable 12.
It has been determined through impedance measurements that when probe 13 is applied to the mouth of a patient, the probe/patient combination exhibits a complex impedance on the order of 150+j200 Ohms. Impedance transformer 14 serves to match this complex impedance with the 50 Ohm impedance of coaxial cable 12 and therefore the output impedance of the AM modulator 34 and output filter 39. This promotes power transmission, and minimizes reflections. In one embodiment, inductor 79 is 0.68 microHenry, and capacitor 81 is 47 picoFarads.
FIG. 7 presents the detailed schematic of the emission sensor 53 of the present invention. Emission sensor 53 includes antenna 82 which is capable of detecting electromagnetic fields at the frequency of the carrier oscillator 32. The signal induced by antenna 82 is applied to a simple diode detector formed by diode 83, capacitor 84 and resistor 85. The demodulated low frequency signal is then applied to the base of a transistor 86 operating as a switch. The output is a low level signal line 87 which is connected to microprocessor 21. Emission sensor 53 is used at the beginning of a treatment session to detect whether probe 13 is emitting electromagnetic fields of the carrier frequency. If so, microprocessor 21 produces on display 17 an indication that the proper electromagnetic field is being produced.
Emission sensor 53 is also connected to the power supply circuitry through EXT DC IN line 115 (see also, FIG. 10). When external dc power is applied, line 115, which is connected to the base of transistor 86, turns transistor 86 on, thus providing an indication to microprocessor 21 that external dc power is applied.
Referring now to FIG. 8, presented is a schematic of the power sensor 54 used to sense the ratio of the power applied to the patient through probe 13 to the power reflected from the patient. This ratio is indicative of the efficiency of power transfer from the application system 11 to the patient, and may be used to assess patient treatment compliance. Power sensor 54 may also be used to monitor the level of power being applied to the patient.
Power sensor 54 includes bi-directional coupler 88 which can be, for example, a model KDP-243 bi-directional coupler available from Synergy Microwave Corporation. Bi-directional coupler 88 operates to couple a portion of the energy emitted by application system 11 through output connected 78 and carried by coaxial cable 12 into detecting circuits 89 and 90.
Output connector 78 is connected to a primary input of bi-directional coupler 88 and co-axial cable 12 is connected to a primary output of bi-directional coupler 88. Bi-directional coupler 88 includes two secondary outputs, each of which are connected to respective detecting circuits 89 and 90. Detecting circuit 89 functions to detect the amount of power applied to the patient, and detecting circuit 90 functions to detect the amount of power reflected from the patient. Detecting circuit 89 is connected through resistive divider 94 to the positive input of differential amplifier 91. Detecting circuit 90 is connected through resistive divider 92 to the negative input of differential amplifier 91. The output of differential amplifier 91 is indicative of the difference between the power transmitted to the patient by application system 11, and the power reflected from the patient, and thus is indicative of an amount of power absorbed by the patient. The output of differential amplifier 91 is applied to an analog to digital converter (ADC) or comparator 93, the output of which connected to microprocessor 21 through power sense line 56.
As described in more detail below with reference to the flow chart of FIGS. 11 a-d, microprocessor 21 operates to analyze the signal appearing on power sense line 56 to determine and control the amount of power applied to the patient, and to assess patient treatment compliance, and possibly to record indicia of the patient treatment compliance on application storage device 52 for later analysis and assessment by a physician or other clinician.
FIG. 9 presents a detailed schematic of the information output circuit 17. Microprocessor 21 controls the display module 109 of information output circuit 17 via data bus 23 and address bus 22 and controls the sound control circuit 110 by an I/O line 100. The display module 109 may be an intelligent LED display module PD3535, available from Siemens or a LCD graphics module available from Epson. The sound control circuit 110 may be a buzzer as shown in FIG. 9 or it may be an advanced speech synthesizer.
Referring now to FIG. 10, presented are the details of the power supply circuit used in the application system 11 of the present invention.
During operation of application system 11, power is derived from rechargeable battery 95 which may be, for example, a six volt rechargeable Ni--Cd battery, or the like. Battery 95 is connected through relay 99 to relay 98. The coil of relay 98 is powered by transistor 106 which is controlled by the output of NAND gate 102.
NAND gates 102, 103, 104 and 105 are configured to form a resettable latch. When on button 18 is depressed, the latch turns on transistor 106 which activates the coil of relay 98. When off button 19 is depressed, the latch is reset thus turning transistor 106 off, and removing power from the coil of relay 98. Microprocessor 21 may also reset the latch by pulling low momentarily on the Auto-Off line 107. This helps to save unnecessary power consumption when the system 11 is being left in an idle state.
When the coil of relay 98 is powered, battery 95 is connected to voltage regulator 97 which provides regulated voltage Vcc which is used to power various components of application system 11.
Connector 96 is provided to accommodate an external ac/dc adapter (not shown) which is used to charge battery 95. When an external dc adapter is connected to connector 96, voltage regulator 101 produces a regulated voltage which powers the coil of relay 99. This causes battery 95 to be disconnected from voltage regulator 97, and causes the output of voltage regulator 101 to be connected to the input of voltage regulator 97, thus permitting application system 11 to be powered by the external dc adapter. An indication of the existence of external dc voltage is applied to emission sensor 53 (FIG. 7) through EXT DC IN line 115.
If external dc power is connected (determined by emission sensor 53 when application system 11 is initially powered), microprocessor 21 executes the battery charging control routine, stops controllable generator 29 and disables the carrier oscillator 32. It also sends a signal to the battery charging control 57 and turns on the fast charging circuits. A message is displayed on display 17 or on a separate light emitting diode indicating that the battery is being charged.
During the battery charging routine, microprocessor 21 constantly monitors the battery voltage from the -dV detector 58 via data bus 23. Once the required -dV is detected, Ni--Cd battery 95 has reached its full charge condition, microprocessor 21 switches off the fast charge circuit and automatically removes power from the system 11. -dV detector 58 may be configured, for example, including a MAX166 digital to analog converter available from Maxim Integrated Products, Inc.
The battery voltage is constantly monitored by the battery voltage monitor 108. Once the battery voltage drops to a predetermined low level (the voltage level at which the output emission power drops by 3% of the calibrated value), a signal is provided to microprocessor 21 which in turn stops the emission and provides an error message on the display 17. Battery voltage monitor 108 may be, for example, a voltage supervisory integrated circuit available from Texas Instruments or SGS Thompson.
Referring now to FIGS. 11 a-d, presented are flow charts of the operation of the application system 11 of FIGS. 1 and 2, in accordance with the method of the present invention. In practice, the flowcharts of FIGS. 11 a-d are encoded in an appropriate computer program and loaded into the operating program storage portion of microprocessor 21 in order to cause microprocessor 21 to control the function of application system 11.
Referring to FIG. 11a, microprocessor 21 starts execution of the program when switch 18 is activated. In block 111, microprocessor 21 initializes the circuits by stopping the wave form address generator 41, disabling the carrier oscillator 32 and displaying a welcome message to the user on display module 109.
In block 112, the source of dc power is immediately checked afterinitialization. If an external dc power source is connected, for example an ac/dc adapter, it is assumed that system 11 should function as a Ni--Cd battery charger. Microprocessor 21 passes control to block 113 which switches on the fast charge mode of the battery charging control 57 and monitors the battery voltage via the -dV detector 58 in the control loop including blocks 111 and 116. Once the Ni-Cd battery 95 reaches its full-charged state as detected by -dV detector 58, microprocessor 21 switches off the fast charging current in block 117 and automatically switches off system 11 in block 118.
If decision block 112 determines that external dc source is not connected, system 11 is powered by the internal battery 95. The battery voltage monitor 108 monitors the battery voltage at all times and provides information to microprocessor 21 for use in decision block 119. If the battery level drops to a predetermined low level, microprocessor 21 displays an error message on the display 109 in block 121. This is to inform the user to re-charge the battery before using the system again. It also switches off system 11 automatically in block 122 if there is no user response as determined by timing loop 123.
Referring now to FIG. 11b, after the battery level is checked, microprocessor 21 checks in block 124 if application storage device 52 is connected to system 11 via interface 16. If application storage device 52 is not connected, microprocessor 21 prompts for the application storage device 52 via information on display 109 in block 126. The application storage device 52 must be connected within a predetermined time limit as determined by block 127, or microprocessor 21 switches system 11 off in block 128.
Once block 124 determines that application storage device 52 is in place, microprocessor 21 reads an identification code in block 129 and checks if application storage device 52 is genuine and valid in block 131. If not, an error message is displayed in block 132 and system 11 is switched off after a predetermined time limit.
If a valid application storage device is connected, microprocessor 21 reads the control information in block 133 and stores the control information in the internal RAM area. Application information such as the type of treatment may be displayed on display 17 in block 134 for user re-confirmation. Microprocessor 21 then pauses and waits in block 136 for input from the user to start the application.
The user starts the application by pressing the on switch 18 again. Microprocessor 21 generates a test emission in block 137 by controlling the controllable generator 29 and prompts the user to check the emission with emission sensor 53 in block 138. Microprocessor 21 then checks the emission sensor input for the indicative signal in block 139. If the emission is not detected within a predetermined time limit as determined by block 142, microprocessor 21 displays a corresponding error message in block 143 and switches off system 11 in block 144 after a predetermined idle time as determined by block 146.
If the emission is detected within the predetermined time limit determined by block 142, control a passes to block 147 where microprocessor 21 executes the application software routine shown in detail in the flowchart of FIGS. 11d and 11e.
The application software routine takes in the control information, interprets the information and controls the controllable generator 29 to generate the corresponding modulation wave form, frequency, power level, duration and duty cycle.
Referring to FIGS. 11d and 11e, microprocessor 21 starts the routine by first setting up a total treatment time counter in block 151 which keeps tracks of the timing of the actual application. It then gets and interprets the first block of modulating frequency data in block 152. Then, in block 153 the modulation wave form is selected via extended I/O lines 45 and a suitable filter network is selected via the extended I/O lines 50. Also in block 153, the gain of modulation signal buffer amplifier 35 is adjusted in accordance with the power level control information. In block 154, the modulation frequency is controlled via the wave form address generator 41. The emission is then enabled by microprocessor 21 in block 156.
In decision block 157, the battery is checked using battery voltage monitor 108 to determine whether the battery level is acceptable. If not, control passes to block 158 where an appropriate error message is displayed. Then, system 11 is shut down in block 161 after a delay time determined by decision block 159.
If, on the other hand, the battery voltage is acceptable, control passes to decision block 162 where it is determined whether or not application storage device 52 is still inserted in interface 16. If not, control passes to decision block 163 where it is determined whether a predetermined time has expired without the presence of application storage device 52. When the time limit expires, control passes to block 164 where an appropriate error message is displayed, and eventually system 11 is automatically shut down in block 161.
If, on the other hand, decision block 162 determines that application storage device 52 is present within interface 16, control passes to block 166 where application storage device 52 is updated with user compliance information. Control then passes to block 167 where the output of power sensor 54 is read. Control then passes to block 168 where the output of power sensor 54 is assessed to determine a level of power being applied to the patient, and to assess whether or not treatment is being effectively applied. For example, if sensor 54 determines the presence of a large amount of reflected power, this condition may possibly be indicative of probe 13 not being properly connected or not being properly inserted into the mouth of a patient.
If decision block 168 determines that treatment is not being properly applied, control passes to decision block 169 which determines whether a predetermined time limit has been exceeded without detection of proper treatment. If the time limit is exceeded, control passes to block 171 where application storage device 52 is updated with information indicative of non-compliance with the treatment protocol.
If, on the other hand, decision block 168 determines that the treatment it is being properly applied, control passes to block 172 where it is determined whether the end of the particular modulation frequency block being applied has been reached. If not, control returns to decision block 157. If, on the other hand, decision block 172 determines that the end of the modulation frequency block presently being applied has been reached, control passes to decision block 173 where it is determined whether the end of the treatment time has been reached. If so, control returns to block 148 (FIG. 11c). If, on the other hand, decision block 173 determines that the end of the treatment session has not been reached, control passes to block 174 where the next frequency block is read from application storage device 52, and control returns to block 153 for the continuation of the treatment session.
At the end of the application routine, control is returned and the microprocessor 21 displays an ending message in block 148 and switches system 11 off automatically in block 149.
FIGS. 12, 13, 14, 15, 16 and 17 present exemplary configurations for application storage device 52. It should be understood that other configurations for application storage device 52 are also possible, without departing from the spirit and the scope of the present invention.
Referring to FIG. 12, application storage device 52 may comprise a magnetically encoded card 181 which includes a magnetically recordable portion 182 which stores the above-described control information and patient treatment compliance information.
Referring to FIG. 13, application storage device 52 may comprise a semiconductor memory 183 which is connected through terminals 184 to interface 16. Semiconductor memory 183 is used to store the above described application control information and patient treatment compliance information.
Referring now to FIG. 14, application storage device 52 may be in the form of a smart card 186 with the semiconductor hidden behind the contacts 187. The semiconductor may comprise only the memory with some security control logic, or may also include a stand-alone microprocessor that assists in communicating with the host microprocessor 21 via interface 16.
As shown in FIG. 15, application storage device 52 may take the form of a key-shaped structure 188 including semiconductor memory 189 and microprocessor 191 which are operatively connected to electrical terminals 192.
FIG. 16 illustrates application storage device 52 in the form of a compact disk read only memory (CDROM) 193, on which control information is optically encoded.
Finally, as shown in FIG. 17, application storage device 52 may take the form of a punched card 194, in which control information is tangibly embodied in a pattern of punched holes 196.
TREATMENT EXAMPLES
The system of the invention for applying a modulated low-energy electromagnetic emission to a patient, is useful for the treatment of a patient suffering from central nervous system (CNS) disorders. In use of the system, the probe for applying the modulated carrier signal to the patient is connected to the patient, in particular by means of a mouth piece probe placed in the patient's mouth and the modulated low-energy electromagnetic emission is applied to the patient through the probe. At least two low-energy electromagnetic emissions modulated at different frequencies are applied to the patient to achieve beneficial results. Beneficially, several discrete electromagnetic emissions modulated at different frequencies are applied to the patient for a specific treatment of a CNS disorder. The time of treatment, which relates to the amount of the low-energy electromagnetic emission applied to the patient, may vary between wide limits depending on the nature of the disorder being treated and the effect desired. However, in general, the time of treatment would be at least one minute per day and could continue over several hours per day, but would normally be at most one hour per day. Most preferably, the treatment time is at least ten minutes per day which may be divided up into two or more application times, e.g., of from five to forty-five minutes per application time.
EXAMPLE I
TREATMENT OF INSOMNIA
One of the specific CNS disorders which has been very satisfactorily treated with the aid of the system of the invention is sleep disorder, in particular insomnia which is the most important sleep disorder. Clinical insomnia is defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R), from the American Psychiatric Association 1987 (DSM-III-R):
"Diagnostic criteria for Insomnia Disorders
A. The predominant complaint is of difficulty in initiating or maintaining sleep, or of non restorative sleep (sleep that is apparently adequate in amount, but leaves the person feeling unrested).
B. The disturbance in A occurs at least three times a week for at least one month and is sufficiently severe to result in either a complaint of significant daytime fatigue or the observation by others of some symptom that is attributable to the sleep disturbance, e.g., irritability or impaired daytime functioning.
C. Occurrence not exclusively during the course of "Sleep-Wake Schedule Disorder or a Parasomnia."
"Diagnostic criteria for 307.42 Primary Insomnia
Insomnia Disorder, as defined by criteria A, B and C above, that apparently is not maintained by any other mental disorder or any known organic factor, such as a physical disorder, a Psychoactive Substance Use Disorder, or a medication."
The frequencies of modulation for the low-energy electromagnetic emissions applied to the patient for treating insomnia have been found to be effective when comprising two or more frequency modulations selected from the following bandwidths: 1-5 Hz, 21-24 Hz, 40-50 Hz, 100-110 Hz, or 175-200 Hz.
A very specific example of a set of low-energy electromagnetic emissions applied to a patient suffering from insomnia are modulated at the following frequencies and applied sequentially to the patient for the times indicated over a period of 20 minutes per day, three times a week or every day is as follows:
Protocol P40: about 2.7 Hz for about 6 seconds, followed by about a 1 second pause, about 21.9 Hz for about 4 seconds, followed by about a 1 second pause, about 42.7 Hz for about 3 seconds, followed by about a 1 second pause, about 48.9 Hz for about 3 seconds, followed by about a 1 second pause.
A study employing the above protocol P40 set of frequency modulations and times of application was performed to test the efficacy of low-energy emission therapy (LEET) in the treatment of insomnia.
EXAMPLE IA
TREATMENT OF INSOMNIA
The primary endpoints of the study were defined as measures of sleep (total sleep time (TST) and sleep latency (SL)) as measured by polysomnography (PSG). Secondary endpoints (also quantified by PSG) included measures of rapid eye movement (REM), non-REM, number of awakenings after sleep onset, and wake after sleep onset (WASO). Additional measures of individual responses to treatment were derived from the patients' reports.
METHODS:
The study was a placebo-controlled, double-blind, repeated-measures study performed on a total of thirty subjects. Treatment was provided via a 12 V battery-powered device in accordance with the present invention, emanating the P40 protocol.
Forty-six subjects underwent polysomnographic (PSG) evaluation in order to yield the thirty subjects who participated in the study. The subjects who met the PSG criteria were randomized to treatment groups by means of a coin flip. All 30 subject completed the study. Subjects were identified for potential enrollment via television and radio advertisement.
Each study subject completed a number of rating scales prior to entry into and throughout the study. These scales included the Hamilton Anxiety Rating Scale (HARS), the Profile of Mood States (POMS), the Hopkins Symptom Check List (HSCL), and a patient reported sleep rating scale. The HARS, POMS, and HSCL were obtained during the initial psychiatric screening prior to entry, on a weekly basis thereafter, and at completion of the study. Daily sleep logs were maintained by patients throughout the study. Patients received treatment 3 times per week over the 4 weeks of the study, and were randomly assigned to either active or inactive treatment groups, under double-blind conditions. Treatment was performed with patients in a supine position, resting comfortably on a bed in a sleep-recording room with a low level of illumination.
ENTRY CRITERIA:
To qualify for a baseline PSG study, subjects were screened for chronic insomnia of a non-medical etiology. Patients with active medical illness, psychiatric diagnoses (DSM-III-R), alcohol/drug addiction, or active use of benzodiazepines and/or tranquilizers were excluded.
Entry into the study required patients to be suffering from chronic insomnia (more than six months) and to meet at least 2 of the 3 established PSG sleep criteria: sleep latency of greater than 30 minutes duration; total sleep time (TST) of less than 360 minutes per night; sleep efficiency (total sleep time/total recorded time) of less than 85%. Subjects were asked to go to bed in the laboratory at their regular bedtime and were allowed to sleep "ad libitum". The study was ended by the technician only if the time in bed was greater than 8.5 hours and the subject at that time was lying in bed awake.
STATISTICAL METHODS:
For purposes of statistical analysis, a Student's t-test was performed comparing the difference in the change scores (post-pre) between the treatment groups. Where appropriate, analyses were adjusted for baseline values using linear regression.
RESULTS:
Base Line Evaluation
Of the 30 consenting, eligible patients, 15 were randomly assigned to each of the treatment groups. In the active treatment group, there were 4 men and 11 women (mean age of 39 years). In the inactive treatment group there were 6 men and 7 women (mean age of 41 years). The mean age of the subjects did not differ significantly between groups.
At baseline, by definition, all patients met criteria for severe insomnia. Although the study groups had comparable patient reported TST durations at baseline, the placebo group had a significantly longer TST at baseline when measured by PSG. Both groups had prolonged sleep latency periods at baseline (>20 mins) as determined by both patient reported and PSG measures. Pre-treatment sleep parameters are summarized in Table II.
Post-Treatment Evaluation: Interval Changes
All 30 patients completed the trial. In the placebo group, the PSG TST decreases slightly at the conclusion of the study, compared with baseline values (from 337.0.+-.57.2 to 326.0.+-.130.5 TST change of -11.0.+-.122.8, p=0.74). Similarly, the pre- and post-patient reported measures of TST were nearly identical in the placebo group (from 269.0.+-.73.6 to 274.3.+-.103.2, TST change of 5.+-.122, p=0.87). In contrast, the PSG measured TST increased in the active group by nearly 90 minutes (from 265.9.+-.67.5 to 355.8.+-.103.5, TST increase of 89.9.+-.93.9, p=0.002). This finding is consistent with the patient reported improvement reported by the active treatment group (from 221.7.+-.112.3 to 304.0.+-.144.7, TST increase of 82.3.+-.169.0 minutes, p=0.08).
Also worth noting is that, while the proportion of REM sleep in the placebo group increased only slightly from 17.3 to 18.7% of total sleep time, in the active group, it increased from 16.3 to 20.9% of the total sleep time. The patient reported measure of sleep latency improved by more than 50% in the active treatment group during the study (from 145.8.+-.133.2 to 70.7.+-.67.9, p=0.03) while sleep latency increased slightly in the placebo group during the study period (from 71.3.+-.41.2 to 82.8.+-.84.8, p-0.58).
SIDE EFFECTS:
Side effects are summarized in Table I. One patient in the active treatment group reported increased dreaming. No other side effects were reported.
TABLE I
______________________________________
SIDE EFFECTS
Side Effect Active Placebo
______________________________________
Mild Headache 0 0
Average Headache 0 0
Tingling Sensation 0 0
Worsening of Sleep 0 0
Nausea 0 0
Uncomfortable sensation in mouth
0 0
Fatigue 0 0
Fever 0 0
Increased Dreaming 1 (3%) 0
Metallic Taste 0 0
Dizziness 0 0
Lightheadedness 0 0
______________________________________
CONCLUSIONS:
Subjects enrolled in this study demonstrated severely disturbed sleep criteria by both patient reported and PSG measures. The active treatment group exhibited an improvement of 34% in PSG TST, while the placebo group demonstrated a 3% decrease in PSG TST. The significant difference in TST changes between groups from baseline was not explained solely by the significantly different baseline TST of the active and placebo groups. Adding the baseline TST in a regression model using treatment as a predictor did not adequately account for the difference in TST between the treatment groups.
Patient reported measurements confirmed the PSG findings, with a 37% improvement in the active group TST compared with a 2% improvements in the control group. Other PSG and patient reported measures of sleep indicated consistently greater improvement in the active group compared with the placebo group. Those results indicate that LEET therapy (using the P40 program) on an every-other-day basis, successfully treats insomnia by both lengthening the total duration of sleep and shortening sleep latency. Furthermore, patients felt that their sleep patterns were improved. Post-treatment sleep parameters are summarized in Table III.
TABLE II
______________________________________
PRETREATMENT SLEEP PARAMETERS
Values shown represent mean .+-. standard deviation.
Measurements are derived from 1 night PSG obtained prior to
initiation of therapy.
PSG REPORT OF
SLEEP: PSG ANALYSIS N = 15 per group
Active Placebo p = Value
______________________________________
Total Sleep Time
265.9 .+-. 67.5
337.0 .+-. 67.2
0.004 (mins.)
Sleep Latency (mins.)
63.9 .+-. 64.1
46.6 .+-. 45.3
0.400
_________________________________
TABLE III
POST-TREATMENT SLEEP PARAMETERS
Values shown represent mean .+-. standard deviation.
Interval changes are reported as PSG data obtained at the end of the study (day 28)-PSG data obtained prior to the initiation of treatment.
PSG POST-TREATMENT SLEEP PARAMETERS
(1 Month) N = 15 per group
Active Placebo p = Value
______________________________________
Total Sleep Time
355.8 .+-. 103.5
326.0 .+-. 130.5
0.494 (mins.)
Change TST 99.9 .+-. 93.9 -11.0 .+-. 4122.8
0.017 (mins)
Sleep Latency
23.1 .+-. 12.8
27.0 .+-. 18.9
0.520
(mins)
Change SL (mins)
-40.8 .+-. 57.8
-19.8 .+-. 37.9
0.250
______________________________________
PATIENT REPORTS OF SLEEP:
SLEEP LATENCY (mins) N = 15 per group
Pre Post Change p = Value
______________________________________
Active Mean 145.8 70.7 -75.0 0.0307
Standard Deviation 133.2 67.9 121.0
Control Mean 71.3 62.8 11.5 0.5813
Standard Deviation 41.2 84.8 78.9
p = value 0.055 0.670 0.028
______________________________________
PATIENT REPORTS OF SLEEP: TOTAL SLEEP TIME (mins) = 15 per group
Pre Post Change p = Value
______________________________________
Active Mean 221.7 304.0 82.3 0.0804
Standard Deviation 112.3 144.7 169.2
Control Mean 269.0 274.3 5.3 0.8683
Standard Deviation 73.6 103.2 122.3
p = Value 0.183 0.523 0.164
______________________________________
EXAMPLE IB
TREATMENT OF INSOMNIA
Another double blind, patient-reported study was also designed to test the efficiency of low-energy emission therapy (LEET) in the treatment of insomnia of non-medical etiology.
The primary PSG of the study was to detect differences between the treatment groups in perceived sleep measures (total sleep time and sleep latency), as reported by the subjects.
METHODS:
The study was preformed on a total of 30 subjects. Treatment was provided using the device of the present invention with the P40 protocol powered by a 12-volt battery. All patients completed all phases of the study. In the inactive treatment group there were 8 males and 7 females (mean age of 40 years). In the active treatment group there were 6 males and 9 females (mean age of 39 years). There were no significant differences in age between the active treatment and inactive treatment populations.
Each study subject completed a number of rating scales prior to entry into and throughout the study. These scales included the Hamilton Anxiety Rating Scale (HARS), the Profile of Mood States (POMS), the Hopkins Symptom Check List (HSCL), and a patient reported sleep rating scale. The HARS, POMAS, and HSCL were obtained during the initial psychiatric screening prior to entry, on a weekly basis thereafter, and at completion of the study. Daily patient reported sleep rating scales were maintained by patients throughout the study. Patients received treatment 3 times per week over the 4 weeks of the study and were randomly assigned to either active or inactive treatment groups, under double-blind conditions. Treatment was performed with patients in a supine position, resting comfortably on a bed in a sleep-recording room with a low level of illumination. Subjects continued to record sleep log data for two weeks after discontinuation of treatment.
ENTRY CRITERIA:
Patients between 20 and 50 years of age were recruited into the study. Entry into the study required patients to meet at least 2 of the 3 established sleep criteria: patient reported sleep latency of greater than 30 minutes; patient reported total sleep time of less than 360 minutes; and patient reported sleep efficiency of less than 85% (calculated as TST/total time in bed). Patients with active medical illnesses, psychiatric illnesses (according to DSM-III-R), drug or alcohol dependence were excluded.
STATISTICAL METHODS:
For the purposes of statistical analysis, a Student's t-test was performed comparing the difference of the change scores (post-pre) between each of the treatment groups.
RESULTS:
Throughout the course of the study, subjects were asked to estimate their total sleep time and sleep latency. A comparison was made between the patient reported sleep latency and the patient reported total sleep time at the time of the telephone interview, and the patient reported sleep latency and patient reported total sleep time obtained in the morning following the last night of treatment. A highly significant difference was seen for total sleep time (two-sided p=0.0021), with a more than threefold increase in the active group compared with the placebo group. The active treatment group also exhibited a >50% decrease in sleep latency as compared with the baseline. Patient reports of sleep are summarized in Table IV.
TABLE IV
__________________________________
PATIENT REPORTS OF SLEEP:
SLEEP LATENCY AND TOTAL SLEEP TIME FOR STUDY
Pre Post Change p = Value
______________________________________
PATIENT REPORTED DATA:
SLEEP LATENCY (mins) N = 15 per group
Active
Mean 53.8 25.1 -28.7 0.0778
Standard Deviation
54.7 25.2 58.4
Control
Mean 70.0 58.53 -11.5 0.5710
Standard Deviation
67.0 71.0 77.0
p = value 0.474 0.105 0.498
PATIENT REPORTED DATA: TOTAL
SLEEP TIME (mins) N = 15 per group
Active
Mean 238.0 401.0 163.0 0.0001
Standard Deviation
58.3 80.8 87.0
Control
Mean 264.0 315.5 51.5 0.0498
Standard Deviation
81.9 112.2 93.0
p = value 0.325 0.024 0.002
______________________________________
No statistically significant differences were seen between the two groups for any other measured parameter. There was no first or second night rebound insomnia as assessed by changes in either total sleep time or sleep latency. Furthermore, there is no evidence of rebound effect during the two weeks following discontinuation of treatment. Rebound insomnia analyses are summarized in Table V.
TABLE V
______________________________________
REBOUND INSOMNIA ANALYSES FOR STUDY
FIRST DAY REBOUND INSOMNIA
ANALYSIS OF STUDY
PRE = DAY 26
POST = DAY 27 N = 15 Per Group
TOTAL SLEEP TIME (min) N = 15 Control
Pre Post Change p = Value
______________________________________
Activ
Mean 401.0 371.8 -27.9 0.17
Standard Deviation
80.8 118.8 71.1
Control
Mean 315.5 330.7 15.1 0.51
Standard Deviation
112.2 110.3 86.3
p = value 0.024 0.34 0.16
Active
Mean 25.1 32.5 5.7 0.15
Standard Deviation
25.1 32.1 13.8
Control
Mean 58.5 51.2 -7.3 0.72
Standard Deviation
71.1 52.6 76.1
p = value 0.01 0.26 0.53
______________________________________
*N = 14 for Active Day 27
SECOND DAY REBOUND INSOMNIA
ANALYSIS OF STUDY
PRE = DAY 26
POST = DAY 28 N = 15 Active
TOTAL SLEEP TIME (min) N = 15 Control
Pre Post Change p = Value
______________________________________
Active
Mean 401.0 355.7 -43.9 0.086
Standard Deviation
80.8 103.6 88.4
Control
Mean 315.5 320.5 5.0 0.85
Standard Deviation
112.2 100.5 99.1
P = Value 0.024 1 0.36 0.17
Active
Mean 25.1 41.4 14.6 0.098
Standard Deviation
25.1 39.8 30.8
Control
Mean 59.5 75.25 16.7 0.44
Standard Deviation
71.1 82.4 81.7
p = value 0. 1 0 0. 17 0.93
______________________________________
N = 14 for Active Day 28
REBOUND INSOMNIA ANALYSIS OF STUDY
PRE = DAY 26
POST = DAY 40 N = 15 Active
TOTAL SLEEP TIME (min) N = 15 Control
Pre Post Change p = Value
______________________________________
Active
Mean 401.0 342.9 56.8 0.0094
Standard Deviation
80.8 91.0 69.7
Control
Mean 315.5 323.7 8.1 0.68
Standard Deviation
112.2 79.0 74.4
p = value 0.024 1 0.55 0.02
Active
Mean 25.1 32.0 5.2 0.55
Standard Deviation
25.1 41.9 32.1
Control
Mean 58.6 32.0 -26.5 0.11
Standard Deviation
71.1 28.9 59.9
p = value 0.10 1.00 0.087
______________________________________
N = 14 for Active Day 40
SIDE EFFECTS:
Side effects for the study are summarized in Table VI.
TABLE VI
______________________________________
SIDE EFFECTS DATA FOR STUDY
(N = 30)
Side Effect Active Placebo
______________________________________
Mild Headache 0 1 (3%)
Average Headache 1 (3%) 0
Tingling Sensation 0 0
Worsening of Sleep 0 0
Nausea 0 1 (3%)
Uncomfortable sensation in mouth
0 0
Fatigue 0 0
Fever 0 0
Increased Dreaming 2 (6%) 0
Metallic Taste 0 0
Dizziness 0 0
Lightheadedness 0 1 (3%)
______________________________________
CONCLUSIONS:
Treatment with LEET using a battery powered system is highly effective in the treatment of insomnia, based on patient reported measurement of total sleep time.
PATIENT REPORTS OF SLEEP: Combined meta-analysis for the above two insomnia studies.
A meta-analysis of the patients' reports of sleep from the two studies is provided in Table VII. These studies were identical in terms of inclusion and exclusion criteria and study design (4-week, double-blinded, placebo-controlled). This analysis, performed on data from 60 patients (30 per group) demonstrates a 52 minute decrease in sleep latency, in the active group versus no change in the inactive group (p=0,025). Total sleep time increased by 128 minutes in the active group versus 28 minutes in the placebo group (p=0.005).
TABLE VII
______________________________________
PATIENT REPORTS OF SLEEP:
SLEEP LATENCY AND TOTAL SLEEP TIME FOR
the Above Two Insomnia Studies
PATIENT RESPONSE DATA: SLEEP
LATENCY (mins) N = 30 Active
Pre Post Change p = Value
___________________________________
Active
Mean 99.8 47.9 -51.9 0.0062
Standard Deviation
110.4 55.4 96.2
Control
Mean 70.7 70.7 0.0 0.9991
Standard Deviation
54.6 77.9 77.5
p = value 0.203 0.199 0.025
PATIENT RESPONSE DATA: TOTAL SLEEP TIME (mins) N = 30 Active
Active
Mean 229.8 352.5 122.7 0.0001
Standard Deviation
88.3 125.3 138.4
Control
Mean 266.5 294.9 28.4 0.1648
Standard Deviation
76.8 108.0 109.3
p = Value 0.091 0.062 0.005
______________________________________
EXAMPLE II:
TREATMENT OF GENERALIZED ANXIETY DISORDER AND PANIC ATTACKS
As discussed above, several discreet electromagnetic emissions modulated at different frequencies are applied to a patient for a specific treatment of a CNS disorder. Based on the statistically significant improvements in total sleep time and sleep latency reported above, a low-energy emission therapy (LEET) program has been developed for a further CNS disorder, more closely defined as generalized anxiety disorders and panic attacks. For this indication, it has been determined that frequency modulations of the low-energy electromagnetic emissions should be within the following bandwidths: 1-5 Hz, 14-17 Hz, 40-50 Hz, and 175-200 Hz. More specifically, a variety of discreet modulations are selected from the above bandwidths and are applied for different times, one specific example comprising: about 1.4 Hz for about 40 seconds, about 2.8 Hz for about 20 seconds, about 3.4 Hz for about 15 seconds, and a separate group comprising: about 3.4 Hz for about 15 seconds, about 14.6 Hz for about 4 seconds, about 42.7 Hz for about 2 seconds, about 48.9 Hz for about 2 seconds, and about 189.7 Hz for about 1 second.
For example, the first group of frequencies mentioned may be applied to the patient sequentially for a period of about 15 minutes during the morning of each day of treatment, and the second group of frequencies may be applied to the patient sequentially for a period of about 30 minutes in the evening of each day of treatment.
Results obtained in treating patients suffering from anxiety and employing the above dosage criteria are reported below.
METHODS:
Subjects were recruited. After obtaining informed consent, subjects were .interviewed with the Structured Clinical Interview for DSM-III-R Diagnosis (SCID), and symptoms were rated using structured interview versions of the Hamilton Anxiety Scale (Ham-A) (Hamilton, "The Assessment of Anxiety States by Rating," Br J. Med. Phychol., 32:pp. 50-55, 1959), and the 31 item Hamilton Depression Rating Scale (Ham-D) (Hamilton, "A Rating Scale for Depression," J. Neurol. Neurosurg. Phychiat., 53:pp. 56-62, 1960). A physical examination was performed and blood was drawn from each patient for laboratory screening.
Subjects meeting the following requirements were entered into the study:
Inclusion criteria:
1. Age 18-65
2. Able to give informed consent
3. Meets DSM-III criteria for Generalized Anxiety Disorder or Adjustment Disorder with Anxious Mood for at least three months' duration.
4. Hamilton Anxiety Scale (HAM-A) equal or greater than 18
Exclusion criteria:
1. Meets DSM-III-R criteria for Substance Abuse in past three months.
2. Known contraindication to low intensity magnetic field (including pregnant patients or those planning to become pregnant in near future)
3. Meets DSM-III-R criteria for Current Mania, Hypomania, or Mixed-Episode Depression, Dysthymia, or Cyclothymia.
4. History of Panic Disorder, Obsessive Compulsive Disorder, Schizophrenia,or Schizoaffective Disorder
5. Acute suicidal ideation at screening interview
6. Use of anxiolytic medication within one week of screening visit
7. Dosage of other psychoactive agents not stable during preceding 12 weeks
8. Has started new psychotherapy in the preceding six months
9. Plans to begin new psychotherapy during the course of the study
Subjects were given oral and written instructions for home use of the LEET device. Treatment consisted of daily exposures of 15 minutes each morning and 30 minutes each evening. The devices were pre-programmed to provide selected AM frequency RFEM waves via an antenna which the subject placed against the roof of their mouths. Subject were instructed to use the devices while recumbent with their eyes closed. All ratings were performed under open conditions. After six weeks of treatment, the devices were collected. Patients returned for follow-up visits in the second and fourth weeks after discontinuing treatment.
RESULTS:
Results are reported for the four women and six men who entered the protocol. As Table VIII illustrates, mean Ham-A improved from 23.4 to 8.1 after the first week of treatment. By the third week of treatment, nine of the ten subjects showed improvement on the Ham-A of at least 50% of their baseline scores. Improvement was generally sustained through the sixth week. After discontinuation, the benefit of treatment appeared sustained in some subjects through the post-treatment follow-up. Although many subjects experienced some increase in Ham-A after discontinuing treatment, no subject reported rebound anxiety. Mean scores on Ham-D also improved from 15.01 at baseline and remained less than 6 after the first week of treatment.
TABLE VIII
MEAN HAMILTON ANXIETY
SCALE SCORES, ALL SUBJECTS
Baseline
Week 1
Week 2
Week 3
Week 4
Week 5
Week 6
Post Tx + 2 Wks
Post Tx + 4
__________________________________________________________________________
Wks
23.4 8.1 5.3 5.3 4.4 4.4 4.4 6.0 8.1
__________________________________________________________________________
DISCUSSION:
The results are noteworthy for several reasons. First, LEET is an entirely new treatment paradigm which offers an attractive side effect profile and the potential to treat anxiety and related disorders. Second, the results are encouraging both in the magnitude of the effect and in the percentage of patients who achieved a clinically significant improvement. Third, the possibility that all instances of observed efficacy are due to placebo response is diminished by the duration of the observed improvement and that several of the patients had failed to improve in prior controlled studies and in previous open treatment with high potency benzodiazepines and/or antidepressants. Further research under double-blind conditions is indicated to further establish the efficacy of LEET and to clarify its role in clinical practice.
Although the invention has been described with reference to certain embodiments, it will be understood by those of skill in this art that additions, deletions and changes can be made to these embodiments, without departing from the spirit and scope of the present invention.
