Alexander
RICKARD, et al.
L-Arginine vs Plaque
L-Arginine vs Plaque
http://ns.umich.edu/new/releases/22876-naturally-occurring-amino-acid-could-improve-oral-health
May 06, 2015
Naturally
occurring amino acid could improve oral health
ANN ARBOR — Arginine, a common amino acid found naturally in foods, breaks down dental plaque, which could help millions of people avoid cavities and gum disease, researchers at the University of Michigan and Newcastle University have discovered.
Alexander Rickard, assistant professor of epidemiology at the U-M School of Public Health, and colleagues, discovered that in the lab L-arginine—found in red meat, poultry, fish and dairy products, and is already used in dental products for tooth sensitivity—stopped the formation of dental plaque.
"This is important as bacteria like to aggregate on surfaces to form biofilms. Dental plaque is a biofilm," Rickard said. "Biofilms account for more than 50 percent of all hospital infections. Dental plaque biofilms contribute to the billions of dollars of dental treatments and office visits every year in the United States."
Biofilm
grown in unsupplemented saliva.

Biofilm grown in saliva supplemented with 500 mM L-arginine.


Biofilm grown in saliva supplemented with 500 mM L-arginine.

Dental biofilms are the culprits in the formation of dental caries (cavities), gingivitis and periodontal disease. Surveys indicate that nearly 24 percent of adults in the United States have untreated dental caries, and about 39 percent have moderate-to-severe periodontitis, a number that rises to 64 percent for those over age 65.
Most methods for dental plaque control involve use of antimicrobial agents, such as chlorhexidine, which are chemicals aimed at killing plaque bacteria, but they can affect sense of taste and stain teeth. Antimicrobial treatments have been the subject of debate about overuse in recent years.
Pending further clinical trials to verify their lab findings, the researchers said L-arginine could take the place of the current plaque-controlling biocide substances including chlorhexidine and other antimicrobials.
"At present, around 10-to-15 percent of adults in the Western world have advanced periodontitis, which can lead to loose teeth and even the loss of teeth. Therefore, there is a clear need for better methods to control dental plaque," said Nick Jakubovics, a lecturer at Newcastle University's School of Dental Sciences.
Their findings are reported in the current issue of PLOS ONE.
The mechanism for how L-arginine causes the disintegration of the biofilms needs further study, the researchers said. It appears arginine can change how cells stick together, and can trigger bacteria within biofilms to alter how they behave so that they no longer stick to surfaces, they said.
In conducting their research, team members used a model system they introduced in 2013 that mimics the oral cavity. The researchers were able to grow together the numerous bacterial species found in dental plaque in the laboratory, using natural human saliva.
"Other laboratory model systems use one or a small panel of species," Rickard said. "Dental plaque biofilms can contain tens to hundreds of species, hence our model better mimics what occurs in the mouth, giving us great research insight."
Other researchers include Ethan Kolderman, Deepti Bettampadi, Derek Samarian and Betsy Foxman of U-M and Scot Dowd of Molecular Research LP.
http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0121835
PLOS x ( May 6, 2015 )
DOI: 10.1371/journal.pone.0121835
L-Arginine
Destabilizes Oral Multi-Species Biofilm Communities
Developed in Human Saliva
Ethan Kolderman, Deepti Bettampadi, Derek Samarian, Scot E. Dowd, Betsy Foxman, Nicholas S. Jakubovics, Alexander H. Rickard
Ethan Kolderman, Deepti Bettampadi, Derek Samarian, Scot E. Dowd, Betsy Foxman, Nicholas S. Jakubovics, Alexander H. Rickard
Abstract
The amino acid L-arginine inhibits bacterial coaggregation, is involved in cell-cell signaling, and alters bacterial metabolism in a broad range of species present in the human oral cavity. Given the range of effects of L-arginine on bacteria, we hypothesized that L-arginine might alter multi-species oral biofilm development and cause developed multi-species biofilms to disassemble. Because of these potential biofilm-destabilizing effects, we also hypothesized that L-arginine might enhance the efficacy of antimicrobials that normally cannot rapidly penetrate biofilms. A static microplate biofilm system and a controlled-flow microfluidic system were used to develop multi-species oral biofilms derived from pooled unfiltered cell-containing saliva (CCS) in pooled filter-sterilized cell-free saliva (CFS) at 37oC. The addition of pH neutral L-arginine monohydrochloride (LAHCl) to CFS was found to exert negligible antimicrobial effects but significantly altered biofilm architecture in a concentration-dependent manner. Under controlled flow, the biovolume of biofilms (µm3/µm2) developed in saliva containing 100-500 mM LAHCl were up to two orders of magnitude less than when developed without LAHCI. Culture-independent community analysis demonstrated that 500 mM LAHCl substantially altered biofilm species composition: the proportion of Streptococcus and Veillonella species increased and the proportion of Gram-negative bacteria such as Neisseria and Aggregatibacter species was reduced. Adding LAHCl to pre-formed biofilms also reduced biovolume, presumably by altering cell-cell interactions and causing cell detachment. Furthermore, supplementing 0.01% cetylpyridinium chloride (CPC), an antimicrobial commonly used for the treatment of dental plaque, with 500 mM LAHCl resulted in greater penetration of CPC into the biofilms and significantly greater killing compared to a non-supplemented 0.01% CPC solution. Collectively, this work demonstrates that LAHCl moderates multi-species oral biofilm development and community composition and enhances the activity of CPC. The incorporation of LAHCl into oral healthcare products may be useful for enhanced biofilm control.
Patents
: Arginine vs Plaque
WO2015048146
COMPOSITIONS AND METHOD FOR DESTABILIZING, ALTERING, AND DISPERSING BIOFILMS
COMPOSITIONS AND METHOD FOR DESTABILIZING, ALTERING, AND DISPERSING BIOFILMS
Inventor: RICKARD ALEXANDER, et al.
The present disclosure relates to compositions and methods for destabilizing biofilms, altering biofilm 3D structure, and dispersing biofilms, in order to enhance biofilm cell removal and/or sensitivity to other agents (e.g., environmental or co-applied treatments). In particular, the present disclosure relates to the use of L-arginine in the removal and/or sensitization (e.g., to antimicrobials) of microorganisms in medical, industrial, domestic, or environmental applications, as well as treatment of bacterial infections (e.g., in biofilms).
BACKGROUND OF THE INVENTION
A biofilm is a well-organized community of microorganisms that adheres to surfaces and is embedded in slimy extracellular polymeric substances (EPSs). EPS is a complex mixture of high-molecular-mass polymers (> 10,000 Da) generated by the bacterial cells, cell lysis and hydrolysis products, and organic matter adsorbed from the substrate. EPSs are involved in the establishment of stable arrangements of microorganisms in biofilms
(Wolfaardt et al. (1998) Microb. Ecol. 35:213-223; herein incorporated by reference in its entirety), and extracellular DN A (eDNA) is one of the major components of EPSs (Flemming et al. (2001) Water Sci. Technol. 43:9-16; Spoering et al. (2006) Curr. Opin. Microbiol. 9: 133-137; each herein incorporated by reference in its entirety). Bacteria living in a biofilm usually have significantly different properties from free-floating (planktonic) bacteria of the same species, as the dense and protected environment of the film allows them to cooperate and interact in various ways. One benefit of this environment is increased resistance to detergents and antibiotics, as the dense extracellular matrix and the outer layer of cells protect the interior of the community. In some cases antibiotic resistance can be increased a thousand-fold (Stewart et al. (2001) Lancet 358: 135-138; herein incorporated by reference in its entirety). Biofilms can be formed in various bacterial species (e.g., Acinetobacter sp. (e.g., A. baylyi, A. baumannii), Staphylococcus aureus, Stenotrophomonas maltophilia, Escherichia coli (e.g., E. coli K-12). The formation of biofilms by such species is a major determinant of medical outcome during the course of colonization or infection. For example, Acinetobacter spp. frequently colonize patients in clinical settings through formation of biofilms on ventilator tubing, on skin and wound sites, medical tubing, and the like, and are a common cause of nosocomial pneumonia.
As biofilms are complex structures formed of various elements, their removal or disruption traditionally requires the use of dispersants, surfactants, detergents, enzyme formulations, antibiotics, biocides, boil-out procedures, corrosive chemicals, mechanical cleaning, use of antimicrobial agents, inhibiting microbial attachment, inhibiting biofilm growth by removing essential nutrients and promoting biomass detachment and degradation of biofilm matrix (Chen XS, P.S.: Biofilm removal caused by chemical treatments. Water Res 2000;34:4229-4233; herein incorporated by reference in its entirety). However, such classical removal or disruption methods are not efficacious or feasible in all situations where biofilm formation is undesirable.
Additional methods for undesirable bacteria in biofilms are needed.
SUMMARY OF THE INVENTION
The present disclosure relates to compositions and methods for destabilizing biofilms, altering biofilm 3D structure, and dispersing biofilms, in order to enhance biofilm cell removal and/or sensitivity to other agents (e.g., environmental or co-applied treatments). In particular, the present disclosure relates to the use of L-arginine in the removal and/or sensitization (e.g., to antimicrobials) of microorganisms in medical, industrial, domestic, or environmental applications, as well as treatment of bacterial infections (e.g., in biofilms).
Embodiments of the present invention provide compositions (e.g., pharmaceutical, commercial, health care, etc.), systems, uses, and methods that result in one or more of: inducing cell-damage, killing cells, disrupting intra-cellular processes leading to
deregulation/loss of homeostasis, disrupting cell-cell adhesion, inducing three dimensional rearrangement of architecture, disrupting cell-cell signaling, disrupting cell-cell metabolic interactions, disrupting adhesion to surfaces, reducing the pathogenic potential of biofilms, reducing biofilm mass, decreasing the proportion of pathogenic bacteria in a biofilm, increasing the proportion of beneficial bacteria in a biofilm, or preventing growth of a microorganism in a biofilm, comprising: contacting bacteria in a biofilm with cell-free L- arginine at a concentration of at least 1 mM, wherein the contacting kills or inhibits the growth of microorganisms and/or alters the 3D arrangement of the cells in the biofilm, which can damage bacteria by preventing them from interacting with others and/or exposing them to deleterious environmental effects. In some embodiments, the microorganism is a bacterium. In some embodiments, the bacteria are in a coaggregate. In some embodiments, the biofilm is a dental biofilm. In some embodiments, the bacteria are in a coaggregate or biofilm with a plurality of different bacterial species (e.g., of Streptococcus and Actinomyces, such as, for example, S. gordonii and A. oris). In some embodiments, the L-arginine prevents coaggregation or promotes de-adhesion/dispersion of said bacteria. In some embodiments, the L-arginine is present at a concentration of at least 1 mM (e.g., at least 10 mM, at least 50 mM, at least 100 mM, 200 mM, at least 250 mM, at least 300 mM, at least 350 mM, at least 400 mM, at least 450 mM, at least 500 mM, at least 600 mM, at least 700 mM, at least 800 mM, at least 900 mM, or at least 1 M). In some embodiments, the bacteria are in multi- species oral biofilms (e.g., dental plaque in saliva). In some embodiments, L-arginine disrupts biofilms grown in saliva without antimicrobial activity. In some embodiments, the method further comprises contacting the bacteria with cetylpyridinium chloride (CPC).
Additional embodiments comprise the use of a composition comprising L-arginine at a concentration of at least 1 mM to induce one or more of: inducing microbial cell-damage, killing cells, disrupting intra-cellular processes leading to deregulation/loss of homeostasis, disrupting cell-cell adhesion, inducing three dimensional rearrangement of architecture, disrupting cell-cell signaling, disrupting cell-cell metabolic interactions, disrupting adhesion to surfaces, reducing the pathogenic potential of biofilms, reducing biofilm mass, decreasing the proportion of pathogenic bacteria in a biofilm, increasing the proportion of beneficial bacteria in a biofilm, or preventing growth of a microorganism in a biofilm. In some embodiments, the composition further comprises at cetylpyridinium chloride (CPC).
Further embodiments provide a plasmid that reports expression or concentration of a component of a biofilm or planktonic cell population, where the plasmid comprises either a first marker under the control of a constitutive promoter or a second marker under the control of a promoter induced by the component. In some embodiments, the marker is a fluorescent marker (e.g., GFP or Mcherry). In some embodiments, the first promoter is a streptococcal ribosomal promoter (e.g., a S. gordonii DL1 50S ribosomal protein (SGO l 192) promoter). In some embodiments, the second promoter is S. gordonii catabolite control protein A (SGO 0773), or S. gordonii argC or arcA promoter.
Additional embodiments provide a streptococcal cell (e.g., S. gordonii) comprising the plasmid. In some embodiments, the cell is in a biofilm.
Some embodiments provide methods and uses of monitoring concentration of a component (e.g., arginine or AI-2) of a biofilm or planktonic cell culture, comprising: a) contacting a streptococcal cell with the plasmid described herein; and b) measuring the level of the marker. In some embodiments, the level of expression of the marker is correlated to the level of the component. In some embodiments, the method further comprises the step of contacting the cell with a test compound (e.g. a drug that kills or inhibits or is suspected of killing or inhibiting the growth of the cell).
Additional embodiments are described herein.
DESCRIPTION OF THE FIGURES
Figure 1 shows the role of arginine in dental plaque growth. High and low concentrations of L-arginine cause biofilm destabilization and results in many cells to disperse/de-adhere from the biofilm leaving behind dead/damage unresponsive cells.

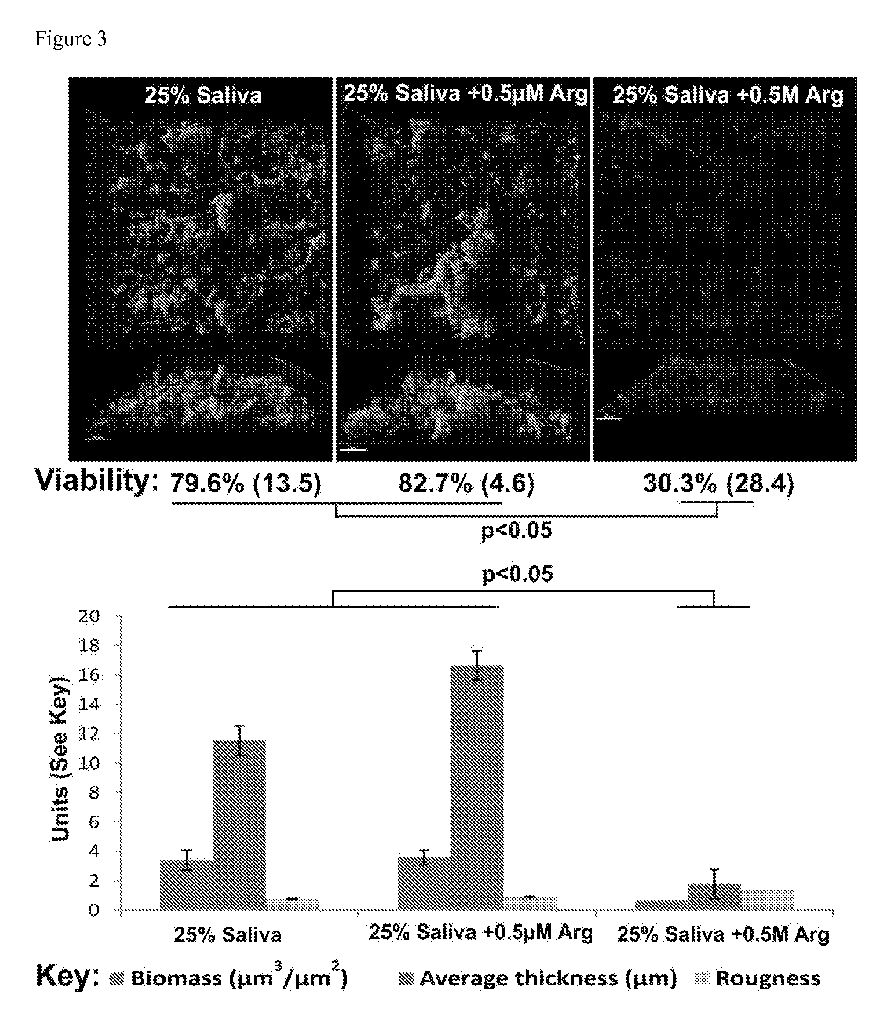
Figure 3 shows S. gordonii bio films in saliva with or without 0.5 mM or 0.5 M (500 mM) arginine (Arg).
Figure 4 shows disruption of arcR reduces biofilm formation by S. gordonii.
Figure 5 shows the effect of L-arginine on species composition of saliva derived community developed in pooled filter sterilized saliva. (A) Showing the increase in bacterial diversity (operational taxonomic units, OTU) caused by prolonged exposure of oral multi- species biofilms to 500mM L-arginine. Black-colored bars represent data derived from the analysis of biofilms developed in flowing non-supplemented saliva while the grey-colored bars represent data derived from the analysis of biofilms developed in 500 mM supplemented saliva. (B) Showing changes in phyla (color coding as before). (C) Showing changes in composition of genera (key is from left to right in order from bottom to top in bar; Neisseria, Granulicatella, Streptococcus, etc).
Figure 6 shows the effect of growing multi-species biofilms in increasing concentrations of L-arginine under static conditions. Representative 3D renderings of 20 h- old oral biofilms grown from a cell-containing saliva (CCS) inoculum in the static biofilm system containing cell free saliva (CFS) supplemented with different concentrations of L- arginine monohydrochloride (LAHC1). Upper renderings (Ai-Hi) are of the x-y plane.
Middle renderings (?2-?2) are of the x-z plane. Lower renderings (A3-H3) represent an angled view (x-y-z). Bars represent 50 µ??. Associated table shows changes in mean percentage cell viability.
Figure 7 shows that L-arginine destabilizes the architecture of multi-species oral biofilms grown in saliva under flowing conditions in a microfluidics channel. Representative 3D renderings and biofilm characteristics derived from computational image analysis of oral biofilms developed for 20 h in different concentrations of L-arginine monohydrochloride (LAHC1) in the Bio flux™ flowing saliva biofilm system.
Figure 8 shows that 500mM L-arginine destabilizes preformed multi-species oral biofilms of differing developmental age.
Figure 9 shows that L-arginine destabilizes pre-formed multi-species oral biofilm communities and in doing so can enhance the penetration of CPC (0.01 or 0.05%). Specifically, this figure shows that 500mM L-arginine enhances the penetration and killing of CPC so that less CPC is required as compared to when used in the absence of L-arginine.
Figure 10 shows fold induction of bio luminescence by Vibrio harveyi BB170 which is responsive to AI-2. AI-2 is shown to be produced in increasing amounts as L-arginine concentration increases. The AI-2 data are normalized to the "control" (non-supplemented saliva).
Figure 11 shows that application of L-arginine but not D-arginine destabizes and prevents growth of bacterial communities.
Figure 12 shows (A) modifiable plasmid (pPElOlO) to allow the generation of fluorescent Streptococcus gordonii DL1 (GFP or Mcherry) and (B) an example of two promoter that allow the evaluation of the differential expression of GFP fluorescence in S. gordonii DL1 biofilms in response to exogenous ly added AI-2.
DEFINITIONS
To facilitate an understanding of the present invention, a number of terms and phrases are defined below:
As used herein the term "biofilm" refers to any three-dimensional, (e.g., matrix- encased) microbial community displaying multicellular characteristics. Accordingly, as used herein, the term biofilm includes surface-associated biofilms as well as biofilms in suspension, such as floes and granules. Biofilms may comprise a single microbial species or may be mixed species complexes, and may include bacteria as well as fungi, algae, protozoa, or other microorganisms. In some embodiments, biofilms comprise coaggregating organisms. In some embodiments, biofilms comprise a single organism or multiple organisms that do not coaggregate.
As used herein, the term "host cell" refers to any eukaryotic or prokaryotic cell (e.g., bacterial cells such as E. coli, yeast cells, mammalian cells, avian cells, amphibian cells, plant cells, fish cells, and insect cells), whether located in vitro or in vivo. For example, host cells may be located in a transgenic animal.
As used herein, the term "prokaryotes" refers to a group of organisms that usually lack a cell nucleus or any other membrane-bound organelles. In some embodiments, prokaryotes are bacteria. The term "prokaryote" includes both archaea and eubacteria.
As used herein, the term "subject" refers to individuals {e.g., human, animal, or other organism) to be treated by the methods or compositions of the present invention. Subjects include, but are not limited to, mammals {e.g., murines, simians, equines, bovines, porcines, canines, felines, and the like), and most preferably includes humans. In the context of the invention, the term "subject" generally refers to an individual who will receive or who has received treatment for a condition characterized by the presence of bio film-forming bacteria, or in anticipation of possible exposure to biofilm-forming bacteria.
As used herein the term, "in vitro" refers to an artificial environment and to processes or reactions that occur within an artificial environment. In vitro environments include, but are not limited to, test tubes and cell cultures. The term "in vivo" refers to the natural environment {e.g. , an animal or a cell) and to processes or reaction that occur within a natural environment.
As used herein, the term "virulence" refers to the degree of pathogenicity of a microorganism (e.g., bacteria or fungus), e.g., as indicated by the severity of the disease produced or its ability to invade the tissues of a subject. It is generally measured experimentally by the median lethal dose (LD50) or median infective dose (ID50). The term may also be used to refer to the competence of any infectious agent to produce pathologic effects.
As used herein, the term "effective amount" refers to the amount of a composition (e.g., a composition comprising L-arginine) sufficient to effect beneficial or desired results. An effective amount can be administered in one or more administrations, applications or dosages and is not intended to be limited to a particular formulation or administration route. In some embodiments, the effective amount is at least 1 mM (e.g., 10 mM, 50 mM, 100 mM, 200 mM, 300 mM, 400 mM, 500 mM, 750 mM, 1000 mM or more).
As used herein, the term "administration" refers to the act of giving a drug, prodrug, or other agent, or therapeutic treatment (e.g., compositions comprising L-arginine) to a physiological system (e.g., a subject or in vivo, in vitro, or ex vivo cells, tissues, and organs). Exemplary routes of administration to the human body can be through the eyes (ophthalmic), mouth (oral), skin (transdermal), nose (nasal), lungs (inhalant), oral mucosa (buccal), ear, by injection (e.g., intravenously, subcutaneously, intratumorally, intraperitoneally, etc.), topical administration and the like.
As used herein, the term "treating a surface" refers to the act of exposing a surface to one or more compositions comprising L-arginine. Methods of treating a surface include, but are not limited to, spraying, misting, submerging, and coating.
As used herein, the term "co -administration" refers to the administration of at least two agent(s) (e.g. , L-arginine in combination with an antimicrobial agent) or therapies to a subject. In some embodiments, the co-administration of two or more agents or therapies is concurrent. In other embodiments, a first agent/therapy is administered prior to a second agent/therapy. Those of skill in the art understand that the formulations and/or routes of administration of the various agents or therapies used may vary. The appropriate dosage for co-administration can be readily determined by one skilled in the art. In some embodiments, when agents or therapies are co -administered, the respective agents or therapies are administered at lower dosages than appropriate for their administration alone. Thus, coadministration is especially desirable in embodiments where the co-administration of the agents or therapies lowers the requisite dosage of a potentially harmful (e.g., toxic) agent(s).
As used herein, the term "wound" refers broadly to injuries to tissue including the skin, subcutaneous tissue, muscle, bone, and other structures initiated in different ways, for example, surgery, (e.g., open post cancer resection wounds, including but not limited to, removal of melanoma and breast cancer etc.), contained post-operative surgical wounds, pressure sores (e.g., from extended bed rest) and wounds induced by trauma. As used herein, the term "wound" is used without limitation to the cause of the wound, be it a physical cause such as bodily positioning as in bed sores or impact as with trauma or a biological cause such as disease process, aging process, obstetric process, or any other manner of biological process. Wounds caused by pressure may also be classified into one of four grades depending on the depth of the wound: i) Grade I: wounds limited to the epidermis; ii) Grade II: wounds extending into the dermis; iii) Grade III: wounds extending into the subcutaneous tissue; and iv) Grade IV: wounds wherein bones are exposed (e.g., a bony pressure point such as the greater trochanter or the sacrum). The term "partial thickness wound" refers to wounds that are limited to the epidermis and dermis; a wound of any etiology may be partial thickness. The term "full thickness wound" is meant to include wounds that extend through the dermis.
As used herein, "wound site" refers broadly to the anatomical location of a wound, without limitation.
As used herein, the term "dressing" refers broadly to any material applied to a wound for protection, absorbance, drainage, treatment, etc. Numerous types of dressings are commercially available, including films (e.g., polyurethane films), hydrocoUoids (hydrophilic colloidal particles bound to polyurethane foam), hydrogels (cross-linked polymers containing about at least 60% water), foams (hydrophilic or hydrophobic), calcium alginates (nonwoven composites of fibers from calcium alginate), and cellophane (cellulose with a plasticizer) (Kannon and Garrett (1995) Dermatol. Surg. 21 : 583-590; Davies (1983) Burns 10: 94; each herein incorporated by reference). The present invention also contemplates the use of dressings impregnated with pharmacological compounds (e.g., antibiotics, antiseptics, thrombin, analgesic compounds, etc). Cellular wound dressings include commercially available materials such as Apligraf®, Dermagraft®, Biobrane®, TransCyte®, Integra® Dermal Regeneration Template®, and OrCell®.
As used herein, the term "toxic" refers to any detrimental or harmful effects on a subject, a cell, or a tissue as compared to the same cell or tissue prior to the administration of the toxicant.
As used herein, the term "pharmaceutical composition" refers to the combination of an active agent {e.g., L-arginine) with a carrier, inert or active, making the composition especially suitable for diagnostic or therapeutic use in vitro, in vivo or ex vivo. The terms "pharmaceutically acceptable" or "pharmacologically acceptable," as used herein, refer to compositions that do not substantially produce adverse reactions, e.g., toxic, allergic, or immunological reactions, when administered to a subject.
As used herein, the term "topically" refers to application of the compositions of the present invention to the surface of the skin and mucosal cells and tissues {e.g., alveolar, buccal, lingual, masticatory, or nasal mucosa, and other tissues and cells which line hollow organs or body cavities).
As used herein, the term "pharmaceutically acceptable carrier" refers to any of the standard pharmaceutical carriers including, but not limited to, phosphate buffered saline solution, water, emulsions {e.g., such as an oil/water or water/oil emulsions), and various types of wetting agents, any and all solvents, dispersion media, coatings, sodium lauryl sulfate, isotonic and absorption delaying agents, disintrigrants {e.g., potato starch or sodium starch glycolate), and the like. The compositions also can include stabilizers and
preservatives. For examples of carriers, stabilizers, and adjuvants. (See e.g., Martin, Remington's Pharmaceutical Sciences, 15th Ed., Mack Publ. Co., Easton, Pa. (1975), incorporated herein by reference). In certain embodiments, the compositions of the present invention may be formulated for veterinary, horticultural or agricultural use. Such formulations include dips, sprays, seed dressings, stem injections, sprays, and mists. In certain embodiments, compositions of the present invention may be used in any application where it is desirable to alter (e.g., inhibit) the formation of biofilms, e.g., food industry applications; consumer goods (e.g., medical goods, goods intended for consumers with impaired or developing immune systems (e.g., infants, children, elderly, consumers suffering from disease or at risk from disease), and the like.
As used herein, the term "medical devices" includes any material or device that is used on, in, or through a subject's or patient's body, for example, in the course of medical treatment {e.g., for a disease or injury). Medical devices include, but are not limited to, such items as medical implants, wound care devices, drug delivery devices, and body cavity and personal protection devices. The medical implants include, but are not limited to, urinary catheters, intravascular catheters, dialysis shunts, wound drain tubes, skin sutures, vascular grafts, implantable meshes, intraocular devices, heart valves, and the like. Wound care devices include, but are not limited to, general wound dressings, biologic graft materials, tape closures and dressings, and surgical incise drapes. Drug delivery devices include, but are not limited to, needles, drug delivery skin patches, drug delivery mucosal patches and medical sponges. Body cavity and personal protection devices, include, but are not limited to, tampons, sponges, surgical and examination gloves, contact lenses, and toothbrushes. Birth control devices include, but are not limited to, intrauterine devices (IUDs), diaphragms, and condoms.
As used herein, the term "therapeutic agent," refers to compositions that decrease the infectivity, morbidity, or onset of mortality in a subject (e.g., a subject contacted by a biofilm- forming microorganism) or that prevent infectivity, morbidity, or onset of mortality in a host contacted by a biofilm- forming microorganism. As used herein, therapeutic agents encompass agents used prophylactically, e.g. , in the absence of a bio film- forming organism, in view of possible future exposure to a bio film-forming organism. Such agents may additionally comprise pharmaceutically acceptable compounds {e.g. , adjuvants, excipients, stabilizers, diluents, and the like). In some embodiments, the therapeutic agents of the present invention are administered in the form of topical compositions, injectable compositions, ingestible compositions, and the like. When the route is topical, the form may be, for example, a solution, cream, ointment, salve or spray.
As used herein, the term "pathogen" refers to a biological agent that causes a disease state {e.g., infection, cancer, etc.) in a host. "Pathogens" include, but are not limited to, viruses, bacteria, archaea, fungi, protozoans, mycoplasma, prions, and parasitic organisms.
As used herein, the term "microbe" refers to a microorganism and is intended to encompass both an individual organism, or a preparation comprising any number of the organisms.
As used herein, the term "microorganism" refers to any species or type of microorganism, including but not limited to, bacteria, archaea, fungi, protozoans, mycoplasma, and parasitic organisms.
As used herein, the term "fungi" is used in reference to eukaryotic organisms such as the molds and yeasts, including dimorphic fungi.
The terms "bacteria" and "bacterium" refer to all prokaryotic organisms, including those within all of the phyla in the Kingdom Procaryotae. It is intended that the term encompass all microorganisms considered to be bacteria including Mycoplasma, Chlamydia, Actinomyces, Streptomyces, and Rickettsia. All forms of bacteria are included within this definition including cocci, bacilli, spirochetes, spheroplasts, protoplasts, etc. Also included within this term are prokaryotic organisms that are Gram-negative or Gram-positive. "Gram- negative" and "Gram-positive" refer to staining patterns with the Gram-staining process, which is well known in the art. (See e.g., Finegold and Martin, Diagnostic Microbiology, 6th Ed., CV Mosby St. Louis, pp. 13-15 (1982)). "Gram-positive bacteria" are bacteria that retain the primary dye used in the Gram-stain, causing the stained cells to generally appear dark blue to purple under the microscope. "Gram-negative bacteria" do not retain the primary dye used in the Gram-stain, but are stained by the counterstain. Thus, Gram-negative bacteria generally appear red.
The term "non-pathogenic bacteria" or "non-pathogenic bacterium" includes all known and unknown non-pathogenic bacterium (Gram-positive or Gram-negative) and any pathogenic bacterium that has been mutated or converted to a non-pathogenic bacterium. Furthermore, a skilled artisan recognizes that some bacteria may be pathogenic to specific species and non-pathogenic to other species; thus, these bacteria can be utilized in the species in which it is non-pathogenic or mutated so that it is non-pathogenic.
As used herein, the term "non-human animals" refers to all non-human animals including, but are not limited to, vertebrates such as rodents, non-human primates, ovines, bovines, ruminants, lagomorphs, porcines, caprines, equines, canines, felines, aves, etc.
As used herein, the term "cell culture" refers to any in vitro culture of cells, including, e.g., prokaryotic cells and eukaryotic cells. Included within this term are continuous cell lines (e.g., with an immortal phenotype), primary cell cultures, transformed cell lines, finite cell lines (e.g., non-transformed cells), bacterial cultures in or on solid or liquid media, and any other cell population maintained in vitro.
The term "coating" as used herein refers to a layer of material covering, e.g., a medical device or a portion thereof. A coating can be applied to the surface or impregnated within the material of the implant.
As used herein, the term "antimicrobial agent" refers to composition that decreases, prevents or inhibits the growth of bacterial and/or fungal organisms. Examples of antimicrobial agents include, e.g., antibiotics and antiseptics.
The term "antiseptic" as used herein is defined as an antimicrobial substance that inhibits the action of microorganisms, including but not limited to a-terpineol, methylisothiazolone, cetylpyridinium chloride, chloroxyleneol, hexachlorophene,
chlorhexidine and other cationic biguanides, methylene chloride, iodine and iodophores, triclosan, taurinamides, nitrofurantoin, methenamine, aldehydes, azylic acid, silver, benzyl peroxide, alcohols, and carboxylic acids and salts. One skilled in the art is cognizant that these antiseptics can be used in combinations of two or more to obtain a synergistic or additive effect. Some examples of combinations of antiseptics include a mixture of chlorhexidine, chlorhexidine and chloroxylenol, chlorhexidine and methylisothiazolone, chlorhexidine and (a-terpineol, methylisothiazolone and a-terpineol; thymol and chloroxylenol; chlorhexidine and cetylpyridinium chloride; or chlorhexidine, methylisothiazolone and thymol. These combinations provide a broad spectrum of activity against a wide variety of organisms.
The term "antibiotics" as used herein is defined as a substance that inhibits the growth of microorganisms, preferably without damage to the host. For example, the antibiotic may inhibit cell wall synthesis, protein synthesis, nucleic acid synthesis, or alter cell membrane function.
Classes of antibiotics include, but are not limited to, macro lides (e.g., erythromycin), penicillins (e.g., nafcillin), cephalosporins (e.g., cefazolin), carbapenems (e.g., imipenem), monobactam (e.g., aztreonam), other beta- lactam antibiotics, beta-lactam inhibitors (e.g., sulbactam), oxalines (e.g. linezolid), aminoglycosides (e.g., gentamicin), chloramphenicol, sufonamides (e.g., sulfamethoxazole), glycopeptides (e.g., vancomycin), quinolones (e.g., ciprofloxacin), tetracyclines (e.g., minocycline), fusidic acid, trimethoprim, metronidazole, clindamycin, mupirocin, rifamycins (e.g., rifampin), streptogramins (e.g., quinupristin and dalfopristin) lipoprotein (e.g., daptomycin), polyenes (e.g., amphotericin B), azoles (e.g., fluconazole), and echinocandins (e.g., caspofungin acetate).
Examples of specific antibiotics include, but are not limited to, erythromycin, nafcillin, cefazolin, imipenem, aztreonam, gentamicin, sulfamethoxazole, vancomycin, ciprofloxacin, trimethoprim, rifampin, metronidazole, clindamycin, teicoplanin, mupirocin, azithromycin, clarithromycin, ofloxacin, lomefloxacin, norfloxacin, nalidixic acid, sparfloxacin, pefloxacin, amifloxacin, gatifloxacin, moxifloxacin, gemifloxacin, enoxacin, fleroxacin, minocycline, linezolid, temafloxacin, tosufloxacin, clinafloxacin, sulbactam, clavulanic acid, amphotericin B, fluconazole, itraconazole, ketoconazole, and nystatin. Other examples of antibiotics, such as those listed in Sakamoto et al, U.S. Pat. No. 4,642, 104 herein incorporated by reference will readily suggest themselves to those of ordinary skill in the art.
As used herein, the term "sample" is used in its broadest sense. In one sense, it is meant to include a specimen or culture obtained from any source, as well as biological and environmental samples. Biological samples may be obtained from animals (including humans) and encompass fluids, solids, tissues, and gases. Biological samples include blood products, such as plasma, serum and the like. Such examples are not however to be construed as limiting the sample types applicable to the present invention.
DETAILED DESCRIPTION OF THE INVENTION
The present disclosure relates to compositions and methods for destabilizing biofilms, altering biofilm 3D structure, and dispersing biofilms, in order to enhance biofilm cell removal and/or sensitivity to other agents (e.g., environmental or co-applied treatments). In particular, the present disclosure relates to the use of L-arginine in the removal and/or sensitization (e.g., to antimicrobials) of microorganisms in medical, industrial, domestic, or environmental applications, as well as treatment of bacterial infections (e.g., in biofilms).
A biofilm is an aggregate of microorganisms in which cells adhere to each other and/or to a surface. These adherent cells are frequently embedded within a self-produced matrix of extracellular polymeric substance (EPS). Biofilm EPS, also referred to as slime, is a polymeric conglomeration generally composed of extracellular DNA, proteins, and polysaccharides in various configurations and of various compositions. Biofilms may form on living or non-living surfaces, and represent a prevalent mode of microbial life in natural, industrial and clinical settings. The microbial cells growing in a biofilm are physiologically distinct from planktonic cells of the same organism, which, by contrast, are single cells that may float or swim in a liquid medium.
Microbial biofilms form in response to many factors including but not limited to cellular recognition of specific or non-specific attachment sites on a surface, nutritional cues, or in some cases, by exposure of planktonic cells to sub-inhibitory concentrations of antibiotics. When a cell switches to the biofilm mode of growth, it undergoes a phenotypic shift in behavior in which large suites of genes are differentially regulated (Petrova et al., J. Bacteriol. 2012 May;194(10):2413-25; Stoodley et al, Annu Rev Microbiol. 2002;56: 187- 209).
Although the present invention is not limited by any type of biofilm, biofilm formation typically begins with the attachment of free-floating microorganisms to a surface. These first colonists adhere to the surface initially through weak, reversible Van der Waals forces. If the colonists are not immediately separated from the surface, they can anchor themselves more permanently using cell adhesion structures such as pili.
Initial colonists commonly facilitate the arrival of other cells by providing more diverse adhesion sites and beginning to build the matrix that holds the biofilm together. Some species are not able to attach to a surface on their own but are often able to anchor themselves to the matrix or directly to earlier colonists. It is during this colonization that the cells are able to communicate via quorum sensing, for example, using such compounds as N-acyl homoserine lactone (AHL). Once colonization initiates, the biofilm grows through a combination of cell division and recruitment. The final stage of biofilm formation is known as development although herein the terms "formation" and "development" are used interchangeably. In this final stage, the biofilm is established and may only change in shape and size. The development of a biofilm may allow for an aggregate cell colony (or colonies) to be increasingly antibiotic resistant.
Dispersal of cells from the biofilm colony is an essential stage of the biofilm lifecycle.
Dispersal enables bio films to spread and colonize new surfaces. Enzymes that degrade the biofilm extracellular matrix, such as dispersin B and deoxyribonuclease, may play a role in biofilm dispersal (Whitchurch et al. (2002) Science 295: 1487; herein incorporated by reference in its entirety). Biofilm matrix degrading enzymes may be useful as anti-biofilm agents (Kaplan et al. (2004) Antimicrobial Agents and Chemotherapy 48 (7): 2633-6; Xavier et al. (2005) Microbiology 151 (Pt 12): 3817-32; each herein incorporated by reference in its entirety). A fatty acid messenger, cis-2-decenoic acid, can induce dispersion and inhibit growth of biofilm colonies. Secreted by Pseudomonas aeruginosa, this compound induces dispersion in several species of bacteria and the yeast Candida albicans (Davies et al. (2009) Journal of Bacteriology 191 (5): 1393-403; herein incorporated by reference in its entirety).
Biofilms are ubiquitous and are usually found on solid substrates submerged in or exposed to some aqueous solution, although they can form as floating mats on liquid surfaces and also on the surface of leaves, particularly in high humidity climates. Given sufficient resources for growth, a biofilm will quickly grow to be macroscopic. Many types of microbes can form biofilms, e.g., bacteria, archaea, protozoa, fungi and algae. Biofilms may comprise a single type of microbe (monospecies biofilms), or, commonly, multiple types. In some mixed species biofilms, each group performs specialized metabolic functions.
Biofilms form in environments including but not limited to: substrates (e.g., rocks, pebbles) in natural bodies of water (e.g., rivers, pools, streams, oceans, springs); extreme environments (e.g., hot springs including waters with extremely acidic or extremely alkaline pH; frozen glaciers); residential and industrial settings in which solid surfaces are exposed to liquid (e.g., showers, water and sewage pipes, floors and counters in food preparation or processing areas, water-cooling systems, marine engineering systems); hulls and interiors of marine vessels; sewage and water treatment facilities (e.g., water filters, pipes, holding tanks); contaminated waters; within or upon living organisms (e.g., dental plaque, surface colonization or infection of e.g., skin, surfaces of tissues or organs or body cavities or at wound sites; plant epidermis, interior of plants); on the inert surfaces of implanted devices such as catheters, prosthetic cardiac valves, artificial joints, and intrauterine devices; and the like.
Biofilms are involved in a wide variety of microbial infections in the body. Infectious processes in which biofilms have been implicated include but are not limited to urinary tract infections, catheter infections, middle-ear infections, formation of dental plaque and gingivitis, contact lens contamination (Imamura et al. (2008) Antimicrobial Agents and Chemotherapy 52 (1): 171-82; herein incorporated by reference in its entirety), and less common but more lethal processes such as endocarditis, infections in cystic fibrosis, and infections of permanent indwelling devices such as joint prostheses and heart valves (Lewis et al. (2001) Antimicrobial Agents and Chemotherapy 45 (4): 999-1007; Parsek et al. (2003) Annual Review of Microbiology 57: 677-701; each herein incorporated by reference in its entirety). Bacterial biofilms may impair cutaneous wound healing and reduce topical antibacterial efficiency in healing or treating infected skin wounds (Davis et al. (2008) Wound Repair and Regeneration 16 (1): 23-9; herein incorporated by reference in its entirety).
Coaggregation is the highly specific recognition and adhesion of genetically distinct bacteria mediated by complementary protein adhesins and polysaccharide receptors on the cell surface of coaggregating cells (Kolenbrander, Annu Rev Microbiol 54, 413-437, 2000; Rickard et al., Trends Microbiol 11, 94-100, 2003a). This phenomenon is distinct from autoaggregation, which is the recognition and adhesion of genetically identical bacteria to one another (Khemaleelakul et al. , J Endod 32, 312-318, 2006; Rickard et al. , FEMS
Microbiol Lett 220, 133-140, 2003b; Van Houdt & Michiels, Res Microbiol 156, 626-633, 2005). Coaggregation was first described between human dental plaque bacteria in 1970 (Gibbons & Nygaard, Arch Oral Biol 15, 1397-1400, 1970), and work over the last two decades has shown that it also occurs between bacteria isolated from the human gut, the human urogenital tract, in wastewater floes, and freshwater biofilms (Ledder et al. , FEMS Microbiol Ecol 66, 630-636, 2008; Phuong et al, J Biotechnol, 2011; Reid et al, Can J Microbiol 34, 344-351, 1988; Rickard et al, Appl Environ Microbiol 66, 431-434, 2000; Simoes et al, Appl Environ Microbiol 74, 1259-1263, 2008). Coaggregation has also been shown to occur among numerous taxonomically distinct freshwater species (Rickard et al , Appl Environ Microbiol 68, 3644-3650, 2002; Rickard et al, 2003b, supra; Rickard et al, Appl Environ Microbiol 70, 7426-7435, 2004b; Simoes et al, Appl Environ Microbiol 74, 1259-1263, 2008) and in planktonic and biofilm populations (Rickard et al , J Appl Microbiol 96, 1367-1373, 2004a). Studies of coaggregation between Sphingomonas (Blastomonas) natatoria and Micrococcus luteus demonstrated that the ability of a species to coaggregate alters dual-species biofilm development in both flowing and static environments (Min & Rickard, Appl Environ Microbiol 75, 3987-3997, 2009; Min et al, Biofouling 26, 931-940, 2010). Coaggregation may mediate biofilm development, architectural changes, and alterations in the species composition (Hojo et al, J Dent Res 88, 982-990, 2009;
Kolenbrander et al, Periodontal 2000 42, 47-79, 2006; Rickard et al, 2003a, supra). In addition, coaggregation may play a role in promoting or hindering the integration of pathogenic species into freshwater biofilms (Buswell et al, Appl Environ Microbiol 64, 733- 741, 1998). Evidence to support such a possibility can be found in studies of dental plaque biofilms where coaggregation has been indicated to promote the integration of oral pathogens such as Porphyromonas gingivalis (Kolenbrander et al, Periodontal 2000 42, 47-79, 2006; Whitmore & Lamont, Mol Microbiol 81, 305-314, 2011).
Dental plaque is composed of hundreds of species of bacteria that can collectively cause oral and systemic diseases (Jakubovics et al, Oral diseases. 2010;16(8):729-39; Kuo et al, Public Health. 2008;122(4):417-33.). During dental plaque development, bacteria sense and respond to numerous exogenous bacterial- or environmental-derived chemicals which alter their ability to establish themselves within these biofilms.
Oral biofilms cause major problems throughout both industrialized and developing countries. Data from recent surveys indicate that 23.7% of US adults have untreated dental caries while 38.5% of adults have moderate to severe periodontitis (National Center for
Health Statistics. Health, United States, 2011 : With Special Feature on Socioeconomic Status and Health. Hyattsville, MD: 2012; Eke et al, Journal of dental research. 2012;91(10):914- 20). Untreated dental caries also affects between 15-20% of children up to 19 years, while periodontitis is a major problem in the elderly population, where 64% of adults over 65 years have moderate to severe forms of the condition (National Center for Health Statistics. Health, United States, 2011 : With Special Feature on Socioeconomic Status and Health. Hyattsville, MD: 2012; Eke et al, Journal of dental research. 2012;91(10):914-20). Clearly, new methods for controlling dental plaque-related diseases are urgently needed. Dental plaque is a finely balanced homeostatic bacterial community (Marsh et al., Periodontology 2000. 2011 ;55(1): 16-35). Embodiments of the present invention provide a broad-acting intervention that alters the balance of such oral bacterial communities and is more effective at controlling dental plaque -related diseases than strategies that target individual species. Dental plaque contains an interactive "aware" community of microbes. On cleaned tooth-surfaces, dental plaque develops through a microbial succession (Jakubovics et al, supra; Kolenbrander et al, Nature reviews Microbiology. 2010;8(7):471-80; Kolenbrander et al., Periodontology 2000. 2006;42:47-79 23, 24). Initial colonizers, predominantly
Streptococcus species, adhere to salivary pellicle and produce thin layers of bio film that support the integration of other species through coaggregation, cell-cell signalling, and metabolite recognition (Jakubovics et al, supra; Kolenbrander et al, Nature reviews
Microbiology. 2010;8(7):471-80; Hojo et al, Journal of dental research. 2009;88(11):982-90; Rickard et al., Trends Microbiol. 2003;11(2):94-100; Bowden et al., Advances in dental research. 1997;1 l(l):81-99). Coaggregation involves specific recognition and adhesion between bacteria and brings different species in close proximity. This increases the potential to exchange cell-cell signalling molecules or metabolites (Kolenbrander et al., Nature reviews Microbiology. 2010;8(7):471-80; Hojo et al, supra). For example, the signalling molecule autoinducer-2 (AI-2)mediates mutualistic growth of the coaggregating partners Streptococcus oralis and Actinomyces oris, and facilitates the development of bio films containing the coaggregating partners S. gordonii and S. oralis (Cuadra-Saenz et al, Microbiology.
2012;158(Pt 7): 1783-95; Rickard et al, Molecular microbiology. 2006;60(6): 1446-56.). Examples of important metabolites include hydrogen peroxide, which is produced by some streptococci, and inhibits other species including mutans streptococci (Zhu et al, Oxid Med Cell Longev. 2012;2012:717843), and lactate which is produced by streptococci and used by coaggregating Veillonella species for energy (Egland et al, Proceedings of the National
Academy of Sciences of the United States of America. 2004; 101(48): 16917-22). Arginine is important in metabolite exchange (Jakubovics et al., supra) but, unlike other mechanisms of communication, it also disrupts coaggregation between oral bacteria and appears to have a major impact upon biofilm structure (Edwards et al., Oral microbiology and immunology. 2007;22(4):217-24; Sato et al, J Microbiol Immunol Infect. 2012; Ellen et al, Oral microbiology and immunology. 1992;7(4): 198-203.).
Thus, arginine, although receiving limited attention to date, is a key global moderator of biofilm development and a pivotal component in the onset of caries or periodontal disease (Nascimento et al., Oral microbiology and immunology. 2009;24(2):89-95). The concept of 'nutritional virulence', whereby bacterial systems for acquiring nutrients from the host are considered key factors for pathogenesis, is emerging as an important paradigm for infectious diseases (Abu Kwaik et al, Cellular microbiology. 2013;15(6):882-90). Amino acids in particular are often a growth-limiting resource for bacteria. Even when species possess all the genes required for amino acid biosynthesis, they may be functionally auxotrophic in certain conditions. For example, Staphylococcus aureus possesses the full genetic pathway encoding the biosynthesis of L-arginine from L-glutamate, yet cannot grow in vitro without L-arginine (Nuxoll et al, PLoS pathogens. 2012;8(1 l):el003033). Similarly, S. gordonii can
biosynthesize L-arginine anaerobically but is a functional arginine auxotroph in aerobic conditions (Jakubovics et al., supra). Oral streptococci have varied requirements for amino acids in vitro (Cowman et al, Applied microbiology. 1975;30(3):374-80; Cowman et al, Journal of dental research. 1978;57(1):48; Terleckyj et al, Infect Immun. 1975;11(4):656- 64), and it is not well understood how these nutritional deficiencies restrict growth in dental plaque. Early colonizing bacteria obtain most of their nutrients from saliva (Bowden et al., Advances in dental research. 1997; 1 l(l):81-99). Human saliva can contain up to 40 µ? free arginine (Van Wuyckhuyse et al, Journal of dental research. 1995;74(2):686-90). S. gordonii is unable to grow aerobically in <25 µ? L-arginine (Jakubovics et al., supra). Arginine restriction of S. gordonii growth can be overcome by coaggregation with A. oris (Jakubovics et al, supra). Expression of S. gordonii arginine biosynthesis genes is strongly down- regulated in coaggregates compared with monocultures, indicating that coaggregation relieves low-arginine stress. Further, coaggregation with A. oris supported growth of S. gordonii under arginine-limited conditions. Therefore, inter-bacterial interactions are important for growth in saliva.
I. Therapeutic Methods
Experiments conducted during the course of development of embodiments of the present disclosure demonstrated that high concentrations of arginine abrogate biofilm formation by S. gordonii. Biofilm formation was highly sensitive to millimolar levels of arginine in a dose-dependent manner.
Arginine concentrations in saliva are thought to stay low due to continuous uptake into cells and turnover by bacterial arginine deiaminase systems (ADS's), which catabolise arginine to ATP, ammonia and L-glutamine (Van Wuyckhuyse et al., Journal of dental research. 1995;74(2):686-90). The production of ammonia increases the local pH of plaque, which protects against caries (Liu et al., International journal of oral science. 2012;4(3): 135- 40.). The ADS's of the opportunistic pathogens Pseudomonas aeruginosa and S. aureus are up-regulated in biofilms and these systems are essential to protect cells from oxygen and glycolysis-derived acids (39, 40). The ADS's of oral streptococci have been shown to influence biofilm formation in mixed-species systems, where removal of L-arginine by S. intermedius ADS inhibits biofilm formation by the periodontal pathogen P. gingivalis (Cugini et al, Microbiology. 2013;159(Pt 2):275-85). Production of ADS's is controlled at the level of transcription by ArgR/AhrC family regulators such as ArcR (Liu et al., Applied and environmental microbiology. 2008;74(16):5023-30; Zeng et al., Journal of bacteriology. 2006;188(3):941-9). ArcR is important for S. gordonii biofilm formation in nutrient-rich growth media.
Further experiments described herein demonstrated that L-arginine reduces the pathogenic potential of biofilms by reducing the biofilm biomass and reducing the total amount and proportion of pathogens (e.g., without direct antimicrobial activity); and L- arginine augments/enhances the activity of antimicrobials such as CPC. This is through enhancing access of antimicrobial by loosening biofilm and also by altering the growth-rate of the bacteria; L-arginine causes cell-cell signaling dysregulation; L-arginine is a combinational treatment that up-regulates metabolism, alters cell-cell signaling, and inhibits cell-cell adhesion; and L-arginine increases the proportion of beneficial bacteria that can combat the negative effects of potential pathogens such as S. mutans.
Accordingly, embodiments of the present invention provide compositions (e.g., pharmaceutical or research compositions or kits) comprising L-arginine (e.g., alone or in combination with CPC) and pharmaceutical, industrial, or research methods of using L- arginine in the treatment and prevention of bacterial infections (e.g., dental plaque) and in decontamination of surfaces (e.g., surfaces of medical devices).
In some embodiments, the present disclosure provides compositions and methods for using L-arginine to disrupt cell-cell interactions (adhesion) within a biofilm, disrupt bacterial homeostasis, induce cell-damage and killing, disrupt intra-cellular processes leading to deregulation/loss of homeostasis, disrupt cell-cell adhesion in biofilms, biofilm 3D rearrangement of architecture, disrupt cell-cell signaling, disrupt cell-cell metabolic interactions, and/or disrupt adhesion to surfaces. In some embodiments, L-arginine induces one or more of the above and these work individually and collectively to kill and/or reduce cell-activity and reduce biofilm biomass. In some embodiments, this also allows for improved killing with antimicrobials (e.g. L-arginine - antimicrobial agents or cocktails).
In some embodiments, the present invention provides compositions comprising L- arginine, alone or in combination with a pharmaceutically acceptable carrier or other desired delivery material (e.g., cleaner or disinfectant, etc.).
In some embodiments, the present disclosure provides compositions (e.g., dental care compositions such as toothpaste, mouthwash, etc.) comprising L-arginine in combination with e.g., CPC.
Pharmaceutical compositions and formulations for topical administration may include transdermal patches, ointments, lotions, creams, gels, drops, suppositories, sprays, liquids, mouthwash, and powders. Conventional pharmaceutical carriers, aqueous, powder or oily bases, thickeners and the like may be necessary or desirable.
Compositions and formulations for oral administration include powders or granules, suspensions or solutions in water or non-aqueous media, capsules, sachets or tablets.
Thickeners, flavoring agents, diluents, emulsifiers, dispersing aids or binders may be desirable.
Compositions and formulations for parenteral, intrathecal or intraventricular administration may include sterile aqueous solutions that may also contain buffers, diluents and other suitable additives such as, but not limited to, penetration enhancers, carrier compounds and other pharmaceutically acceptable carriers or excipients.
Pharmaceutical compositions of the present disclosure include, but are not limited to, solutions, emulsions, and liposome-containing formulations. These compositions may be generated from a variety of components that include, but are not limited to, preformed liquids, self-emulsifying solids and self-emulsifying semisolids.
The pharmaceutical formulations, which may conveniently be presented in unit dosage form, may be prepared according to conventional techniques well known in the pharmaceutical industry.
The compositions may additionally contain other adjunct components conventionally found in pharmaceutical compositions. Thus, for example, the compositions may contain additional, compatible, pharmaceutically-active materials such as, for example, antipruritics, astringents, local anesthetics or anti-inflammatory agents, or may contain additional materials useful in physically formulating various dosage forms of the compositions, such as dyes, flavoring agents, preservatives, antioxidants, opacifiers, thickening agents and stabilizers. However, such materials, when added, should not unduly interfere with the biological activities of the components of the compositions. The formulations can be sterilized and, if desired, mixed with auxiliary agents, e.g., lubricants, preservatives, stabilizers, wetting agents, emulsifiers, salts for influencing osmotic pressure, buffers, colorings, flavorings and/or aromatic substances and the like which do not deleteriously interact with the active agents of the formulation.
In some embodiments, the pharmaceutical composition contains a) L-arginine, and b) one or more other agents useful in killing or preventing the growth of microorganisms (e.g., antibiotics) or impacting the growth, formation or health impact or microorganisms in bio films.
In some embodiments, the present invention provides kits, pharmaceutical compositions, or other delivery systems for use of L-arginine in treating or preventing bacterial infections or biofilms present on surfaces (e.g., dental plaque). The kit may include any and all components necessary, useful or sufficient for research or therapeutic uses including, but not limited to, L-arginine, pharmaceutical carriers, and additional components useful, necessary or sufficient for treating or preventing bacterial infections. In some embodiments, the kits provide a sub-set of the required components, wherein it is expected that the user will supply the remaining components. In some embodiments, the kits comprise two or more separate containers wherein each container houses a subset of the components to be delivered. Optionally, compositions and kits comprise other active components in order to achieve desired therapeutic effects.
In some embodiments, L-arginine is used to kill bacteria in coaggregates or biofilms. The compositions comprising L-arginine described herein find use in the killing or inhibition of growth of a variety of microorganisms (e.g., pathogenic bacteria or fungi). In some embodiments, L-arginine or compositions comprising L-arginine find use in the treatment of bacterial infections in or on the body (e.g., bacterial infections in coaggregates or biofilms). In some embodiments, L-arginine or compositions thereof are used to treat bacterial infections in wounds, sepsis, pathogenic bacterial infections in the stomach or intestine, and the like.
In some embodiments, pharmaceutical compositions are administering in a maintenance or ongoing manner (e.g., one or more times a day, two or more times a day, one or more times a week, etc.). In some embodiments, compositions are administered continuously (e.g., via a skin patch, bandage, or time release formulation). In some embodiments, compositions are administered once, twice, 5 times, 10 times or more. In some embodiments, compositions are administered over a period of weeks, months, years or indefinitely
In some embodiments, L-arginine or compositions comprising L-arginine find use in the decontamination of medical devices (e.g., catheters, speculums, and the like) or implantable medical devices (e.g., pacemakers, internal defibrillators, artificial joints or bones and the like).
In some embodiments, L-arginine or compositions comprising L-arginine find use in the decontamination of surfaces (e.g., surfaces comprising biofilms). Examples include but are not limited to, household surfaces, hospital or clinical surfaces (e.g., exam tables, operating rooms, etc.), and the like.
In some embodiments, L-arginine or compositions comprising L-arginine find use in the decontamination or protection of food or food preparation areas. For example, in some embodiments, L-arginine is applied to a food after harvest to protect against future contamination or treat existing contamination.
In some embodiments, L-arginine or compositions comprising L-arginine find use in treating and/or preventing dental carries and gum disease. In some embodiments, L-arginine is added to mouthwash, toothpaste, or other oral care products.
II. Screening compositions and methods
Embodiments of the present disclosure provide compositions and methods for determining levels of biofilm components (e.g. arginine or AI-2) in biofilms or planktonic cells. Arginine is internalized by streptococci and sensed through the action of three different, but related, regulatory proteins: ArcR, AhrC and ArgR. These alter their conformation when they bind to arginine so that they either bind to promoter sequences or are released from promoters.
Accordingly, embodiments of the present disclosure provide a plasmid that reports expression or concentration of a component in a biofilm or planktonic cell culture. In some embodiments, the plasmid comprises a first detectable (e.g., fluorescent) marker under the control of a bacterial promoter that is constitutively expressed or a second marker (e.g., a different color fluorescent label), that is induced by the component in the biofilm (e.g., arginine or AI-2 present in the biofilm or cell culture). The present disclosure is not limited to particular promoters or reporter genes. Examples of fluorescent markers include, but are not limited to, luciferase, chloramphenicol acetyltransferase, green fluorescent protein (GFP), or Mcherry. In some embodiments, the promoter is a streptococcal promoter (e.g., a promoter active in S. gordonii such as, e.g., S. gordonii DL1 50S ribosomal protein (SGO l 192), S. gordonii argC or arcA promoters, or catabolite control protein A (SGO 0773)). In some embodiments, the 50S ribosomal protein promoter or other streptococcal ribosomal promoters or lactococcal promoters such as usp45 serve as control promoters for constitutive expression of the first marker. In some embodiments, catabolite control protein A promoter is a reporter of AI-2. In some embodiments, argC or arcA promoters are responsive to arginine.
In some embodiments, the present disclosure provides methods of using the plasmids described herein to monitor concentration of components (e.g., arginine or AI-2) of a biofilm or planktonic cell population (e.g., in a streptococcal spp. such as S. gordonii), comprising: a) contacting a streptococcal cell with the promoters described herein; and b) measuring the level of marker. In some embodiments, the level of signal from the marker under the control of the promoter induced by the external biofilm component or the constitutive promoter is compared to level of signal from known quantities/concentrations of the component (e.g., a standard curve). In some embodiments, the level of fluorescence is then be correlated in a fluorimeter or imaging system.
In some embodiments, the reporter plasmids and methods find use in research, screening (e.g., drug screening), and diagnostic applications. For example, in some embodiments, test compounds (e.g., antimicrobial drugs) are added to a biofilm or planktonic cell population and the effect of the test compound on levels of biofilm components (e.g., arginine) is assayed.
EXPERIMENTAL
The following examples are provided in order to demonstrate and further illustrate certain preferred embodiments and aspects of the present invention and are not to be construed as limiting the scope thereof.
Example 1 This example relates to bio films formed by single species of bacteria. In these systems, L-arginine has diverse effects on bacterial gene regulation, phenotype, metabolism and biofilm formation.
Arginine sensing triggers gene regulation.
S. gordonii contains the full genetic pathway for biosynthesis of L-arginine. However, like S. aureus, it is a functional arginine auxotroph under laboratory conditions (Jakubovics et al, supra, Nuxoll et al, PLoS pathogens. 2012;8(1 l):el003033). A shift from arginine - replete medium to arginine-deficient medium resulted in a marked change in phenotype of S. gordonii. In addition to genes previously shown to be regulated by coaggregation, there was a significant decrease in the expression of several well-characterized cell surface adhesins (Table 1). In general, metabolism was decreased with the exception of the arginine biosynthesis pathway, which was strongly up-regulated (Figure 2). These data are consistent with the development of a specialized biofilm dispersal cell state, similar to that produced by the dimorphic aquatic bacterium Caulobacter crescentus, upon nutrient limitation (England et al., Journal of bacteriology. 2010;192(3):819-33.). This example relates to biofilms formed by single species of bacteria. In these systems, L-arginine has diverse effects on bacterial gene regulation, phenotype, metabolism and biofilm formation.
Biofilm formation in an environmentally germane microfluidic biofilm system.
The effects of arginine-dependent dosing of S. gordonii biofilms is assessed in a modified Bioflux microfluidics system. Human saliva is collected and pooled from >6 different volunteers (Cuadra-Saenz et al, Microbiology. 2012;158(Pt 7): 1783-95), diluted to 25%, and additional supplements (e.g. sucrose, haemin, BHI) added as required to simulate different conditions. Note, we grow robust biofilms in even non-supplemented saliva and pooling the saliva minimizes variation in salivary components. To obtain an indication of free arginine levels in pooled saliva, arginine is measured in >3 different pooled saliva samples using standard techniques (Jakubovics et al, supra; Van Wuyckhuyse et al., Journal of dental research. 1995;74(2):686-90). The 24 channels of the Bioflux system are coated with saliva, inoculated with monocultures of S. gordonii (or multi-species biofilms harvested form saliva) and biofilms are developed at 37°C with cell free saliva. After 20 h, cells are stained with live/dead stain and visualized using a Leica SPE CLSM. Biomass and structural biofilm parameters are quantified using COMSTAT (Heydorn et al., Microbiology. 2000; 146 ( Pt 10):2395-407), ImageJ (Collins, Biotechniques. 2007;43(1 Suppl):25-30), and IMARIS software (Bitplane, Switzerland). Figure 3 shows that high concentrations of arginine (500 mM) significantly reduce the extent of bio film present. Arginine and bacterial biofilm formation.
Arginine has been shown to play a key role in biofilm formation and host colonization by Gram-positive and Gram-negative bacteria. For example, at physiological concentrations found in cystic fibrosis sputum, arginine promotes P. aeruginosa biofilm formation (Bernier et al., Research in microbiology. 2011;162(7):680-8). Arginine was the only amino acid that prevented swarming by P. aeruginosa, and thus plays a pivotal role in promoting a sessile lifestyle in this species (Bernier et al, supra). In S. aureus, the final gene in the arginine biosynthesis pathway, argH, is essential for virulence in a mouse kidney abscess model, indicating that arginine is restricted in vivo (Nuxoll et al., supra). Many oral streptococci are auxotrophic or conditionally auxotrophic for arginine (Jakubovics et al., supra, Cowman et al. Applied microbiology. 1975;30(3):374-80; Terleckyj et al, Infect Immun. 1975;11(4):656- 64; Cowman et al, Applied microbiology. 1974;27(l):86-92; Rogers et al, Journal of general microbiology. 1990;136(12):2545-50). The importance of the S. gordonii arginine
biosynthesis pathway for growth in salivary biofilms is investigated using an argH mutant and a complemented strain. The present invention is not limited to a particular mechanism. Indeed, an understanding of the mechanism is not necessary to practice the present invention. Nonetheless, it is contemplated that arginine is a limiting nutrient for L-arginine biosynthesis- deficient streptococci growing as single-species colonies in salivary biofilms. Further, it is contemplated that coaggregation with other species in the biofilm overcomes the effects of L- arginine restriction but is subject to disruption by adding elevated amounts of L-arginine.
Arginine catabolism in biofilms.
In biofilm cells of P. aeruginosa, arginine metabolism is up-regulated
compared with planktonic cells, due to an increase in expression of genes encoding the arginine deiminase system (ADS) (Xu et al, PloS one. 2013;8(2):e57050; Sauer et al, Journal of bacteriology. 2002; 184(4): 1140-54). The ADS is a key component of anaerobic metabolism in this organism. The switch to ADS-mediated anaerobic fermentation is linked to increased susceptibility of P. aeruginosa to ciprofloxacin and tobramycin (Borriello et al., Antimicrobial agents and chemotherapy. 2004;48(7):2659-64). The ADS is also important in the pathogenicity of S. aureus. Indeed, the US A300 pathogenic lineage of S. aureus has acquired a genetic element termed the Arginine Catabolic Mobile Element (ACME), which contains genes encoding an ADS pathway and is thought to be a key factor in promoting growth and survival on the skin (Otto et al., International journal of medical microbiology: IJMM. 2013). As such, the ACME may be important for the high transmissibility of USA300 strains.
High concentrations of arginine negatively affect the ability of certain oral bacteria to colonize oral bio films. Arginine has been shown to inhibit several different coaggregation interactions between oral bacteria (Edwards et al., Oral microbiology and immunology. 2007;22(4):217-24; George et al, Oral microbiology and immunology. 1992;7(5):285-90. PubMed PMID: 1494452; Kaplan et al, Molecular microbiology. 2009;71(l):35-47;
Takemoto et al., Journal of periodontal research. 1993;28(l):21-6.; Nagata et al., Journal of dental research. 1990;69(8): 1476-9.). Data described herein shows that the disruption of coaggregation within oral bio films leads to the release of bacteria from the biofilm. Nutrients induce dispersion of P. aeruginosa biofilms and this phenomenon involves genes required for arginine metabolism (Sauer et al., Journal of bacteriology. 2004 186(21): 7312-7326). Figure 4 shows that in S. gordonii the gene encoding ArcR, the regulator of arginine deiminase system genes, is required for biofilm formation by S. gordonii since a strain lacking the arcR gene does not form strong biofilms. Therefore L-arginine is a central regulator of biofilm formation.
Example 2
This example describes the impact of L-arginine on oral bio films that contain species grown under conditions representative of the human oral cavity. Using a microfluidic-based approach, using human saliva as the inoculum and 25% filter- sterilized human saliva as the nutrient source, it was demonstrated that HCL-balanced L-arginine (LAHCL) destabilizes oral biofilms in a concentration dependent manner. Destabilization was expressed as loss of biofilm structure and change in bacterial community membership, as determined by confocal laser scanning microscopy and 454 pyrosequencing. Very limited antimicrobial effects were evident and only detected as a consequence of biofilm perturbation, and the optimal concentration for destabilization was between 50 and 500mM. No substantial changes in pH were recorded, due to the use of HC1 balanced L-arginine (L-arginine monohydro chloride) and the buffering capacity of human saliva.
As traditional approaches to control oral biofilms rely heavily on antimicrobials and L-arginine was demonstrated a destabilizing effect, synergy with other antimicrobials to more effectively inactivate biofilm cells was investigated. Mixing L-arginine with cetylpyridinium chloride (CPC) resulted in at least five times greater biofilm inactivation (by live/dead staining). Taken collectively, it was demonstrated that L-arginine has broad oral biofilm destabilizing effects under conditions representative of the human mouth. Such effects are used to remove biofilms or enhance traditional CPC-based antimicrobial treatment strategies. Results are shown in Figures 5-11. Figure 5 shows changes in community composition of L-arginine (500 mM) treated bio films. Figure 6 shows the effect of L-arginine (CLSM) on multi-species bio films of bacteria in saliva derived community developed in pooled filter sterilized saliva in a static (non-flowing) microplate system. 500mM L-arginine destabilized multi-species oral biofilm communities to reduce biofilm biomass (and therefore total numbers of bacteria, including pathogens) and also makes the biofilm more diffuse with respect to architechture. No substantial killing was observed although there is a slight statistically significant increase in red "signal" indicating that the non-responsive
dead/damaged cells are left behind in the biofilm.
Figure 7 shows Representative 3D renderings and biofilm characteristics derived from computational image analysis of oral biofilms developed for 20 h in different concentrations of L-arginine monohydro chloride (LAHC1) in the Bioflux™ flowing saliva biofilm system. Green signal indicates viable live cells and red signal indicates damaged/dead cells.
Associated table shows changes in cell viability, biofilm biomass, thickness, and roughness. Data derived from at-least 18 renderings across three experiments and standard deviations are shown in brackets. *P<0.05; **P<0.01; **P<0.001 : significant differences from the CFS control.
Figure 8 shows that 500mM L-arginine destabilizes pre-formed multi-species oral biofilms of differing developmental age.
Figure 9 shows that L-arginine destabilizes multi-species oral biofilm communities to enhance the penetration of CPC. As a consequence, lower CPC concentrations are used to achieve the same level of killing/inactivation/cell damage.
Figure 10 shows fold induction of luciferase production in the Vibrio harveyi reporter strain BB170 normalized to a positive control (BB152). A control run of plain CFS (cell-free saliva) with the given concentrations of arginine was compared to microfluidics efflux, which contains any secreted molecules from bacteria grown with the given concentration of L- arginine. Values were produced by first taking averages of 4 trials at each concentration and dividing them by the average for the negative control, creating an induction number. The amount of AI-2 produced from multi-species biofilms increases as the concentrations of arginine used to treat them increases. This indicates that L-arginine energizes bacterial communities, because AI-2 is a proxy for metabolism. High AI-2 may also have a destabilizing effect on the community, which could explain or contribute to the structural changes seen in the biofilms. Figure 11 shows that application of L-arginine disrupts bacterial biofilm communities but D-arginine does not have the same effect. Therefore the destabilizing effects of arginine are specific to the L-form.
In conclusion, these examples demonstrate that L-arginine reduces the pathogenic potential of bio films by reducing the biofilm biomass and reducing the total amount and proportion of pathogens; L-arginine augments/enhances the activity of antimicrobials such as CPC. This is through enhancing access of antimicrobial by loosening biofilm and also by altering the growth-rate of the bacteria; L-arginine causes cell-cell signaling dysregulation; L- arginine is a combinational treatment that up-regulates metabolism, alters cell-cell signaling, and inhibits cell-cell adhesion; and L-arginine increases the proportion of beneficial bacteria that can combat the negative effects of pathogens such as S. mutans. The proportion of Veillonella species are increased in biofilms as are the proportions of non-cariogenic streptococci.
Example 3
A plasmid (See e.g., Figure 12) was utilized to monitor expression of reporter genes in the presence of arginine or AI-2. The ??????? backbone described in England et al. (Proc Natl Acad Sci U S A. 2004 Nov 30;101(48): 16917-22. Epub 2004 Nov 16) was modified to express fluorescent genes such as GFP or Mcherry in S. gordonii DL1 or other species of streptococci. Specifically, promoters from differentially regulated genes are inserted upstream of a GFP or Mcherry gene harbored on ??????? (or similar streptococcal plasmid shuttle vector system) to be differentially expressed (Figure 12A and 12B). An example was performed (Figure 12B) showing minimal differential expression by a 50S ribosomal protein (SGO l 192; gene rplJ responsible for the highly abundant 50S ribosomal protein L10) when exposed to exogenously added autoinducer-2 (AI-2) as compared to drastically different gene expression by the catabolite control protein A (SGO 0773; ccpA). The plasmid provides constitutive -producing fluorescent probes for monitoring bacteria in biofilms and reporter systems that report arginine (or AI-2) concentrations in biofilms such as those found in dental plaque biofilms.
US2011236508
L-ARGININE-BASED FORMULATION FOR ORAL ABSORPTION
L-ARGININE-BASED FORMULATION FOR ORAL ABSORPTION
A formulation comprising large quantities of l-arginine and/or fat plaque dissolving agents which is palatable, stench free, and does not evoke nausea. The formulation is adapted to facilitate the adsorption of l-arginine to the blood system and to introduce high levels of l-arginine and/or other fat plaque dissolving agents such as EDTA, its derivatives or its salts into the blood system which are sufficient for effectively dissolving fat plaques in the artery. The formulation comprises at least 10% (w/w) L-arginine, edible organic acids, emulsifier(s), preservatives, flavorings, ethanol and water. Other embodiments may further include chromium salts, and EDTA or its derivatives and their salts
DE102010003280
Oral-, dental care- and -cleaning products, useful for plaque reduction and/or caries prevention...
Oral-, dental care- and -cleaning products, useful for plaque reduction and/or caries prevention...
Oral-, dental care- and -cleaning products comprise at least one oligo- or polypeptide of vegetable origin, in which the molar ratio of basic amino acids (arginine, histidine and lysine) to acids and semi-acidic amino acids (aspartic acid, glutamic acid, tyrosine and cysteine) is greater than 1. An independent claim is included for reducing plaque and/or preventing caries, comprising packing the products in the form of a toothpaste and using for cleaning the teeth using a toothbrush, or packing in the form of a mouthwash and using for rinsing the oral cavity.
US5874068
Stabilized antiplaque and antigingivitis oral compositions containing N alpha -alkyl-L-arginine alkyl ester salts
Stabilized antiplaque and antigingivitis oral compositions containing N alpha -alkyl-L-arginine alkyl ester salts
Also published as: WO9929289
An antiplaque and antigingivitis effective oral composition containing a stabilized N alpha -acyl acidic amino acid ester salt is disclosed. Also disclosed is a method for inhibiting plaque buildup in the oral cavity with an oral composition containing the stabilized N alpha -acyl acidic amino acid ester salt.
JP3803695
ANTIMICROBIAL PREPARATION
ANTIMICROBIAL PREPARATION
Also published as: JP3803695
PURPOSE: To obtain an antimicrobial preparation exhibiting excellent antimicrobial activity against the aggregate and lump of microorganisms, such as a biofilm or plaque, which can substantially not be controlled with an antimicrobial agent a lone. CONSTITUTION: The antimicrobial preparation contains 0.001-10wt.% of arginine or its derivative and 0.001-10wt.% of a compound exhibiting antimicrobial activity. The further addition of 0.005-5wt.% of at least a surfactant selected from a nonionic surfactant and an amphoteric surfactant to the antimicrobial preparation gives the more excellent antimicrobial effect. The compound exhibiting the antimicrobial activity includes cationic antimicrobial agents (e.g. cetylpyridinium chloride), fluorides, natural antimicrobial agents (e.g. thymol, oil-soluble glycyrrhiza extract, a polyphenol), trichlosan, and isopropylmethylphenol.; The nonionic surfactant is preferably a polyethylene oxide-polypropylene oxide block copolymer, and the amphoteric surfactant is preferably a palm oil fatty acid amide propylbetaine.
JPH09286712
COMPOSITION FOR ORAL CAVITY
COMPOSITION FOR ORAL CAVITY
PROBLEM TO BE SOLVED: To obtain a composition for oral cavity, capable of promoting absorption of a cationic disinfectant to the surface of tooth and excellent in prevention of formation of dental plaque and prevention of decayed tooth. SOLUTION: This composition for oral cavity is obtained by blending a cationic disinfectant such as gluconic acid chlorohexidine with an N long chain acyl basic amino acid lower alkyl ester or its salt such as N-cocoil-L-arginine ethylester-pyrrolidone carboxylic acid salt and a pH adjuster such as citric acid so as to keep pH to 5.5 to 6.5. Thereby, largest absorption amount of the disinfectant is obtained, especially in a region of pH5.5 to 6.5 and absorption to dental surface is promoted in the region.
JP3566374
COMPOSITION FOR ORAL CAVITY
COMPOSITION FOR ORAL CAVITY
PURPOSE:To obtain a composition for oral cavity having an excellent suppression effect of pH reduction of a bacterial plaque, constituted by containing arginine, canavanine or their salts, and a carbon dioxide producing agent or carbon dioxide. CONSTITUTION:Arginine, canavanine which is an arginine analog, and their salts of 0.05-20wt.%, and a carbon dioxide producing agent or carbon dioxide of 0.5-20wt.% are contained in this composition. Further, a calcium ion capturing agent of 0.05-50wt.% is contained in it as indispensable component, and the other optional components which are added in an usual composition for oral cavity, are added to adjust pH of 5-10, when it is dissolved in water of 10 fold amount.; By combining arginine, canavanie or their salts having a high safety and the carbon dioxide producing agent or carbon dioxide, with a manifestation of suppressing effect of lactic acid production, together with promoting effect of its decomposition, a reduction of pH of a bacterial plaque is suppressed. Since, even taking a food containing sucrose at the same time, the pH lowering is suppressed, a cariogenicity is reduced, and it is useful in fields of quasi-drug, a cosmetic and a food, and capable of changing a bacterial flora in the oral cavity to a flora rich with a goody bacteria.
EP0711543
/ CN1156022 / CN1093756
Oral preparations
Oral preparations
The present invention relates to oral preparations having anti-caries activity. The compositions comprise pyruvic acid or an orally-acceptable salt thereof, and urea and/or arginine or a derivative thereof. This combination induces a pH-rise in the plaque.