
rexresearch.com
rexresearch.com
Shark Repellants
Here's some food for thought to cud about whilst the lawyers or elasmobranchi circle for the kill-nibble...
Wikipedia : Shark Repellant
NBC News : Researchers tout shark repellent
SharkTec
Sharkshield
Brian Handwerk : New Shark Repellent Uses Chemical Signals
Stroud's Shark Repellant Patents ;
US2014173966 -- ELASMOBRANCH-REPELLING MAGNETS AND METHODS OF USE
US2012085018 -- Elasmobranch-repelling magneto-electropositive fishing hook
US2010203154 -- Elasmobranch-Repelling Compounds, Methods of Use and Devices
US2010016346 -- ELASMOBRANCH-REPELLING COMPOUNDS AND METHODS OF USE
US2007256623 -- Elasmobranch-repelling electropositive metals and methods of use
More Shark Repellant Patents
http://en.wikipedia.org/wiki/Shark_repellent
Shark Repellent
A shark repellent is any method of driving sharks away from an area. Shark repellents are a category of animal repellents. Shark repellent technologies include magnetic shark repellent, electropositive shark repellents, electrical repellents, and semiochemicals.
Shark repellents can be used to protect sharks by driving them away from areas where they are likely to be killed by human beings; in this case, the shark repellent serves as a conservation method.
There is evidence that surfactants such as sodium lauryl sulfate can act as a shark repellent at concentrations on the order of 100 parts per million. However, this does not meet the desired "cloud" deterrence level of 0.1 parts per million.[1][2]
Research indicates that sharks will avoid an area when they smell chemical released by dead and dying sharks. Six chemicals were synthesized from shark glands and tissues and used in experiments. Sharks immediately reacted once they detected these chemicals. To quote a 2004 Associated Press article, "Fisherman and scientists have long noted sharks stay away if they smell a dead shark."[3]
Recent Advances
There have been significant advances in the research, development, and testing of aerosol shark repellents that repel sharks through replicating the chemicals that sharks emit when they die or are in danger.[4] The scientists behind these advances, Dr.Eric Stroud and Dr. Patrick Rice, operate a company dedicated for the research and development of such shark repellents.[5] Their organization, SharkDefense, is geared towards saving both humans and sharks.[6] Sharkdefense does not sell any products, but they are partnered with a company called SharkTec LLC, which sells Sharkdefense approved products at sharktecdefense.com [7]
History
Some of the earliest research on shark repellents took place during the Second World War when military services sought to minimize the risk to stranded aviators and sailors in the water. Studies at the time, combined with historical research, revealed that about the only thing that will drive sharks away is the odor of another dead shark. Efforts were made to isolate the active principles in dead shark bodies that repelled other sharks. Eventually, it was determined that certain copper compounds, such as copper sulfate[citation needed] and copper acetate ,[8] in combination with other ingredients, could mimic a dead shark and drive live sharks away from human beings in the water. For years, a combination of copper acetate and a black dye to obscure the user was supplied to sailors and aviators of the United States Navy as a shark repellent. Known as "Shark Chaser," it was first packaged in cake form using a water soluble wax binder and rigged to life vests. The Navy employed Shark Chaser extensively between 1943 and 1973. It is believed[8] that the composition does repel sharks in some situations, but not in all, with about a 70% effectiveness rating.
Today, the search for an ideal shark repellent is ongoing. Some research, based on semiochemicals, looks promising. Electrical devices that disturb a shark's sensitive ampullae of Lorenzini are also partially effective.
http://www.nbcnews.com/id/5560773/ns/technology_and_science-science/t/researchers-tout-shark-repellent/
7/30/2004
Researchers tout shark repellent
SAN JUAN, Puerto Rico — Excited by the scent of blood, a dozen sharks dart about in a frenzy as a researcher dips a pole in the sea and squirts out a clear, yellowish substance. Within seconds, the sharks jerk their snouts away and vanish.
Researchers say they finally have found a potent repellent to drive away sharks, after testing off Bimini island in the Bahamas. It’s a goal that’s eluded scientists for decades.
If proven effective, the repellent one day might protect divers, surfers and swimmers. But researchers say that would require much more study. First they hope it can protect sharks — in decline worldwide due to overfishing — by reducing the numbers caught needlessly by long-line commercial fishermen.
“You introduce this chemical, and they all leave,” said lead researcher Eric Stroud, a 30-year-old chemical engineer from Oak Ridge, N.J. “It works very, very well.”
The repellent, called A-2 because it was the second recipe tried, is derived from extracts of dead sharks that Stroud gathered at New Jersey fish markets and piers. Fishermen and scientists have long noted sharks stay away if they smell a dead shark.
“We have something that really works, but research remains,” said Samuel Gruber, a University of Miami marine biologist and shark expert who is helping conduct tests at the Bimini Biological Field Station.
Tests have found the repellent effective on three species: the Caribbean reef, blacknose and lemon sharks. Studies are needed on other species such as the great white, mako and oceanic whitetip.
Gruber said the repellent seems to carry a chemical messenger that triggers a flight reaction. He said more studies are needed to pinpoint the active molecule among a dozen or so.
A dose of 4 fluid ounces is enough to scare away feeding sharks, Stroud said, keeping them away from a fish head for two hours with just a few drops per minute. In contrast, sharks didn’t respond to a red dye in control tests.
The researchers presented their work in May during a meeting of the American Society of Ichthyologists and Herpetologists in Norman, Okla. Films of their tests captured images of sharks splashing the surface as they turn to flee.
They hope to make a slow-dissolving repellent for use in baits and fishing nets, and to guard equipment on submarines and oil exploration vessels that sharks have damaged in the past.
The repellent, though nontoxic, is apparently so disagreeable to sharks it can revive them from semiconsciousness. Some species slip into a hypnotic state if turned belly-up, and tests found the repellent brought captive sharks out of that trance.
Repellent research began in World War II, when the U.S. Navy created “Shark Chaser” for sailors and downed pilots. Mixed with black dye, it was made of copper acetate, which scientists thought would smell like a rotting shark. Studies later showed it wasn’t that effective.
A promising find came in 1972, when University of Maryland shark expert Eugenie Clark discovered that a Red Sea fish, the Moses sole, secreted a milky substance that repelled sharks.
The finding caused a stir, and soon the makers of Coppertone suntan lotion contacted Clark, hoping to market it. She said she discouraged them, saying initial research couldn’t back up such a use.
Years of study followed by Gruber and others. In the end, though, the repellent derived from the sole wasn’t practical because it had to be squirted into a shark’s mouth to be effective.
Clark — who at 82 still works at Mote Marine Laboratory in Sarasota, Fla. — said the latest findings could be a welcome way to reduce accidental killing of sharks, though she is skeptical of human use, saying few would be carrying the repellent at the rare moment it’s needed.
“I’d be happy to see somebody work it out, but I don’t see it as a practical solution,” she said.
Anti-shark items on the market now include cages, steel mesh suits and a device called the Shark Shield, which when worn by divers or surfers emits an electric field. The device’s Australian maker acknowledges it can’t guarantee total effectiveness.
In most cases, the danger of attack is extremely slight. The International Shark Attack File, at the Florida Museum of Natural History, recorded 55 unprovoked attacks worldwide last year, including four deaths.
Stroud got the idea to pursue a repellent after several 2001 shark attacks drew widespread attention, including one that nearly killed an 8-year-old boy near Pensacola, Fla.
Stroud and engineer Mike Herrmann do lab work in a New Jersey warehouse, relying on donations of less than $500,000 from two private benefactors.
They have a patent pending and are starting a company, Shark Defense Inc., to eventually market the repellent.
http://www.sharktecdefense.com/pages/the-scientist
The Science Behind SharkTec's Shark Repellent Sprays
HOW A SHARK HUNTS:
Sharks have an amazing sense of smell (most sharks can detect blood and animal odors from many miles away) which is one of the reasons they are such effective predators. In addition to smell, sharks also rely on their sense of taste. Typically before a shark commits to its prey it will first give a "test bite". The shark has very sensitive taste buds in its mouth which can quickly decipher whether the potential meal is within its ordinary diet; in the case of humans the shark will often reject this prey after the first bite.
GAME-CHANGING DISCOVERY:
Using what is called Semiochemicals derived from decomposed sharks SharkTec is able to offer a natural product which triggers a flight reaction in sharks. Essentially living sharks will instinctually stay far-far away from the area where they can smell and taste another dead shark!
PROVEN, TESTED RESULTS:
The theory of repelling living sharks by using semiochemicals as shark repellents was proposed by Baldridge (1990) and by Rasmussen and Schmidt in 1992. In 2001, investigation of these possibilities led Eric M. Stroud (SharkTec’s partner) to begin qualitative analysis on semiochemical extractions using captive juvenile sharks. In 2003, with the help of Dr. Samuel Gruber, Grant Johnson, and the Bimini Biological Field Station, the team was able to document a number of successful field tests on wild feeding sharks. The results of these field tests were first presented at the 2004 Joint Meeting of Ichthyologists and Herpetologists 26 – 31 May, 2004, in Norman, OK. Since then the product has continued to return compelling results and has been feature on well known publications such as Discovery Channel.
http://www.youtube.com/user/Sharkshield
Shark Shield
Luke Tipple, a Marine Biologist, Discovery Channel Host and Shark Diver presents the Shark Shield FREEDOM7, the only scientifically proven and independently tested electronic shark deterrent designed to reduce the risk of an unwanted shark encounter.
Shark Shield devices create a powerful electrical field which induces spasms in predatory shark's highly sensitive electrical receptors. Used by professional and navy divers around the world, the FREEDOM7 is a safety device providing peace of mind while supporting the conservation of shark
https://www.sharkshield.com/
Technology
An Ocean of Respect
Drawn by the call of the ocean, sharks or no sharks, we’re still going out there! Our solution is scientific, lightweight and powerful. With Shark Shield, we’ve replaced fear with awe.
Shark Shield has taken 20 years of tireless scientific research to evolve into what it is today: proven, reliable and non-evasive shark deterrent technology. And underlying it all is a deep respect for sharks, the great predators of the ocean. Now you can have the peace of mind to swim, surf, kayak and dive without fear. It’s our playground, but it’s their home!
In 1995 it was discovered that sharks have a heightened sensitivity to close-range, low frequency electrical fields. Two decades of intensive global research has developed this knowledge into a reliable and essential piece of equipment that protects visitors to the ocean and the predators that live there.
Shark Shield consists of two electrodes which when both are submerged emit a three dimensional electronic field that surrounds the user. When a shark comes to within a few meters of the Shark Shield, the strong electronic pulses emitted by the device cause the shark to experience muscle spasms.
This does NOT harm the shark in any way, but merely causes it to experience a high level of discomfort. From testing, the closer the shark is to the Shark Shield field, the more spasms occur in the sharks’ snouts, which results in it turning away from the electronic field, thereby protecting the user.
When you’re out there surfing, you don’t want to be thinking about predators. Now with Shark Shield connected to your board, all you have to think about is your next wave! The low drag antennae in the water below you will emit an electrical pulse that is keeps you safe and does no lasting harm to the creature.
Sharks have highly sensitive electrical receptors called Ampullae of Lorenzini located in their snouts. These sense electrical current and are used to detect prey, but only at very close distances. Once the shark is out of the affected area, it no longer feels the electrical impulse.
There are no long-term adverse effects to the shark and as a result Shark Shield devices support the conservation of sharks by removing the need for culling or other lethal means of managing human and shark interactions.
Shark Shield devices do not affect other ocean creatures.
Proven Protection
When you strap a Shark Shield to your ankle, board or kayak, you are strapping on the credibility and belief of some of the worlds leading oceanic organizations: from the Natal Sharks Board in South Africa, to the Australian Elite Military and the US Coast Guard.
You can review published test results in our Scientific Research section. The video below shows Ian “Shark” Gordon testing Shark Shield and the effectiveness of the technology on even the largest of sharks.
As a result of continuous research and investment over the past ten years, Shark Shield has lodged numerous patents relating to electrical shark deterrents.
Scientific Overview
Shark Deterrent Research by Kwazulu Natal Shark Board
The electrical wave-form used in the Shark Shield is based on a technology originally invented by the Kwazulu Natal Shark Board of South Africa in the 1990's.
Predatory sharks have small gel filled sacs knows as ‘Ampullae of Lorenzini’ on their snouts. They use these short range sensors when feeding or searching for food.
Shark Shield is a three-dimensional electrical wave form which creates an unpleasant sensation impacting the shark’s ‘Ampullae of Lorenzini’. When the shark comes into proximity of the electrical wave form (a few meters in diameter) it experiences non-damaging but uncontrollable muscular spasms causing it to flee the area.
The field is projected from the unit by two electrodes, which create an elliptical field that surrounds the user. Both electrodes must be immersed in the water for the field to be created. The electrode configuration depends on the model of the Shark Shield unit and the diagram below is a mathematical example of how the original POD and SCUBA7 electric field would look if you could see it.
In the video below we discuss how the electrical shark deterrent technology works and provide examples of the electircal field required to offer a level of protection against a shark attack.
From testing, the closer the shark is to the Shark Shield field, the more spasms occur in the sharks’ snouts. This becomes intolerable and the shark then veers away, and usually doesn’t return.
A distinct advantage of the unique electrical wave-form is that it deters sharks and does no lasting harm to the shark. Once the shark is out of the affected area, it no longer feels the effect of the electrical wave form. The video below shows Ian "Shark" Gordon testing Shark Shield and the effectiveness of the technology on even the larges of sharks.
The original technology was released onto the market in 1995 by POD Holdings Ltd, a joint venture company partly owned by the Natal Sharks Board and the South African Government. In addition to being tested by National Military and other authorities, Shark Shield has been extensively tested to the highest standards by scientists and marine biologists over many years.
SHARKSHIELD PATENTS
WO9637099
SHARK REPELLANT DEVICES
Inventor:
CHARTER GRAEME ERNEST ; HARTZENBERG IGNATIUS MARTHINUS
Applicant: NATAL SHARKS BOARD
Also published as: ZA9603377 // MX9601583 // JPH09140293 // BR9602418 // AU5769496
Apparatus for repelling aquatic creatures such as sharks comprising a pair of electrodes (18) for immersion in a body of water, charge storage means such as a capacitor (14) charged to a predetermined voltage by a charging circuit from a source of electrical power, such as a battery, control logic to generate control signals and controllable switch elements (16), such as silicon controlled rectifiers (SCR's), thyristors or the like that have a low on resistance. The thyristors (16) connect the capacitor (14) selectively to the electrodes (18) in response to the control signals, to discharge the capacitor charge into the water, thereby to create an electrical field between the electrodes (18). The charging circuit may be a DC to DC converter (12) that provides an output voltage higher than the battery voltage. The thyristors and associated circuitry are set to discharge the capacitor charge into the body of water in a series of pulses.
BACKGROUND TO THE INVENTION
This invention relates to a method of and apparatus for repelling aquatic creatures such as sharks.
The large nerves in animals such as sharks, contain many thousands of nerve fibres. Some of these fibres are connected to muscles and, when stimulated, cause the muscles to contract. Others run between sense organs and the animal's brain. Artificial stimulation of one of these large nerves by electric pulses applied to the nerve gives rise to the transmission of nerve impulses to the muscles and directly to the brain. The impulses to the muscles give rise to muscular twitching which, together with the direct impulses to the brain, are appreciated as an unnatural sensation apparently arising simultaneously from all the sense organs of the animal. These sensory messages to the brain will, in all probability, startle the animal and drive it away from the source of artificial stimulation.
The applicant's co-pending European Patent Application (publication number 0 631 721) is directed to methods of and apparatus for controlling aquatic animals utilising such artificial stimulation. The patent application describes the use of a pulsed electric field that is set up between electrodes immersed in the water, the pulses having a duration of between 0.1 and 200 ms at a repetition rate of between 1 and 60Hz. The pulses have an amplitude of between 24 and 72V and the rise time of each pulse is preferably less than 0,001us. The polarity of the pulses is reversed periodically by switching the output of the power supply to the electrodes.
When it is intended specifically to control aquatic animals of the sub-group Elasmobranchii, such as sharks the pulses are preferably generated in pulse trains, each comprising a plurality of pulses with a pulse duration of between 0.1 and 3ms. The pulses in each pulse are spaced at intervals of between 1 and 30ms and the pulse trains are repeated at intervals of between 100 and 1000ms. In a preferred version of this embodiment of the invention described in European Patent Application 0 631 721, each pulse in the pulse train has a duration of 2ms and the pulses in each pulse train are spaced at intervals of 20ms, the pulse trains being repeated at a frequency of between 2 and 5Hz. The polarity of successive pulse trains is preferably alternated.
This invention relates to a development on devices of the kind described in European Patent Application 0 631 721.
SUMMARY OF THE INVENTION
According to this invention a method of controlling aquatic animals in a body of water comprises the steps of: immersing at least one first and one second electrode in the body of water; storing electrical charge in charge storage means; and discharging the charge storage means into the body of water via the electrodes, thereby to create an electrical field between the electrodes to repel aquatic animals from the vicinity of the electrodes.
The charge storage means may be a capacitor which is charged to a predetermined voltage by a charging circuit.
The charge storage means is preferably discharged by connecting it to the immersed electrodes via controlled switching elements having a low on resistance.
Further according to the invention apparatus for controlling aquatic animals in a body of water comprises: at least one first and one second electrode for immersion in a body of water; charge storage means for storing electrical charge; a charging circuit for charging the charge storage means from a source of electrical energy; control means for generating control signals; and at least one controllable switch element having a low on resistance and arranged to connect the charge storage means selectively to the first and second electrodes in response to the control signals, to discharge the charge storage means into the body of water, thereby to create an electrical field between the electrodes to repel aquatic animals from the vicinity of the electrodes.
The charge storage means may comprise a capacitor.
The charging circuit may be a DC to DC converter operable from a battery, the DC to DC converter providing an output voltage substantially higher than the battery voltage.
The control means may include timing means for generating the control signals at a predetermined rate and driver means for applying the control signals to control terminals of the switch elements with sufficient energy to operate the switch elements.
The switch elements are preferably silicon controlled rectifiers (SCR's), thyistors or the like that are selected for desirably low on resistance characteristics.
The characteristics of the switch elements and associated circuitry are preferably selected for the charge storage means to discharge into the body of water in a series of pulses, each pulse having a rise time as close to instantaneous as is possible.
BRIEF DESCRIPTION OF THE DRAWINGS
In the drawings:
Figure 1 is a circuit diagram of the electrode switching circuitry forming part of the device of
the invention;
Figure 2 is a circuit diagram of the control circuitry for the device; and
Figures 3 and 4 are oscilloscope traces illustrating some of the preferred electrode pulse wave forms.


DESCRIPTION OF EMBODIMENTS OF THE INVENTION
The circuits illustrated in the drawings are intended for use in a personal protection device that is to be worn or carried by the user. Such a device will of necessity be relatively small and light and it will, in most applications, rely on battery power. This is not intended to limit the scope of the invention to battery powered devices.
Referring to Figure 1, electrical power (either battery or mains power) is applied at 10 to a power converter 12.
The circuitry of Figure 1 illustrates battery power applied to a DC to DC converter 12. While the voltage will be determined by the eventual application, a typical input voltage for a personal protection device is 12V battery voltage input to the DC to DC convertor 12 where it is converted to 60V to 200V DC. In a preferred form of the invention, the output voltage is 84V, but environmental factors, such as water temperature and salinity might determine that a different (or variable) output voltage be used.
The DC voltage is applied to a capacitor 14 where it is stored pending discharge to a number of silicon controlled rectifiers (SCRs) 16 connected in a cross fired bridge configuration.
Under the control of the control circuitry illustrated in
Figure 2, the SCRs 16 switch power to a set of electrodes 18. In use the electrodes 18 are immersed in water which serves as an electrolyte or load.
The output voltage can be controlled in dependence on the conduction characteristics of the water surrounding the electrodes. For instance, the applicant has found that reducing water temperature reduces the water conductivity.
A temperature sensor may therefore be used to control the resistance of a variable resistor in circuit with the DC to DC convertor 12 to vary the output voltage in dependence on the water temperature.
The control circuitry illustrated in Figure 2 consists, essentially, of a timer circuit 20, a control logic circuit 30 and a driver circuit 40.
The timer circuit includes an integrated circuit timer 22 which supplies regularly timed clock pulses to the control logic circuitry 30.
The control logic circuitry 30 generates control signals which are applied to the driver circuit 40.
The control logic circuitry 30 is set to apply alternating firing signals to the gates 42 of a pair of metal-oxide semiconductor field effect transistors (MOSFETs) 44 which drain to a pair of dual secondary pulse transformers 46.
The output pulses from the pulse transformers are applied to the SCR gates.
The control logic circuitry 30 is set to output alternating pulses to the MOSFET gates 42 thereby activating the MOSFETs 44 to apply pulses sequentially to each of the pulse transformers 46.1 and 46.2.
The first secondary 48.1 of the pulse transformer 46.2 is connected to SCR 1 (16.1). The second secondary 48.4 of the pulse transformer 46.2 is connected to SCR 4 (16.4).
In the same way, the first secondary 48.2 of the pulse transformer 46.1 is connected to SCR 2 (16.2) and the second secondary 48.3 is connected to SCR 3 (16.3).
The pulse transformers have a turns ratio which is calculated to provide control signals to the gates of the
SCR's 16 having a sufficiently high voltage and sufficient energy to switch the SCR's hard on rapidly. In the preferred form of the invention, the SCR gates are supplied with a switching pulse that is in excess of the maximum SCR rated gate pulse.
The SCR's are switched hard on rapidly in order to permit the fastest possible discharge of the capacitor 14 in order to obtain an output pulse at the electrodes with a desirably short rise time as is illustrated in the waveform diagrams of figures 3 and 4.
The pulses may have a duration of between 100us and 200ms at a repetition rate of between 0.5 and 60Hz. In this regard, a single repetition of an alternating or commutated pulse device is an "up" and a down pulse.
The pulse duration and shape (see figures 3 and 4) is determined largely by the impedance of the load (sea water). The rise time of each pulse is ideally as close to instantaneous as the circuit will permit and preferably less than 0,2us.
The control logic circuit 30 applies control pulses alternately to the gates of the MOSFET's 44.1 and 44.2, so that the secondary windings of the pulse transformers 46.1 and 46.2 produce output pulses alternately.
Thus, a control pulse applied to the gate of the MOSFET 44.2 causes relatively high voltage control pulses to be applied to the gates of the SCR 1 (16.1) and SCR 4 (16.4), causing an output pulse of nominal positive polarity to be generated between the electrodes 18.1 and 18.2. The next control signal, applied to the gate of the MOSFET 44.1, causes control pulses to be applied to the gates of
SCR 2 (16.2) and SCR 3 (16.3), causing an output pulse of nominal negative polarity to be generated between the electrodes. Thus, alternate pulses at the electrodes 18.1 and 18.2 have opposite polarity, effectively doubling the peak to peak output voltage of the unit.
Where peak output voltage is of secondary importance, a simpler circuit employing a single switch element can be used instead.
http://news.nationalgeographic.com/news/2004/07/0729_040729_sharkrepellent_2.html
July 29, 2004
New Shark Repellent Uses Chemical Signals
Brian Handwerk
Researchers say they have developed a shark repellent that uses apparently natural chemical signals to shift the animals from hunting mode to flight mode. If it proves to be effective and environmentally safe to use, it could soon become standard-issue for everyone who comes into contact with the marine predators—from surfers to commercial fishers.
Eric Stroud is a chemist and cofounder of the New Jersey based Oak Ridge Shark Lab. He began looking for an effective repellent during 2001, when some well-publicized incidents caused a media feeding frenzy known as the Summer of the Shark (in fact, that season recorded below-average statistics of shark-human encounters).
"As a chemist I was wondering what was being done as far as a repellent," Stroud recalled. "I began looking through a lot of past research and ended up in the area of semiochemicals. That seemed to be promising."
Semiochemicals are chemical "messengers" used in natural behavior and communication between individuals — though the chemicals' exact roles are not completely understood.
Animals or even plants may emit different semiochemicals (including pheromones) which serve as sexual attractants, repellents to potential predators, or inducements to flight mode. A flower, for example, may mimic sexual attractants to draw pollinating insects, while other animals may emit scents that deter predators.
Semiochemicals are currently used in animal-control industries like insect management. They can be used as attractants to lure bugs into traps or as repellents to keep them away.
Semiochemicals are also common in the lives of aquatic animals, said Samuel H. Gruber. "Doc" Gruber is a marine biologist at Florida's University of Miami and a leading shark researcher with decades of hands-on experiences. "Certain kinds of fishes, like minnows, release something when attacked that tells the rest of the school to disperse quickly," he said.
Stroud and assistant Mike Herrmann believed that sharks might possess a similar avoidance chemical that sometimes warns other sharks to stay away. Their task was to isolate that chemical." We took that as our direction and began to investigate the molecular chemistry of shark tissues," Stroud said.
The hands-on results from tests at Gruber's Bimini Biological Field Station in the Bahamas, and elsewhere, have been very promising.
To date, six different species have been effectively repelled by the mixture, which was dropped from a boat into a chum-filled sea of feeding sharks.
"They stop feeding, go into alarm mode, and they rapidly leave," Stroud explained. "Once they detect this, we suspect by olfactory senses, there's definitely a behavioral change, and they either go deep or leave the area.
"I think it's not a question of [affecting the] gills or of pain, it seems to be a signal," Gruber said. "When the shark gets the signal its behavior looks reflexive."
In all tests so far, the chemical has proven nontoxic to sharks.
Fish feeding in the area appear to be totally unaffected, yet sharks detect the substance in even minute proportions.
In the controlled environment of lab tanks, sharks have responded to even 0.1 part per million — for example, they would likely respond to 12 ounces of the chemical in an Olympic-size swimming pool.
The semiochemical is even strong enough to awaken lab sharks from tonic immobility, an induced, "death-feigning" state during which researchers can go so far as to perform surgery without arousing the animal.
Historic Challenge
Shark repellents have been in development for decades — with only limited success. Researchers have tried (and continue to try) everything from chemicals and cages to audio signals and electric fields.
During World War II widespread ocean combat and casualties led to large numbers of human-shark interactions. The Navy issued a chemical repellent called Shark Chaser to protect sailors and airplane pilots.
Sharks also caused operational difficulties. Future chef Julia Child helped the wartime Office of Strategic Services (OSS), the forerunner to today's CIA, cook up repellents that would prevent sharks from prematurely detonating anti-submarine explosives.
None of these wartime repellents was particularly effective.
In the mid-1970s, marine biologist Eugenie Clark tested a natural repellent from acidic protein secretions of the flounder-like Red Sea Moses sole. Gruber also worked on the project in Israel, Egypt, and Japan. However, effective, natural supplies of the secretion were limited and synthetic versions proved expensive and unstable.
In the early 1980s Cold War developments renewed the Navy's lapsed interest in repellents.
"It came to light that the submarine fleet was being challenged by sharks," Gruber recalled. "We had subs, as did the Soviets, cruising around the Atlantic listening for each other with towed sonar arrays, and from time to time they experienced what was called the 'million dollar bite.'
The costly chomp occurred when sharks bit, and damaged, trailing arrays or listening devices known as hydrophones — in these cases, big rubber tubes about 2 inches (5 centimeters) in diameter and half a mile (800 meters) long.
"The sharks were really biting into these things," Gruber recalled, noting that the problem spurred his first involvement in the development of chemical repellents.
In the 1990s the Natal Sharks Board of South Africa developed and patented electronic repellent technology employed by professional divers on their cages.
Australian-based SeaChange Technology currently markets the technology on their Shark Shield line of electronic repellent products for divers, swimmers, and surfers. While some hail the devices as effective, they lack the possible range of uses a semiochemical repellent could offer.
Fisherman's Friend?
Semiochemical repellent could find its way into everything from clothing to fishing tackle.
The substance could be a boon for longline commercial fishing operations like swordfish boats—and for the sharks that they inadvertently catch.
"To make longline fishing a little more selective, to reduce the horrific bycatch, which is sometimes three or five wasted sharks for each targeted species—that would be fantastic," Gruber said.
Though the product must be tested on more species, recreational applications may soon include incorporating the chemical into bathing suits, sunblock, and wet suits.
While the chance of attack will always be very small, those who spend time in the water may breathe a bit easier knowing that they are chemically less appealing to sharks.
Yet the biggest beneficiary may turn out to be the sharks themselves. Helping them avoid human encounters may be critically important to their survival.
Stroud's Shark Repellant Patents
ELASMOBRANCH-REPELLING MAGNETS AND METHODS OF USE
US2014173966
Devices and methods are disclosed for repelling elasmobranchs with high-pull-force magnets, including devices and methods for reducing by-catch in commercial fisheries and protecting humans from attacks by elasmobranchs.
BACKGROUND OF THE INVENTION
[0002] Elasmobranchs represent a significant problem in the commercial fishing industry. Elasmobranchs are often inadvertently caught on fishing hooks and tackle directed at other more commercially valuable kinds of fish. This inadvertent catching of elasmobranchs (or other non-valued fish) is called "by-catch." As many as 100 million elasmobranchs are killed each year as by-catch. This loss of life has resulted in a real threat to several shark species. Currently, as many as 80 species of shark are considered threatened with extinction.
[0003] Further, when elasmobranchs are caught as by-catch, fishing operations receive no return on their investment since the shark is caught on a hook that might have otherwise brought in a marketable fish. Additionally, the fishing tackle on which a shark is caught often must be cut loose for the safety of those working on the fishing vessel causing a loss of both equipment and time.
[0004] Longlining is a commercial fishing method that suffers significant losses from shark by-catch. Longlining uses multiple baited individual fish hooks with leaders strung at intervals along an often very long (2-3 miles) main fishing line. Longline fishing operations routinely target swordfish and tuna. The longline hooks, however, are not selective and elasmobranchs are sometimes caught in greater numbers than the intended catch. The result is great loss of life in elasmobranchs and significant financial losses in the longline industry. Elasmobranchs cause additional losses in the longline fishing industry by scavenging marketable fish caught on longlines before the fish may be retrieved for processing.
[0005] Elasmobranchs also represent a problem in the commercial trawling industry. Trawling is a commercial fishing method that catches fish in nets. Elasmobranchs cause significant losses for trawlers because they scavenge fish caught in trawl nets before they are retrieved for processing. As such, valuable fish are often lost to shark predation. Also, sharks often tear holes in the nets, resulting in partial or complete loss of catch and significant repair costs.
[0006] There has been a long-felt need for methods and devices to deter elasmobranchs from commercial fishing lines and nets. Attempts in the middle of the twentieth century were made to protect trawl nets with electric discharge devices. (Nelson, "Shark Attack and Repellency Research: An Overview," Shark Repellents from the Sea ed. Bernhard Zahuranec (1983) at p. 20). Nevertheless, no commercially effective repellent has yet to be made available for reducing shark by-catch in the commercial fishing industry or for reducing loss of valuable fish or fishing tackle to shark predation. Further, Applicant is unaware of any consideration in the art of the use of magnets to repel elasmobranchs to limit by-catch and other losses from elasmobranchs.
[0007] U.S. Pat. No. 4,667,431 discloses an electric prod for repelling fish. Within the electric prod, the switch for providing electric current to the prod is a reed switch, which contains a magnet. However, the magnet is not a part of the repelling portion of the electric prod.
[0008] An effective shark repellent would not only be valuable to the fishing industry but also would be valuable for protecting humans from shark attacks. No effective repellent has yet to be marketed for limiting the risk of shark attacks faced by humans exposed to elasmobranchs. Over the last 50 years antishark measures employed to protect humans from shark have included electrical repellent devices (Gilbert & Springer 1963, Gilbert & Gilbert 1973), acoustical playbacks (Myrberg et al. 1978, Klimley & Myrberg 1979), visual devices (Doak 1974) and chemical repellents (Tuve 1963, Clark 1974, Gruber & Zlotkin 1982). None of these procedures proved satisfactory in preventing shark attacks. (Sisneros (2001)). As such, the long felt need for an effective repellent has not been satisfied.
[0009] Researchers have historically used several bio-assays to determine if a repellent evokes a flight response in shark. One such bio-assay measures the effect of a repellent on a shark that is immobilized in "tonic immobility." Tonic immobility is a state of paralysis that typically occurs when a shark is subject to inversion of its body along the longitudinal axis. This state is called "tonic," and the shark can remain in this state for up to 15 minutes thereby allowing researchers to observe effects of repellents. After behavioral controls are established, an object or substance that has a repelling effect will awaken a shark from a tonic state. Researches can quantify the strength of a repellent effect from these studies.
[0010] Another bio-assay employs a Y-shaped maze wherein a shark is exposed to a choice between two paths containing the same olfactory stimulus. One path exits the maze without a repellent while the other contains a repellent. If the sharks consistently choose the path without the repellent or consistently become agitated in the path having the repellent, researchers may conclude the repellent is effective.
BRIEF SUMMARY OF THE INVENTION
[0011] Applicant has discovered that a high-pull-force magnet is an effective elasmobranch repellent useful in limiting by-catch as well as protecting humans. High-pull-force magnets, known or hereinafter developed, that are of sufficient strength to repel elasmobranchs are acceptable in aspects of the present invention.
[0012] According to a non-limiting embodiment of the present invention, an apparatus for repelling elasmobranchs is provided comprising a high-pull-force magnet. Preferably, the high-pull-force magnet is a permanent magnet. More preferably, the high-pull-force magnet is a neodymium-iron-boride magnet. According to a non-limiting embodiment of the invention, the high-pull-force magnet may have a nickel coating to protect the magnet from corrosion. High-pull-force magnets in accordance with the present invention may have a shape of a cylinder, a cone, a circle, a cube, a disk, a bar, a sphere, a plate, a rod, a ring, a tube, a stick, a block or other shape. In a non-limiting embodiment of the invention, a high-pull-force magnet may have a hollow portion. In a non-limiting embodiment of the invention, a plurality of high-pull-force magnets may be arranged together in a ring. In another non-limiting embodiment of the invention, an apparatus is provided with a high-pull-force magnet that is capable of spinning.
[0013] High-pull-force magnets of the present invention have a pull force preferably of greater than about 50 pounds, more preferably greater than about 100 pounds, and most preferably greater than about 200 pounds. In a non-limiting embodiment, a high-pull-force magnet has a nominal strength of preferably greater than about 5000 gauss, more preferably greater than about 10,000 gauss, and most preferably greater than about 20,000 gauss. In a non-limiting embodiment, a high-pull-force magnet produces a magnetic strength preferably of about 5 gauss at a distance of about 0.01 m to about 1 m, more preferably of about 5 gauss to about 14,000 gauss at a distance of about 0.01 to about 0.5 m, and most preferably of about 10 gauss to about 320 gauss or greater at a distance of about 0.1 m to about 0.4 m.
[0014] According to a first non-limiting aspect of the present invention, an apparatus is provided comprising a high-pull-force magnet and a buoy, a barge, a net, fishing tackle or any combination thereof. Fishing tackle may comprise a longline, a main line, a gangion, a lead, a weight, a buoy, a net, or any combination thereof.
[0015] According to a second non-limiting aspect of the present invention, an apparatus is provided comprising a high-pull-force magnet and a fish hook. Such fish hook may be individual or attached to longline or mainline and such fish hook may have a single hook or multiple hooks.
[0016] According to a third non-limiting aspect of the present invention, a method is provided for repelling elasmobranchs comprising attaching a high-pull-force magnet to a hook, longline, mainline, fishing tackle, gangion, lead, weight, buoy, net, boat or any combination thereof.
[0017] According to a fourth non-limiting aspect of the present invention, an apparatus is provided comprising a surfboard and a high-pull-force magnet. A high-pull-force magnet may be housed within the surfboard, be attached to the surfboard, or be trailed behind the surfboard in the water.
[0018] In fifth non-limiting aspect of the present invention, a method is provided for repelling elasmobranchs comprising attaching a high-pull-force magnet to a human body or to clothing or accessories associated with a human body. In a preferred technique, a high-pull-force magnet may be attached to a human ankle or wrist or may be attached to a bracelet. A high-pull-force magnet may also be attached to a belt, a weight belt for diving, or flippers for diving and snorkeling.
[0019] In a sixth non-limiting aspect of the present invention, a kit is provided comprising a high-pull-force magnet.
BRIEF DESCRIPTION OF THE DRAWINGS
[0020] The invention will now be described by way of example with reference to the accompanying drawings wherein:
[0021] FIG. 1 illustrates a traditional circle hook attached to a line and a non-limiting preferred zone (I) for locating a high-pull-force magnet in accordance with the present invention.
[0022] FIGS. 2A-C illustrate non-limiting positions within the zone (I) for locating a high-pull-force magnet in accordance with the present invention. FIG. 2A illustrates a high-pull-force magnet attached to the line above the hook. FIG. 2B illustrates a high-pull-force magnet attached to the hook. FIG. 2C illustrates a high-pull-force magnet attached to the hook shank and clear of the hook eye.
[0023] FIGS. 3A-C illustrate non-limiting positions within the zone (I) for locating a high-pull-force magnet on a J- hook in accordance with the present invention. FIG. 3A illustrates a high-pull-force magnet attached to the line above the hook. FIG. 3B illustrates a high-pull-force magnet attached to the hook. FIG. 3C illustrates a high-pull-force magnet attached to the hook shank and clear of the hook eye.
[0024] FIGS. 4A-B illustrate non-limiting positions within the zone (I) for locating a high-pull-force magnet on a treble hook in accordance with the present invention. FIG. 4A illustrates a high-pull-force magnet attached to the line above the hook. FIG. 4B illustrates a high-pull-force magnet attached to the hook.
[0025] FIG. 5 illustrates an exemplary demersal longline with a high-pull-force magnet in accordance with the present invention.
[0026] FIGS. 6A-B illustrate non-limiting devices for repelling elasmobranchs in accordance with the present invention. FIG. 6A illustrates a buoy and high-pull-force magnet and a net with a plurality of high-pull-force magnets in accordance with the invention. FIG. 6B illustrates a barge and a high-pull-force magnet in accordance with the present invention.
[0027] FIGS. 7A-C illustrate non-limiting exemplary surfboards with a high-pull-force magnet in accordance with the invention. FIG. 7A illustrates a surfboard with a high-pull-force magnet embedded in or attached to the surfboard in accordance with the invention. FIG. 7B illustrates a surfboard with a high-pull-force magnet that is capable of spinning in accordance with the invention. FIG. 7C illustrates a high-pull-force magnet or magnets that are capable of spinning when placed in water. Such a spinning high-pull-force magnet may comprise individual magnets attached to a hub that is attached to an axle to allow free spinning of the high-pull-force magnet or magnets attached to the surfboard when water current is present.
[0028] FIGS. 8A-C illustrate exemplary accessories for attaching a high-pull-force magnet to a human or other subject or object. FIG. 8A illustrates a belt or weight belt with a high-pull-force magnet in accordance with the invention. FIG. 8B illustrates a bracelet or wristband with a high-pull-force magnet in accordance with the invention. FIG. 8C illustrates flippers for snorkeling or diving with a high-pull-force magnet in accordance with the present invention.
[0029] FIG. 9 illustrates a plurality of high-pull-force magnets arranged into exemplary bracelets, belts or attachable rings in accordance with the invention.
DETAILED DESCRIPTION OF THE INVENTION
[0030] "By-catch" is any kind of fish that is caught in a fishing operation wherein the fish is not the object of the fishing operation. For example, if the target fish of a longline fishing operation is tuna, an elasmobranch caught on a hook of the longline is by-catch.
[0031] "Elasmobranchs" in this specification means one or more elasmobranchii in the super-orders Galeomorphii and Squalomorphii and orders Squaliforms (dogfish), Carcharhiniformes (requiem sharks), Lamniformes (mackerel sharks), and certain Orectolobiformes (carpet sharks). Elasmobranchs in this specification includes nurse sharks, an Orectolobiforme, but this specification does not include the other carpet sharks, such as wobbegongs.
[0032] "Gauss" is a measure of magnetic field strength. Gauss is a unit of the density of a magnet's flux (or flux density) measured in centimeter-gram-second. A tesla is equal to 10,000 gauss. Gauss and tesla are common units for referring to the power of a magnet to attract (or repel) other magnets or magnetic materials. The Gauss unit describes both the coercivity of a magnet and its saturation magnetization. Gauss describes how strong the magnetic fields are extending from the magnet and how strong of a magnetic field it would take to de-magnetize the magnet.
[0033] "Grade" of a neodymium-iron-boride magnet specifies the quality of material used to construct the magnet. All else being equal, the higher the quality of materials used to construct the magnet, the greater the magnet's strength. In grading neodymium-iron-boride magnets, a lower grade, e.g., N35, does not have as much magnetic strength as a higher grade, e.g. N45.
[0034] "Longline" refers to a fishing line that may extend up to many miles wherein a mainline extends the full length of the longline and individual shorter gangion lines attached to the mainline are spaced at set intervals (perhaps several feet or meters or perhaps 1000 feet or greater apart). Hooks are attached to the individual gangion lines. Hooks may be baited and used to catch target fish. The addition of a magnet of sufficient strength repels elasmobranchs from the baited hooks as well as from the region of the longline generally.
[0035] "Nominal strength" of a magnet is measured in gauss or tesla and reflects the theoretical strength of a magnet at its core. Nominal strength is a function of the grade of a magnet. The higher the grade, the higher the nominal strength. Nominal strength is the strength necessary to demagnetize the magnet.
[0036] "Pull force" is the attractiveness of a magnet to a mild steel flat surface in pounds. The formula for calculating pull force is provided in detail herein.
[0037] "Target fish" is any kind of fish, the catching of which is the object of a fishing operation. For example, the target fish of a longline fishing operation may be tuna. A fish that is caught on the longline that is not tuna would not be a target fish.
[0038] "Tonic immobility" is the state of paralysis that typically occurs when an elasmobranch is subject to inversion of its body along the longitudinal axis of the body, i.e., is belly up. An elasmobranch can remain in this state for up to 15 minutes.
I. HIGH-PULL-FORCE MAGNETS AS REPELLENTS OF ELASMOBRANCHS
[0039] It has been discovered that high-pull-force magnets repel elasmobranchs. High-pull-force magnets comprising a pull force of about 50 pounds or greater introduced into the environment of an elasmobranch have demonstrated repelling action on elasmobranchs. Likewise, magnets comprising a nominal strength of greater than about 0.5 teslas (5000 gauss) have demonstrated repelling action on elasmobranch. Further, magnets producing about 5,000 mG to about 500,000 mG of magnetic strength at a distance of about 0.01 m to about 1 m from the magnet or about 10,000 mG to about 320,000 mG of magnetic strength at a distance of about 0.1 m to about 0.4 m from the magnet have demonstrated repelling action on elasmobranchs.
[0040] High-pull-force magnets may be employed near fishing lines, fish hooks or fishing nets to repel sharks from bait, hooks or nets that have been set for target fish (not sharks). High-pull-force magnets may also be employed near people, animals or objects in the water to repel elasmobranchs from frightening or injuring the people, animals or objects in a particular area.
[0041] Sufficient magnetic force to repel elasmobranchs may be measured in a number of ways. Magnetic force may be measured as pull force, as nominal magnetic strength at the core of the magnet (in gauss) or at a distance of interest from the magnet (in gauss). Any measurement known to an artisan practicing the invention may be useful.
[0042] The high-pull-force magnet may be a permanent magnet or an electromagnet. Magnets made of neodymium-iron-boride (NdFeB) are preferred, given present magnet technology, since these magnets have high pull force relative to their physical size. A coating, such as nickel, may protect permanent high-pull-force magnets from corrosion in water. A preferred NdFeB magnet, in accordance with the present invention, may have a grade of about N38 through about N50 or greater.
[0043] A high-pull-force magnet for repelling elasmobranchs may comprise the shape of a cylinder, a cone, a circle, a cube, a disk, a bar, a sphere, a plate, a rod, a ring, a tube, a stick, a block, a tapered cone, or any other shape. The high-pull-force magnet may further comprise a hollow portion for stringing, like beads, on a fishing hook, line, belt, bracelet or rings. A high-pull-force magnet comprising a cylinder with a diameter of about 4 inches to about 8 inches and a thickness of about 1 inch to about 4 inches is preferred. A magnet with a diameter of about 4 inches and a thickness of about 1.5 inches is most preferred.
[0044] High-pull-force magnets having a pull force of about 50 pounds or greater have demonstrated repelling activity on elasmobranch species at distances as great or greater than 0.3 m from the elasmobranches. Further, a longline fitted with a series of seven magnets set more than 100 feet apart has shown repelling activity across an entire longline of about 2000 feet. As such, high-pull-force magnets in accordance with the invention may be used to repel elasmobranches. The repelling activity of high-pull-force magnets may be useful in the commercial fishing industry to reduce elasmobranch by-catch and predation, and useful to repel elasmobranchs from humans in the environment of an elasmobranch or repel elasmobranchs from an area of interest.
[0045] The mode of action of high-pull-force magnets on elasmobranchs is not fully understood. While not wishing to be bound by any particular theory, one plausible theoretical explanation for this surprising finding of repellent activity of high-pull-force magnets is the possibility that electrical eddy currents are generated by an elasmobranch moving through the strong magnetic field created by the high-pull-force magnet. The resulting eddy currents may over stimulate ampullae of Lorenzini (known to be used by elasmobranchs for navigation and orientation) causing the ampullae of Lorenzini to disorient the elasmobranch or otherwise signal danger to the elasmobranch causing aversive behavior.
[0046] Several species of sharks have demonstrated the ability to sense magnetic fields (Kalmijn, 1978; Ryan, 1980; Klimley, 1993; 2002) but were not repelled by the use of such magnets. The ampullae of Lorenzini organ within sharks is used to detect weak electrical fields at short ranges, which functions in the final stages of prey capture: usually when a shark is inches from its prey. A shark's prey emits weak electric fields that are detectable to the shark. As a shark approaches prey, the shark can sense the weak electric field emitted therefrom. In the natural environment, the detection range of the shark's ampullae of Lorenzini is effective only within inches of an object. As magnetic field strength is increased elasmobranchs sense the magnetic field at much greater distances, such as 0.3 m or greater. When a plurality of magnets are introduced across a large area or region (such as along a fishing longline) sharks may sense a powerful magnetic field at close range spanning an area/length of 1000 feet.
[0047] Magnetic fields generated by high-pull-force magnets such as permanent magnets are effective repellents for elasmobranchs, excluding certain carpet sharks in the Orectolobidae family. It is believed that high-pull-force magnets are not effective repellents against certain carpet sharks, particularly spotted wobbegongs (Orectolobus maculatus), because they ambush predators and rely more on visual, olfaction, and lateral line clues than this magnetic sense. This species of shark is found chiefly in Australia and Indonesia, and does not represent significant by-catch species or species that are known to be aggressive against humans. Magnets, however, are effective against nurse sharks in the Orectolobiform family.
[0048] While not wishing to be bound by a particular theory, the flux of a permanent magnet, such as an NdFeB magnet, may correlate with the detection range of the ampullae of Lorenzini. Since, the magnetic flux from a magnet decreases at the inverse cube of the distance from the magnet, at only a few meters distance the magnetic field exerted by the magnet is less than the Earth's magnetic field. As such, repelling of elasmobranchs with magnets appears to occur within several meters of a high-pull-force magnet. Additionally, if a series of high-pull-force magnets is spaced in a region, a measurable level of repelling appears to occur over the entire region.
[0049] High-pull-force magnets have been demonstrated by Applicant to act as acceptable repellents of elasmobranchs. The repellent activity of high-pull-force magnets has been shown to be better than existing shark-repellent technology with the exception of certain chemical repellents being developed by SHARK DEFENSE LLC that have a greater range of action.
[0050] A. Magnetic Forces
[0051] The force of a magnet may be measured in a variety of ways. Gauss is a unit of the density of a magnet's flux (or flux density) measured in centimeter-gram-second. A tesla is equal to 10,000 gauss. Gauss and tesla are known common units for referring to the power of a magnet to attract (or repel) other magnets or magnetic materials. The Gauss unit describes both the coercivity of a magnet and its saturation magnetization. Basically, it describes how strong the magnetic fields are extending from the magnet and how strong of a magnetic field it would take to de-magnetize the magnet.
[0052] The pull force of a magnet is related to the magnet's nominal strength in gauss or teslas but uses the nominal strength to create a practical measure of a magnets ability to apply a pulling force on materials that are attracted to a magnetic field, such as ampullae of Lorenzini in elasmobranch. Pull force is related to the flux density of the magnet's magnetic field (in gauss or tesla) and the shape of the magnet. Pull force is calculated using the following equation: Pull Force=0.576*Br <2>*(Th)*A <1/2 >where Br=Flux Density in KiloGauss, Th=Thickness of Magnetized Surfaces in inches and A=Surface Area of the magnet in inches. Using this equation, a magnet's pull force may be determined A high pull force value for magnets is greater than about 50 pounds.
[0053] The strength of a magnet's magnetic field is inversely related to the distance an object is from the magnet. As such, magnets of very low strength (or gauss) may repel elasmobranchs if the elasmobranch moves close enough to sense the magnetic field of the magnet. A high-pull-force magnet having sufficient strength to repel an elasmobranch at sufficient distance such that the elasmobranch is deterred from striking a baited hook or coming near a person or other subject is preferred. It is more preferred that a high-pull-force magnet have a pull force of at least 100 pounds to provide sufficient magnetic force to repel an elasmobranch away from a baited hook or a person before the elasmobranch may bight the hook or harm the person. Because an elasmobranch may act to strike a hook or person at a distance from the target, the stronger the high-pull-force magnet, the more effective it will be. It has been reported that magnets have a beneficial health effect in humans and a negative health effect in humans at high power. Applicant makes no representation herein of the safety of use of high-pull-force magnets by humans during short- or long-term use.
II. METHODS AND DEVICES FOR MAGNETIC REPELLENTS
[0054] A. Magnets
[0055] Exemplary and non-limiting high-pull-force magnets in accordance with the invention may be constructed of any material that is capable of generating a magnetic field without requiring an outside energy source (such a permanent ferrous magnet). Magnetism may be generated in any manner known to the skilled artisan who is practicing aspects of the invention.
[0056] There are many varieties of permanent magnet materials including neodymium magnets (which are some of the most powerful permanent magnets known at this time), samarium-cobalt magnets, ceramic magnets, plastic magnets, Alnico magnets as well as traditional ferrous magnets. Any magnetic material having sufficient pull force may be used as a repellent of elasmobranchs.
[0057] Exemplary permanent magnets include neodymium-iron-boride (NdFeB) magnets, ferrous metal magnets, samarium-cobalt magnets, or any other magnetic material. High-pull-force magnets may be flexible or inflexible. High-pull-force magnets may be made of sintered metal powder or of metal or any other magnetizable material.
[0058] A preferred magnetic material for high-pull-force magnets contemplated within an aspect of the invention is NdFeB. NdFeB is a more preferred material than ferrous magnets, flexible magnets or samarium-cobalt magnets. Flexible magnetic strips may be constructed from magnetic powder such as ferrous or other powder mixed with polymer bonding material such as rubber-like material. Samarium-cobalt magnets are less preferred in that they may be more brittle than other magnets.
[0059] In selecting a high-pull-force magnet, a pull force of about 50 pounds or greater is preferred. A pull force of about 100 pounds or greater is more preferred since the impact of the magnetic field will felt at a greater distance from the magnet.
[0060] Neodymium-iron-boride magnets, commonly called "rare earth," "NdFeB," or "NIB" magnets, typically meet or exceed residual inductances greater than about 5,000 gauss, which is preferred. Residual inductance defines how changing magnetic fields generate electric currents and is also measured in gauss.
[0061] In order to maximize high pull force, the surface area of a magnet may be maximized. For example, a 6'' diameter by 2'' thick cylindrical N38 NdFeB magnet (nominal strength 13000 gauss; pull force 1042 pounds) may be effective in repelling elasmobranchs at a range of 6''.
[0062] A plurality of magnets may be employed to repel elasmobranchs. For example, 1'' cube magnets may be arranged in a 12'' long bar and used to repel elasmobranchs. The cube magnets may be of any magnetic material capable of producing sufficient magnetic strength at any distance of interest from the magnet to repel elasmobranchs.
[0063] Alternatively, a plurality of 1'' cube magnets may be arranged linearly with a distance between each magnet. The magnets may be arranged linearly with positive poles facing one another or may be arranged with positive poles facing negative poles. Smaller magnets are also effective in repelling elasmobranchs and may preferably be arranged to maximize surface area presented to an oncoming elasmobranch.
[0064] Metals with special magnetic properties may be used in conjunction with permanent magnets in order to maximize or shape the magnetic flux profile of the magnet and thereby increase the pull force by directing the magnetic force more powerfully at an elasmobranch of choice. For example, holmium metal, which possesses the highest magnetic moment of the known elements, may be used to optimize the magnetic flux profile. A 1.5'' holmium ring with a drilled 0.5'' diameter center, coupled to an NdFeB 1.5'' diameter cylindrical magnet, produced aversive reactions in immobilized sharks when the holmium end was oriented to the shark's nares. Other materials that may also be used, among others, for controlling the shape of the magnetic flux of a magnet may be gadolinium; pyrolytic graphite; mu-metal (a nickel-iron alloy comprising copper and molybdenum that has a very high magnetic permeability and is, therefore, very effective at screening magnetic fields); and bismuth.
[0065] To protect permanent magnets from corrosion when placed in water, permanent magnets may be coated with any coating that will reduce corrosion and preserve the magnetic force of the magnet. For example, magnets may be coated with nickel, rubber, plastic, acrylic, enamel, paint or other coating. Nickel-plated NdFeB magnets are an example of preferred high-pull-force magnets so long as the coating remains intact.
[0066] It may be desirable to encase a magnet in paint. Black paint is a preferred paint color to avoid underwater reflections and flashes of sunlight from the magnet's surface that can act as an attractant. A magnet may also be enclosed in any waterproof housing, such as a polymer coating.
[0067] B. High-Pull-Force Magnets in Combination with Hooks
[0068] A non-limiting aspect of the present invention is the use of high-pull-force magnets to repel elasmobranchs from baited hooks. Exemplary and non-limiting combinations of a high-pull-force magnet and a hook are illustrated in FIGS. 1-4. For example, in FIG. 1, an exemplary and non-limiting circle hook and line ( 100) are illustrated wherein a circle hook ( 140) is attached to a line ( 150) along with an exemplary and non-limiting Zone I in the circle hook and line where a high-pull-force magnet may be placed or affixed. The preferred region (Zone I) for magnet placement along the line ( 150) or shank ( 142) of the hook is any region wherein the affixed or placed magnet does not obstruct the hook gap distance (Zone II). Not more than 20% of the hook gap distance (Zone II) is preferably obstructed by the magnet such that the hook is not prevented from being baited or setting in the corner of the mouth of a target fish. Nevertheless, any arrangement wherein the hook is not prevented from catching target fish is acceptable. Tapered conical designs (not illustrated) are contemplated such that the diameter of the high-pull-force magnet at the hook end is smaller than the diameter of the high-pull-force magnet at the line end of Zone I.
[0069] Exemplary and non-limiting combinations of a high-pull force magnet on a hook and line are illustrated in FIGS. 2A-C. As in FIG. 2A, a high-pull-force magnet ( 210) may be placed in proximity to a circle or offset circle hook ( 240) attached to a line ( 250) so that it rests on the hook eye ( 241) providing an exemplary embodiment such as the hook-magnet combination embodied at 260. As in FIG. 2B, a high-pull-force magnet ( 210) may be placed in proximity to a circle or off-set circle hook ( 240) so that it rests on the shank ( 242) of the hook providing an exemplary embodiment such as the hook-magnet combination embodied at 270. As in FIG. 2C, a high-pull-force magnet ( 210) may be placed on a circle or offset circle hook ( 240) so that it is secured to the outside of the shank ( 242) and the hook eye ( 241) providing an exemplary embodiment such as the hook-magnet combination embodied at 280. A high-pull-force magnet may be affixed outside the shank ( 241) of a hook simply by the magnetic force of the high-pull-force magnet. Vinyl electric tape (not illustrated) may be used to secure the high-pull-force magnet. Black vinyl tape is preferred to reduce reflections of light.
[0070] High-pull-force magnets may be provided in any shape. It is preferred that a magnet's shape not significantly obstruct the hook gap distance (zone II). The magnet may comprise a hole through which a lead, or gangion, or mainline or other filamentous object may pass. Exemplary non-limiting shapes may include a cube or block of any size or other object having at least one plane comprising four right angles and a hole passing through the object such that fishing line or other filament may be passed through to affix the magnet in place on fishing tackle or other object. Alternative, non-limiting shapes may also include cylindrical or other circular, oval or oblong three-dimensional shapes having a hole passing through some portion of the shape. Alternative, non-limiting shapes may also include a hollow pyramid or a hollow trapezoid.
[0071] Alternative, non-limiting shapes may also include a solid cube or similar shape, a solid rectangle or similar shape, a solid bar or similar shape, a solid pyramid or similar shape, a solid trapezoid or similar shape or any other shape. Magnets may be shaped as a ring, a trapezoid, a series of trapezoids, a series of trapezoids arranged in a larger ring pattern, a cone, a tapered cone, a narrow or wide cylinder or in the shape of a Billy club. Preferably, the shape when combined with a hook provides a hook in proximity to a magnet comprising sufficient magnetic field strength to repel elasmobranchs.
[0072] Exemplary and non-limiting combinations of a high-pull-force magnet and a hook are also illustrated in FIGS. 3A-C. As in FIG. 3A, a high-pull-force magnet ( 310) may be placed in proximity to a j-hook ( 340) on a line ( 350) such that it rests on the hook eye ( 341) providing an exemplary embodiment such as the hook-magnet combination embodied at 360. As in FIG. 3B, a high-pull-force magnet ( 310) may be placed in proximity to a j-hook ( 340) such that it rests on the shank ( 342) of the hook providing an exemplary embodiment such as the hook-magnet combination embodied at 370. As in FIG. 3C, a high-pull-force magnet ( 310) may be placed on a j-hook ( 340) such that it is secured to the outside of the shank ( 342) and the hook eye ( 341) providing an exemplary embodiment such as the hook-magnet combination embodied at 380. As described above in the illustration of FIG. 2, magnets may be provided in any shape.
[0073] Exemplary and non-limiting combinations of magnet and hook are also illustrated in FIGS. 4A-B. In FIG. 4A, a high-pull-force magnet ( 410) may be placed in proximity to a treble hook ( 440) on a line ( 450) such that it rests on the hook eye ( 441) providing an exemplary embodiment such as the hook-magnet combination embodied at 460. As in FIG. 4B, a magnet ( 410) may be placed in proximity to a treble hook ( 440) such that it contacts the shank ( 442) of the hook providing an exemplary embodiment such as the hook-magnet combination embodied at 470.
[0074] A hook in accordance with the invention may be any hook that is capable of catching target fish. The hook may comprise stainless steel, steel, galvanized metals, ferromagnetic metals or any other material, metallic or plastic or any other composite.
[0075] A high-pull-force magnet in accordance with an aspect of the invention may comprise any magnetic material.
[0076] C. High-Pull-Force Magnets on Longlines
[0077] An exemplary and non-limiting method of repelling elasmobranchs involving repelling elasmobranchs from longlines in accordance with the invention is illustrated in FIG. 5. A longline ( 500) may be deployed from a boat ( 561) to fish for a target fish of interest. The main line ( 550) of the longline may be attached to a buoy ( 520) and at a set distance from the buoy may be attached to an anchor ( 562). A set of gangions ( 530) with hooks ( 540) may be attached to the mainline beginning at the anchor ( 562) and may be spaced sufficiently to limit interaction between individual gangion lines ( 530). Each hook may have a magnet ( 510) mounted resting on the hook eye ( 541). Alternatively, the magnet may be mounted on a hook shank ( 542) or may be secured to the outside of the hook ( 540). The hooks may be baited. The longline may be a demersal longline such that the main line is proximal to the ocean or otherwise water's floor. The longline may be a pelagic long line, such that the main line is nearer to the surface of the water, suspending in the water column, typically at about 100 to about 500 feet below the surface. In the aspect of the invention where the longline is a pelagic longline, anchors ( 562) may have less weight or may be absent from the longline apparatus. The longline may also be a semipelagic longline wherein the mainline is further down the water column from the surface as compared to a pelagic line but is not proximal to the water's floor or is not proximal to the water's floor on at least one end of the longline. Use of magnets with longlines reduces by-catch of elasmobranchs.
[0078] Longlines comprising magnets may be handled in the commercial environment in a manner similar to those practices known in the art of longline commercial fishing. Because hooks must be carefully managed to control tangling and hooking of objects on a longlining boat, including other portions of the tackle of the longline, commercial fishing operations and those of skill in the art will recognize how to handle longlines with hooks. High-pull-force magnets on longlines likewise may be handled in the same manners as one would consider appropriate in the art to avoid entanglements of magnets or magnets sticking together. The long distances between gangions (often more than 100 feet) allow for commercial fishing operators to provide sufficient distance between magnets to avoid the magnets sticking together during fishing or during handling of tackle. Further, high-pull-force magnets used for longlines are of sufficiently small size and magnetic force that the magnets may be separated from one another by hand if they do become stuck together.
[0079] As described above, high-pull-force magnets of any size may be used in combination with a longline hook so long as the target fish may be caught on the hook. An exemplary high-pull-force magnet on a longline hook may be 2''*0.25''*2''. Smaller high-pull-force magnets are also acceptable. High-pull-force magnets of less than 0.5'' cubed may be appropriate for smaller hook settings. Smaller high-pull-force magnets having sufficiently powerful magnetic fields such as N48 grade NdFeB are more preferred.
[0080] D. High-Pull-Force Magnet Repellents on Buoys, Nets and Barges
[0081] An exemplary and non-limiting method of repelling elasmobranchs with a high-pull-force magnet or a plurality of high-pull-force magnets placed on a buoy or barge or net is illustrated in FIGS. 6A-B. Buoys with high-pull-force magnets as their weighted bases are shown as element 660 and 661 in FIG. 6A. The floating portion of the buoy ( 620) allows the buoy to float while the high-pull-force magnet portion of the buoy ( 610) remains in the water because of its weight. A series of buoys comprising high-pull-force magnets may be placed in a region to repel elasmobranchs or may be placed around a swimming area or rescue area to repel elasmobranchs. A series of buoys with high-pull-force magnets may be accompanied by a series of high-pull-force magnets submerged ( 611) in an area of interest, such as a swimming area. As illustrated in FIG. 6B, very large high-pull-force magnets may be placed on a large floating barge ( 670) comprising a high-pull-force magnet ( 610).
[0082] An exemplary and non-limiting device for repelling elasmobranchs with a plurality of magnets is illustrated in FIG. 6A as element 600, an elasmobranch repelling net apparatus. Buoys ( 660 and 661) may be employed to float a net ( 650) comprising a series of magnets ( 640) held within the net and magnetic rings ( 630) holding the ropes of the net together. The net may be strung to the bottom of the water column using weighted magnets ( 611). The net may be anchored to a specific location to provide a physical barrier. The net may provide a curtain of magnetic field to repel elasmobranchs from an area or to keep elasmobranchs from entering an area of interest, such as a swimming or working area. A net ( 650) comprising magnets such as those illustrated as elements 610, 611, 630 and 640 may also be used to trawl for fish, shrimp or other aquatic species. In another non-limiting aspect of the invention, high-pull-force magnets may be placed in aquaculture cages to repel sharks from predation or scavenging of cultured stock. High-pull-force magnets are useful to prevent damage by elasmobranchs to aquaculture cages, nets or other equipment.
[0083] E. Surfboard Fitted with High-Pull-Force Magnet
[0084] A non-limiting repelling device in accordance with the invention may comprise a surfboard comprising a high-pull-force magnetic device. FIG. 7A illustrates exemplary surfboards in accordance with an aspect of the invention. A surfboard ( 720) may comprise a high-pull-force magnetic device such as a permanent high-pull-force magnet ( 710) imbedded, affixed, attached or otherwise associated in any manner contemplated by one of skill in the art with the surfboard. A permanent high-pull-force magnet may be pressed into a space drilled into the surfboard ( 730). It may also be affixed with glue, waterproof tape, Velcro or any other mechanism known in the art now and hereafter.
[0085] In an alternative non-limiting example in FIG. 7B, a surfboard ( 750) may comprise a high-pull-force magnet or plurality of high-pull-force magnets in association with one another wherein the high-pull-force magnet or magnets are capable of spinning when placed in water ( 740). Such a spinning high-pull-force magnet ( 740) may comprise individual magnets attached to a hub ( 770) that is attached to an axle ( 760) to allow free spinning of the high-pull-force magnet or magnets attached to the surfboard ( 750) when water current is present.
[0086] A high-pull-force magnet may be enclosed in the body of a surfboard or other watercraft or may be trailed behind a surfboard, other watercraft or swimmer.
[0087] F. High-Pull-Force Magnet Repellents on Swimming and Diving Clothing and Accessories
[0088] One exemplary non-limiting aspect of the present invention comprises a magnetic material for producing a magnetic field near a swimmer or diver or other person or object in an elasmobranch environment.
[0089] High-pull-force magnets, such as, for example, high-pull-force NdFeB magnets or other high-pull-force permanent magnets may be worn as a bracelet or a band or otherwise placed in proximity of a person or object. An increase in the number of high-pull-force magnets and an increase in the grade of high-pull-force magnets that may be worn increases the magnetic field around the wearer and increases the repelling activity of the bracelet, band or other magnet article. Research on captive nurse sharks suggests that such a bracelet is effective in repelling sharks. Using a vinyl-walled tank, high-pull-force magnets were waved outside the tank wall near a resting nurse shark inside the tank. The shark had no olfactory, motion, sound, or visual clues. In seven separate observations, the nurse shark always rapidly fled from its resting site once the high-pull-force magnet was waved on the tank wall near the subject.
[0090] In a non-limiting example, an omnidirectional permanent magnetic field may be affixed or arranged near a subject or object exposed to an elasmobranch environment. The permanent magnetic field may be generated from, for example, a permanent magnet or an electromagnet. A permanent magnet may be affixed, for example, to any portion of a swimmer's or diver's body such as the head, the leg, the arm, the torso, the ankle, the wrist, or any other portions of the body.
[0091] FIGS. 8A-C illustrate non-limiting examples of permanent high-pull-force magnets ( 810) attached to a belt ( 801) ( FIG. 8A) or bracelet ( 802) ( FIG. 8B) or flippers ( 803) ( FIG. 8C).
[0092] FIG. 9 illustrates a variety of non-limiting alternative designs for bracelets, belts or rings constructed solely from high-pull-force magnets. A plurality of bar magnets ( 981) ( 982) ( 983), larger spherical magnets of varying sizes ( 984) or smaller spherical magnets ( 985) may be shaped into a bracelet or belt. A plurality of discs ( 986) may be shaped into a bracelet or a belt or any shape that keeps the magnets in proximity to the body. Two concave bar magnets ( 987) may be placed on the ankle or wrist opposite each other such that they are held in place on the ankle or wrist by attractive magnetic forces.
[0093] The bracelets in FIG. 9 may be flexible and may be modulated to fit a portion of the body. Individual magnets of the bracelet may be easily separated and placed on the ankle or wrist.
[0094] The disks ( 986) may be magnetized on their edges and not magnetized on their faces. As such, the disks may be assembled as a ring using magnetic connections on their edges. The disks may be manipulated and may be returned to a circle. As such, they may conform to a ring to attach to any type of clothing, equipment or body part to which a ring may be attached.
[0095] High-pull-force magnets may likewise be attached to clothing or water accessories such as swim trunks, wet suits, headbands, flippers, goggles or other piece of clothing or accessory. High-pull-force magnets may be sewn into such clothing or may be affixed with tape, glue, Velcro or any other mechanism for affixing to clothing or accessories for swimming, diving or otherwise working or playing in water.
[0096] Many human-shark interactions in shallow water, especially around the State of Florida in the United States, are hypothesized to be "mistaken identity" by the shark in water with poor visibility. The blacktip shark ( C. limbatus) and nurse shark ( G. cirratum) are often implicated in these encounters. The sharks do not have an olfactory clue in most of these "mistaken identity" cases. A series of high-pull-force magnets, such as NdFeB high-pull-force magnets or other strong permanent high-pull-force magnets, may be used as means to repel the shark as it approaches within a few inches of the magnets. With a strong high-pull-force magnet, such as NdFeB, or an increased number of high-pull-force magnets, to increase magnetic field strength, repellent activity increases and the chance that a shark will be repelled prior to an investigatory bump or bite is greatly increased.
[0097] The invention is further described with the following non-limiting examples, which are provided to further illuminate aspects of the invention.
III. EXAMPLES
Example 1
Pull Force of High-Pull-Force Magnets
[0098] Some of the high-pull-force magnets that have been used in examples in this application are listed below in Table 1 with calculation of the pull force of the respective high-pull-force magnets based on the geometry, size, grade and nominal strength (conservative BR) of the high-pull-force magnet.
[0000]
TABLE 1
Conservative Pull Force
Geometry Size Grade Br (Gauss) (pounds)
Puck 4'' * 1.5'' N38 13000 521
magnet
Bar 6'' * 2'' * 0.5'' N48 13800 191.31
Hollow 1'' * 1'' with 3/16'' N42 13200 72.75
cylinder hollow center
2 stacked 0.472'' * 1.97'' * N50 14100 46.7
hollow 0.24'' hollow
cylinders center
Cube 1'' * 1'' * 1'' N48 13800 110.5
longlines
[0099] Pull force is descriptive of the attractiveness of a magnet to a steel flat surface. A shark is not a magnetic steel surface, but it does have a surface (likely the ampullae of Lorenzini) that interacts with the magnetic field of the magnet. As such, pull force is an appropriate method for measuring interaction of an elasmobranch with a magnetic field.
Example 2
High-Pull-Force Magnets as Repellents on Longlines
[0100] The following example demonstrates the elasmobranch repellent activity of high-pull-force magnets of greater than about 150 pounds of pull force on long lines. High-pull-force magnet treatments were evaluated on one demersal longline located in the middle of a large lagoon. Adjacent longlines in the same lagoon produced large shark catch (generally greater than two sharks over the 15 hooks on a line).
[0101] Seven hooks on a demersal longline of about 1000 feet were treated with 2''*0.25''*2'' NdFeB N48 magnets (nominal force 14,000 gauss; pull force about 161 pounds). The high-pull-force magnets were secured at even-numbered hooks on the longline, directly above the eye of the hook and strapped to the gangion leader with black vinyl electrical tape. All hooks received bait. If the bait was lost during the experiment, the hook was re-baited while the high-pull-force magnets were not removed or replaced; only the bait was exchanged.
[0102] A large nurse shark of about 250 cm was captured on a control hook (hook with no magnet affixed) after a second re-bait. From earlier longline trials at this spot, a much higher nurse catch was expected on this line, especially since the high-pull-force magnets acted as weights and held the baits closer to the sea floor. However, only one nurse shark was caught. As such, it is believed sharks were repelled from the entire longline by the series of high-pull-force magnets affixed thereto.
[0000]
TABLE 2
1 <st >Set 2nd Re-
Hook Treatment Bait Bait bait Bait Species Caught
1 None Barracuda Barracuda Tuna
2 Magnet Barracuda Barracuda Barracuda
3 None Barracuda Barracuda Barracuda
4 Magnet Barracuda Barracuda Tuna
5 None Barracuda Barracuda Tuna
6 Magnet Barracuda Barracuda Tuna
7 None Barracuda Barracuda Tuna
8 Magnet Barracuda Barracuda Tuna
9 None Barracuda Barracuda Tuna
10 Magnet Barracuda Barracuda Tuna
11 None Barracuda Barracuda Tuna
12 Magnet Barracuda Barracuda Tuna
13 None Barracuda Barracuda Tuna Nurse, 250 cm
14 Magnet Barracuda Barracuda Barracuda
15 None Barracuda Barracuda Tuna
Example 3
High-Pull-Force Magnets as Repellents on Longlines
[0103] The following example demonstrates the elasmobranch repellent activity of high-pull-force magnets of greater than 50 pounds of pull force on long lines. A first demersal longline with eight hook sets was baited with barracuda flesh and placed in open water. No high-pull-force magnets were placed on the hooks. Five sharks were captured on the longline over 24 hours representing 5 separate shark species ranging in size from 97 cm to 240 cm. See Table 3.
[0000]
TABLE 3
Hook Species
1 1. Tiger (F), 235 cm
2. Nurse (F) 231 cm
3. Sharpnose (F), 97 cm
2
3
4 Nurse 240 cm
5
6
7
8
9
10
11
12
13
14 Blacknose 115 cm
15
[0104] A second demersal longline with fifteen hook sets was baited with squid and placed in the same position in open water as the first demersal longline discussed above for 67 hours. The trial with the second demersal longline was run three months after the trial with the first demersal longline. Seven of the fifteen hooks were treated with 1''*1*''*1" neodymium-iron-boride grade N48 cube magnets (pull force of about 110 pounds; nominal force around 14,000 gauss) with the high-pull-force magnet secured to the outside of the hook shank using the magnetic force of the hook and black vinyl electric tape. All hooks received bait. During re-baits, the high-pull-force magnets were not removed or replaced; only the bait was exchanged.
[0105] Two small sharks were caught on the second demersal longline. A blacknose shark of 110 cm was caught on a control line with no magnets. A sharpnose shark of 80 cm was caught on high-pull-force magnet line. The large decrease in shark catch between the first demersal longline trial (five relatively large sharks for their species) and the second demersal longline trial (two relatively small sharks) was ascribed to the presence of magnets along the longline. See Table 4.
[0000]
TABLE 4
Hook # Trtmt Bait Species Caught
1 control squid
2 magnet squid
3 control squid
4 magnet squid
5 control squid
6 magnet squid sharpnose 80 cm
7 control squid blacknose 110 cm
8 magnet squid
9 control squid
10 magnet squid
11 control squid
12 magnet squid
13 control squid
14 magnet squid
15 control squid
[0106] A third demersal longline was set with 15 hooks in the same position as the first and second demersal longlines discussed above. The third demersal longline was set within a day of the second demersal longline. Seven of the eight hooks were fixed with magnets at the same position. Magnets were small NdFeB grade N50 hollow cylinders (12 mm outer diameter*6.1 mm inner diameter). Two magnets were placed on each hook creating a total magnet length of 50 mm. Together the magnets have a pull force of about 47 pounds and a nominal force of 14,100 gauss. The demersal line was placed in the same open water position as both demersal lines in Example 3. Within a 24-hour period, 3 large (>200 cm) tiger sharks were captured, 2 on magnet treatments. The smaller (less powerful) magnets did not repel tiger sharks.
[0107] Since a larger number of sharks (and of larger size) were caught on the first and third longlines, the three trials presented in this example demonstrate that sharks were repelled from the second longline comprising magnets of sufficient magnetic strength to repel sharks. Together, the three longline trials contained in this example demonstrate repelling of sharks by magnets of sufficient magnetic strength to repel sharks across a longline.
Example 4
High-Pull-Force Magnet Terminates Tonic Immobility at Greater than 30 cm Distance
[0108] Preliminary research conducted on the effects of specific magnetic fields on shark behavior suggests that weak magnetic fields (0.3-0.5 Gauss) produced by electromagnets had no significant repelling effect on juvenile nurse sharks, Ginglymostomata cirratum, and juvenile lemon sharks ( Negaprion brevirostris) under tonic immobility, however, very strong magnetic fields (i.e. about 14,000 Gauss or 1.4 Tesla) produced by large (4''diameter*1.5'' height) "rare earth" magnets (neodymium-iron-boride; NdFeB) (13000 gauss, pull force of 521 pounds) had a significant repelling effect on both shark species at distances of 0.3 m or less. Additional experiments on captive sharks in an offshore, sandy bottom, fenced-in enclosure were done with NdFeB high-pull-force magnets buried under the sand. Exposure of the sharks to the buried magnets resulted in "violent reorientation" as the captive sharks came into proximity of the buried high-pull-force magnets.
Example 5
Y-Maze Preference Bioassays
[0109] A Y-maze was constructed to establish a preference test to determine the repellent activity of magnets on elasmobranchs. The maze was constructed of three sections of clear acrylic 8 inch diameter tubing, connected at 33[deg.] angles to form a Y-shape. Slotted guides were secured to the entrances of each tube, to allow the insertion of a moveable door, which obstructs one exit. The entire maze was submerged in a test tank. Sharks were allowed to freely enter the maze and exit the maze. A high-pull-force magnet was placed, south pole facing the maze junction, in an obstructed leg of the maze, preventing an exit from the maze in that direction if that obstructed leg is chosen. The diameter of the tubing was sufficient to allow juvenile nurse sharks, juvenile lemon sharks, and juvenile wobbegong sharks to enter and pass through, but it was small enough to prevent the specimen from turning around within the tube.
[0110] For each trial, uncooked shrimp were used as a reward, and the south pole of a 4'' diameter NdFeB nickel-coated cylindrical high-pull-force magnet (pull force 521 pounds; nominal force about 13000 gauss) was placed in the obstructed leg. One shrimp was positioned midway into the entrance tube to entice the shark to enter the maze. Two shrimp were placed midway into the exit tube, and two shrimp were placed midway into the tube containing the magnet. When the shark entered the maze and reached the Y-junction, the shark was presented with approximately the equal odor gradient from the shrimp in the exit tube and the tube containing the magnet. If the shark chose the maze without the high-pull-force magnet, it was rewarded with two additional shrimp as it exited. If the shark chose the maze with the high-pull-force magnet, it was subjected to an exponentially-increasing magnetic field as it moved down the tube. The shark could only physically back out of the high-pull-force magnet tube and into the junction. Sharks that moved into the magnet and attempted to back out were visible traumatized. Feeding observations regarding the two shrimp in the high-pull-force magnet tube were made.
[0111] Each trial was scored as follows:
+1 Subject enters the maze
+1 Subject exits the maze
+1 Subject takes the first reward shrimp just after entry (teaser)
+2 The unobstructed path is chosen at the junction
+3 At least one reward shrimp in the unobstructed path is taken
-2 The obstructed path is chosen (magnet) at the junction
-3 The specimen enters more than 6'' into the obstructed path and is visibly struggling.
[0119] A perfect score=7 for each trial. If a shark became traumatized and requires removal from the maze for its own safety, a score is calculated up to the point of the rescue. A rescue is made whenever a subject appears to be highly distressed, and a physical injury is likely.
[0120] For example, a nurse shark entered the maze, took its first reward shrimp, and immediately chose the unobstructed path. As it exited, it took its two reward shrimp, and exited the maze without a change in behavior.
[0000]
Score=1+1+2+3=7
[0121] In another example, a nurse shark entered the maze and took its first reward shrimp. It chose the obstructed path but was repelled by the magnet. The shark backed up into the Y-junction; reoriented itself; and exited the unobstructed path without taking the two shrimp available in the unobstructed path.
[0000]
Score=1+1-2+1=1
[0122] In yet another example, a lemon shark entered the maze and took its first reward shrimp. It chose the obstructed path, and then continued down the magnet to within 6'' of the magnet. It became extremely distressed and a rescue was made.
[0000]
Score=1+1-2-3=-3
[0123] In an investigation, three nurse sharks were subjected to the maze. Shark 1 was subjected to the maze five times. Shark 2 was subjected to the maze 5 times but only entered the maze 4 times. Shark 3 was subjected to the maze once but required rescue when it encountered the magnet and subsequently died, apparently from stress related to exposure to the magnet. The magnet in the obstructed maze was a 4''*1.5'' cylindrical NdFeB magnet of grade N48 (13000 gauss, 521 pounds pull force). The results are contained in Table 5.
[0000]
TABLE 5
Obstruction Nurse 2
Exit Nurse 1 (Lg.) (Med.) Nurse 3
Trial 1 L 1 1 -4
Trial 2* L 5 1 (rescue
Trial 3 R 4 3 performed)
Trial 4 L 4 4 Shark would
Trial 5 R 5 Did not enter not
re-enter maze
in
subsequent
trial
[0124] The data suggest that Nurse 1 has learned to navigate the maze, retrieve a reward, and exit without distress. Nurse 2 appears to be learning, but did not re-enter on the fifth trial. Nurse 3 had to be rescued. It was notably distressed by the magnet. Unfortunately, Nurse 3 did not eat after this experience, and subsequently died at about 30 days after the experiment. We did not observe any external injuries on Nurse 3. We attribute this to stress and possibly shock from encounter with the high-pull-force magnet in the maze.
Example 6
N48 Neodymium-Iron-Boride (NdFeB) Nickel-Coated Permanent Magnet Terminate Tonic Immobility
[0125] Juvenile lemon sharks ( Negaprion brevirostris) and juvenile nurse sharks ( Ginglymostoma cirratum) that had been placed in tonic immobility were subjected to the magnetic field of an N48 neodymium-iron-boride (NdFeB) nickel-coated 4''*1.5'' cylinder permanent high-pull-force magnet and were observed. The high-pull-force magnet had the following characteristics:
[0126] Calculated Pull Force 521 pounds
[0127] Residual Induction: 14 KGs
[0128] Coercive Force: 11.0 KOe
[0129] Intrinsic Coercive Force: >=12.0 KOe
[0130] Maximum Energy Produce: 48 MGOe
[0131] Curie Temperature: 320[deg.] C.-330[deg.] C.
[0132] Vickers Hardness: 500-600
[0133] Working Temperature: <-80[deg.] C.
[0134] Temperature Coefficient -0.11% per [deg.] C.
[0135] A DC milligauss magnetometer (Alpha Labs, Inc.) was used to record magnetic field strength during the study. The magnetometer sensor was secured to the top of a nonmagnetic [1/2]'' polyvinyl chloride stake, which was driven vertically into the sand at the test site. The magnetometer sensor was submerged for the study. Water depth did not exceed 36'' at the test site. A meter-long rule was secured to the magnetometer sensor.
[0136] A control test was preformed in order to determine if the activated magnetometer sensor would terminate tonic immobility. The magnetometer was set to zero to compensate for the background magnetic field of the earth, which allowed fluctuations from the permanent magnet to be measured. A juvenile female lemon shark in tonic immobility was held directly at the magnetometer sensor. Tonic immobility did not terminate. The magnetometer readings did not fluctuate when the lemon shark was in proximity to the sensor demonstrating no change in magnetic field strength.
[0137] Two 4'' cylindrical N48 grade NdFeB nickel-coated permanent high-pull-force magnets (nominal strength 14000 gauss, pull force about 521 pounds) were calibrated by observing the magnetic field strength versus distance from the magnet under water. The following data were recorded:
[0000]
TABLE 6
Distance milliGauss (mG)
1.5 m +191
1.0 m +524
0.9 m +700
0.8 m +920
0.7 m +1310
0.6 m >+2000
Because the maximum reading of the magnetometer used in the experiments was 2000 mG, magnetic fields at distances less than 0.6 m from the magnet were calculated using a standard gauss calculation for a cylindrical magnet. In this case, we used the calculator provided at www.arnoldmagnetics.com/mtc/calc_gauss_cyl.htm. The following parameters were in-put into the magnetic field calculator: L=4 in.; D=1.5 in; Br=13,000 G; Z=distance from magnet.
[0138] With a juvenile shark subject to tonic immobility at the magnetometer sensor, the permanent magnet was moved along a stationary rule, level with the shark and the sensor, towards the shark. The high-pull-force magnet was not moved faster than 0.1 m/s toward the shark. The following results were obtained for termination of tonic immobility. (Note: +denotes the north pole, electrically on the gaussmeter.)
[0000]
TABLE 7
Magnetic Distance (m)
Pole to terminate
Facing tonic
Specimen Shark immobility Calculated mG
Juvenile lemon shark, + 0.1 246971
Juvenile lemon shark, - flipped + 0.0 3130415
Juvenile nurse shark, - 0.3 14477
Juvenile nurse shark, + 0.3 14477
Juvenile nurse shark, + 0.2 44154
Juvenile nurse shark, - 0.2 44154
Juvenile nurse shark, + 0.2 44154
Juvenile nurse shark, + 0.2 44154
[0139] Since the movement of the permanent high-pull-force magnet underwater induces an electrical current, the next study moved the tonic shark toward two stationary high-pull-force magnets, each fixed at 1.5m from the sensor. Tonic immobility was terminated when the sharks were brought within 0.2m of the high-pull-force magnet faces.
[0140] It was consistently observed that tonic sharks awoke by turning away from the magnet's face. This was independent of the pole of the high-pull-force magnet, and the orientation of the shark's head toward the magnet. More violent responses occurred when the shark's head was oriented 90 degrees to the high-pull-force magnet face, rather than 0 degrees (nose-to-magnet face).
[0141] The movement of the shark toward the high-pull-force magnet, as well as the movement of the high-pull-force magnet toward the shark might create electric current and awaken the shark. To eliminate this possibility, care was taken not to move the high-pull-force magnets in a rapid manner.
Example 7
Electromagnetic Device with Lower Magnetic Strength Did Not Terminate Tonic Immobility
[0142] In a first experiment using an electromagnetic device, an iron-core electromagnet was secured to the end of a PVC pole, and energized with 12 VDC using a marine wet-cell battery. Current was monitored using a digital multimeter. A tonic juvenile female lemon shark was held at the magnetometersensor, while the tip of the electromagnet was moved. The following results were obtained:
[0000]
TABLE 8
Distance between AMPS to
shark and electromagnet @ Measured
electromagnet 12VDC mG Shark's Response
1.0 m 6.27 A -10 Did not awaken
0.5 m 6.28 A -139 Did not awaken
0.0 m 6.24 A -1700 Did not awaken
0.0 m 6.16 A (reversed >2000 Did not awaken polarity)
[0143] In a second experiment using an electromagnetic device, a commercial 1000 lb.-strength waterproof electromagnet, produced by L OCKNETICS, I NC., was energized with 12V DC using a marine wet-cell battery. According to the product specifications, this magnet draws a consistent 30A at 12 VDC, which exceeded the capability of the digital multimeter. A tonic juvenile female lemon shark was held at the magnetometersensor, while the face of the electromagnet was moved. The following results were obtained:
[0000]
TABLE 9
Distance between AMPS to
lemon shark and electromagnet @ Measured Lemon shark's
electromagnet 12VDC mG Response
1.5 m 30 A -20 Did not awaken
1.0 m 30 A -40 Did not awaken
0.5 m 30 A -280 Did not awaken
0.5 m 30 A, but flickered -280 Did not awake
powered randomly
instead of a constant
supply
0.0 m 30 A >2000 Did not awaken
0.0 m 30 A reversed polarity >2000 Did not awaken
randomly
[0144] These two experiments demonstrate that despite strong electromagnetic fields in close proximity, such fields were not sufficient to terminate tonic immobility in juvenile nurse sharks and lemon sharks. The magnetic field strength was not sufficient to terminate tonic immobility.
[0145] However, as seen above, a powerful field from an NdFeB permanent high-pull-force magnet is sufficient to terminate tonic immobility in juvenile nurse sharks and lemon sharks. It is believed that a field strength of approximately 50 G at least 0.1 m distance from am elasmobranch reliably terminates tonic immobility. 50 gauss is about 100 times the Earth's magnetic field.
Example 8
Bracelet, Belt or Other High-Pull-Force Magnet as Repellent of Shark
[0146] Two lemon sharks in an outdoor pen were placed in tonic immobility. A blinder was placed between the sharks and a magnet having about 191 pounds of pull force and a nominal strength of about 14000 gauss. Upon introducing the magnetic bar up to about 0.2 meters behind the blind, tonic immobility was terminated and the sharks violently moved in orientation away from the high-pull-force magnet.
Example 9
Bracelet as Repellent of Shark
[0147] Research on captive nurse sharks suggests that a high-pull-force bracelet is effective in repelling sharks. Using a vinyl-walled tank, high-pull-force magnets were waved outside the tank wall near a resting nurse shark inside the tank. The shark had no olfactory, motion, sound, or visual clues. In seven separate observations, the nurse shark always rapidly fled from its resting site once the high-pull-force magnet was waved on the tank wall near the subject. When non-magnetic objects were waived at the same position outside the tank, no change in behavior was observed.
Example 10
Target Fish not Repelled by High-Pull-Force Magnets
[0148] Preliminary research conducted on the effects permanent magnetic fields on adult cobia, Rachycentron canadum, suggests that very strong magnetic fields (i.e. >14,000 Gauss or 14 Tesla) produced by "rare earth" magnets (NdFeB) (13,800 gauss, 110 pounds pull force) had little effect on cobia during feeding. Digital video of cobia feeding within 5cm of the "rare earth" high-pull-force magnet was recorded. In three trials sardines were offered to the cobia on PVC tubes with no magnets inside. In three subsequent trials sardines were offered on PVC tubes with a high-pull-force magnet inside. The high-pull-force magnet was composed of 4 discs (1 '' diameter*[1/4]'' height) stacked on top of each other with Teflon(TM) rings between each magnet.
[0149] In another control test, squid was presented to yellowfin tuna in the presence of an NdFeB high-pull-force magnet of grade N48. A horizontal pole with six squid (and a corresponding high-pull-force magnet) hung equally spaced along the pole was presented to the tuna. The pole was lowered into the tank. The tuna took the bait in the presence of the high-pull-force magnets. The tuna were not repelled.
[0150] The ability to selectively repel elasmobranch is useful both for longline fishing applications (to catch target fish and avoid killing elasmobranch) and for human applications, particularly for divers and snorkelers (to repel elasmobranchs and not repel fish).
Elasmobranch-repelling magneto-electropositive fishing hook
US2012085018
A fishing hook with elasmobranch-repelling qualities is disclosed. The fishing hook, comprised of a ferromagnetic material, is rendered repellent to elasmobranchs through the incorporation of an exterior coating of an electropositive metal, and contact or impulse magnetization.
BACKGROUND OF THE INVENTION
[0002] Pelagic longlining fishing is an open-ocean technique that employs a long mainline from which individual hooks are suspended at various depths depending on the target species. The hooks are attached to the main line by monofilament branch lines called gangions or "snoods". Floats are attached to the mainline at regular intervals to keep it elevated horizontally in the water column. A variety of bait types are employed, including whole small fish, Atlantic mackerel and squid, to name a few. Luminescent light sticks are often fastened to the gangions near the baited hooks, making them more attractive to the targeted species and also attracting smaller species on which targeted species feed. The longlines used by the United States domestic pelagic longline fleet range from 20 to 40 miles in length. The depth at which the hooks are set is controlled by the length of the lines attaching the main line to the floats, by the length of the gangions, and by the speed at which the longline gear is set. After a variable "soak time," the gear is retrieved, and the catch is brought on board for cleaning and icing down in the hold. This "one at a time" processing and handling gives longline products a high quality distinction in the marketplace.
[0003] Pelagic shark species such as the blue shark (Prionace glauca) are often attracted to miles of attractive stimuli resulting from the longlines. Shark interactions on pelagic longlines result in substantial inconveniences and adverse economic effects to fishers (Gilman, Clarke, Brothers, Alfaro-Shigueto, Mandelman, Mangel, Petersen, Piovano, Thomson, Dalzell, Donoso, Goren, Werner, 2007). In fisheries with restrictions on shark-finning, a lack of market for shark meat, or a per-trip limit on shark retention, shark interactions cause the following:
Reduced catch of marketable species: When baited hooks are occupied by sharks (referred to as "bycatch") or removed by sharks, there are fewer hooks available to catch marketable target species;
Damage and loss of fishing gear: Sharks bite off terminal tackle (e.g., baited hook, leader, weighted swivel, and line) from branch lines, stretch and chafe branch lines, break the main line, and some shark species will pull the gear down causing branch lines to become entangled often resulting in large quantities of unusable fishing gear;
Risk of injury: It is dangerous for crew to handle caught sharks. There is a risk of being bitten or hit by weights when branch lines containing sharks snap during gear retrieval; and,
Expenditure of time. A majority of fishers consider the time required to remove sharks from gear, retrieve terminal tackle and repair and replace gear as a central concern resulting from shark interactions.
[0008] Responding to this problem, the inventors developed and commercialized two repellent materials which show selective shark repellent abilities for fisheries: Ferromagnets and electropositive metals. Both materials affect the electrosensitive ampullae of Lorenzini organ found only in sharks, as discussed below.
[0009] Elasmobranch fishes (sharks and rays) geolocate using magnetoreception, a method used by a wide variety of marine and terrestrial organisms (Kalmijn, 1973, 1974, 1982, 1984; Phillips, 1986; Carey and Scharold, 1990; Klimley, 1993; Wiltschko and Wiltschko, 1995; Holland et al. 1999). Organisms that employ magnetoreception typically gather information while in motion about geomagnetic parameters such as field intensity and the angle of inclination (Skiles, 1985).
[0010] There are three primary ways in which an animal perceives the Earth's magnetic field: (1) magnetite-based magnetoreception (Kirschvink et al., 2001; Wiltschko et al., 2002) (2) chemical magnetoreception (Ritz et al., 2000), and (3) indirect magnetoreception via electromagnetic induction (Kalmijn, 1982, 1984; Johnsen and Lohmann, 2005). Previous studies hypothesized that elasmobranchs perceive the Earth's geomagnetic fields through indirect magnetoreception via electromagnetic induction, and they use this locational information to navigate within coastal and pelagic environments (Kalmijn 1973, 1974, 1982, 1984; Carey and Scharold, 1990; Klimley 1993; Holland et al. 1999).
[0011] To understand how the process of electromagnetic induction aids elasmobranchs in navigation, it is essential to understand the law of electromagnetic induction proposed by Faraday. The law states that the electromotive force induced in a circuit is directly proportional to the time rate of change of magnetic flux through the circuit. An application of this law employs the classic example of a simple generator (i.e. a coil conductor and a permanent magnet) to demonstrate how the movement of the permanent magnet induces a measurable electromotive force. As a magnetic dipole approaches the coil, the magnetic field exerts an electromotive force on the electrons within the coil, producing an electrical current. For example, on a molecular level, a permanent ferromagnetic material such as Barium-ferrite contains a greater-than-average number of magnetic domains oriented in the same direction, and within each domain, unpaired electrons have their spin aligned in the same direction. The resulting magnetic flux from the permanent magnet induces the movement of electrons in the coil/conductor creating measurable voltages and current.
[0012] A similar phenomenon occurs when an animal swims through a magnetic (or geomagnetic) field. Electromagnetic induction occurs as an animal swims through the geomagnetic field emanating from the center of the earth, which ranges from 0.25-0.65 gauss. The geomagnetic flux causes the free electrons found within an organism's body (similar to a conductive coil) to move, creating an induced voltage and current within the shark.
[0013] Hypothetically, elasmobranchs can perceive the induced voltages, using their acute electrosensory organ known as the ampullae of Lorenzini (Kalmijn, 1966, 1971, 1974, 1984). The electric potential created by the geomagnetic field is different than that of the electric potential found within the conductive gel of the ampullae. The difference in electric potentials initiates the transmission of a signal sent via the afferent neurons to the central nervous system of the elasmobranch. Multiple ampullae distributed across the cephalic (nose) region of the elasmobranch are able to detect the minute differences in the Earth's geomagnetic field enabling the organism to determine its relative geolocation. Studies of the swimming behavior of blue sharks (Prionace glauca; Scharold, 1990) and scalloped hammerheads (Sphyrna lewini, Klimley, 1993) concluded that their directional movement within the referenceless pelagic environment must involve some compass-like mechanism, although the physiological basis for such a mechanism was not described at that time. Meyer et al. (2005) exposed scalloped hammerheads (Sphyrna lewini) and sandbar sharks (Carcharhinus plumbeus) to weak electromagnetic fields (maximum field strength 100 [mu]T), which altered their feeding behavior. This study supported the hypothesis that the ampullae of Lorenzini, a network of gel-filled canals on the head of elasmobranchs which detects electric fields in the final stages of prey capture (Kalmijn, 1971; Kajiura and Holland, 2002, Kajiura, 2003) are also capable of detecting magnetic fields relatively close to that of the Earth's geomagnetic field. The ampullae are essentially low frequency voltmeters, allowing elasmobranchs to detect low frequency electric stimuli, i.e. less than 5 nV/cm in uniform fields and as low as 1 nV/cm in dipole fields Kalmijn 1966, 1971, 1974, 1982; Kajiura 2003; Peters 2007).
[0014] O'Connell (2007, 2008, 2009) found that for nurse sharks (Ginglymostoma cirratum) and southern stingrays (Dasyatis americana), the behavior towards a permanent magnet apparatus was dependant on the treatment type. In the presence of permanent magnets, D. americana and G. cirratum demonstrated a significantly greater number of avoidance behaviors towards the magnet side of the apparatus, while both species fed a significantly greater number of times from the procedural (nonmagnetic) control side. These results suggest that the species tested in this experiment were sensitive to these magnets and were successfully repelled from baited areas containing magnets.
[0015] On May 1, 2006, SharkDefense discovered that highly electropositive metals (EPMs)-metallic elements towards the left side of the periodic table-particularly early-Lanthanide or "rare earth" metals, induced deterrent behavior in juvenile lemon (Negaprion brevirostris) and nurse (Ginglymostoma cirratum) sharks. Subsequent to this discovery, SharkDefense applied for patents in the United States and Canada, which are currently pending. Not all seawater-corrodible metals, such a copper and zinc, are suitable as shark repellent EPMs. Shark repellency is a function of the standard reduction potential available from the metal in basic seawater electrolyte, relative to a shark's skin. The standard cell potential, E0, between the metal and shark skin must be 0.8 eV or greater. If a shark skin reference electrode is not available, a carbon electrode may be substituted. An electromotive force in a standard seawater (pH=8.1) electrolyte with a carbon-metal electrode spacing of at least 0.01 m should yield at least 0.5 eV, indicating satisfactory shark repellent. A standard cell potential is calculated from the half-cell reactions for the metal and the electrolyte. For example, the standard reduction potential of zinc metal in basic electrolyte is 1.246 eV. Adding the -0.828 eV reduction for water, the standard cell potential is +0.418 eV. Zinc metal is not an effective shark repellent. By comparison, the standard reduction potential for yttrium metal (a trivalent EPM and confirmed shark repellent), is 2.85 eV, giving a standard cell potential of 2.022 eV (Bard, 1985). This corresponds closely to actual measurements made with yttrium metal and a shark fin clipping electrode in pH=8.1 seawater at 25[deg.] C.
[0016] In response to the discovery several National Oceanographic and Atmospheric Administration (NOAA), academic and private sector researchers conducted various experiments to evaluate the efficacy of employing EPMs as shark deterrent technology during commercial fishing. The Pacific Islands Fisheries Science Center of the National Marine Fisheries Service, Honolulu, Hi. hosted a Shark Deterrent and Incidental Capture Workshop on Apr. 11, 2008 at the New England Aquarium, Boston, Mass. Researchers were invited to present on a variety of topics, including shark sensory biology, an overview of shark bycatch during pelagic longline fishing and an arsenal of shark deterring technologies offered by Shark Defense. The majority of the research presentations focused on the effects of EPMs on shark behavior and presented evidence on their efficacy as a shark bycatch reduction mechanism during commercial fishing. The following outline the major results presented during the workshop:
[0017] Wang, Swimmer, and McNaughton (2008) reported repelling behavior of Galapagos (C. galapagensis) and sandbar (C. plumbeus) sharks when an EPM (Neodymium-Praseodymium mischmetal; NdPr) was placed on the end of a baited bamboo pole in preliminary studies in Hawaiian waters.
[0018] Stoner and Kaimmer (2007) conducted laboratory investigations on the effects of EPMs on spiny dogfish (S. acanthias) and Pacific halibut (Hippoglossus stenolepis). In a pairwise test with EPMs and inert metal controls, they reported that dogfish attacked and consumed baits protected with cerium (Ce) mischmetal at a significantly lower frequency than controls. Number of approaches before attacking the bait and time to attack the baits was significantly higher in the presence of mischmetal, as were numbers of approaches before first attack. No halibut aversion was reported. Encouraged by the results of the laboratory studies, Kaimmer and Stoner (2008) conducted field investigations using EPMs as a deterrent during commercial fishing for halibut near Homer, Ak. They reported a 17% reduction in spiny dogfish bycatch and a 48% reduction in bycatch of the clearnose skate-another elasmobranch with ampullae of Lorenzini electroreception abilities. They reported no noticeable aversion by the halibut and an associated 5% increase in halibut catch. Increases in halibut catch were most likely due to more hooks available to target species. Stoner and Kaimmer also conducted additional cerium mischmetal EPM trials during 2008 at the Oregon Coastal Aquarium (Newport, Oreg.) to observe the behavior of sharks in the presents of EPMs and lead controls suspended in the water column. Analysis of the video suggested that several species of sharks and rays avoided the EPM more than the lead control.
[0019] Brill et al., (2009) conducted EPM trials using small sandbar sharks (C. plumbeus) in a 3.6 m diameter*0.67 m deep pool. The experimental design consisted of an EPM treatment-three small ingots of NdPr mischmetal suspended in a vertical line immediately below the water surface-and a control-three small lead ingots of similar size and shape and similarly suspended in the water column-placed into the tank with the captive sharks. Their swimming patterns were recorded over one hour intervals and were subsequently digitized using Lolitrack automated video analysis software (Loligo Systems, Tjele, Denmark). They suggested that the NdPr mischmetal clearly exhibited potential to repel sharks and hand potential for reduction of shark bycatch during commercial longline fishing.
[0020] Brill (2009) also reported that in field trials with bottom longline gear, electropositive metal placed within 10 cm of the hooks reduced the catch of sandbar sharks by approximately two thirds, compared to the catch of sharks on hooks in proximity to plastic pieces of similar size and shape.
[0021] Although two 2008 studies involving spiny dogfish were inconclusive, the consensus of the workshop participants was that EPMs were a potential practical and promising shark deterrent technology for application in commercial fisheries.
[0022] While ferromagnets and electropositive metals alone have both demonstrated shark repellency, species-specific behavioral variations have been reported by fishermen using these single materials (e.g., some sharks responded only to magnets and not to metals). For example, in 2008 field studies where spiny dogfish (Squalus acanthias) represent a large component of unwanted catch, Pacific spiny dogfish were repelled by electropositive metals (Stoner, Kaimmer, 2008), while Atlantic spiny dogfish were not (Tallack, Mandelman, 2009). Brown smooth hound sharks (Mustelis henlei) in Baja, Mexico were responsive to magnets but not to electropositive metals. (J. Wang, pers. comm.). In a 2008 International Pacific Halibut Commission field study, unwanted catch of Pacific longnose skates was reduced 48% using electropositive metals (Stoner, 2008), while catch rates remained unaffected for Atlantic butterfly rays and southern stingrays (Dasyatis americana) using electropositive metals (Brill, 2009), yet southern stingrays in both the Florida Keys and South Bimini, Bahamas (D. americana) were responsive to permanent magnets (O'Connell, 2007, 2008, 2009). Current magnetic materials that combine electropositive metals and ferromagnetic metals, such as neodymium-iron-boride (NIB) and samarium-cobalt (SmCo) magnets, are unsuitable for commercial fishery use. NIB magnets are readily corroded by seawater due to the high iron content in its sinter. SmCo magnets offer better corrosion resistance but are brittle and are more expensive compared to ferrite materials.
[0023] The storage and deployment of the aforementioned shark repellent materials add additional challenges for fishermen. These materials must be stored onboard the vessel, and add to the expense when gear is lost due to shark interactions. During deployment, each magnetic or electropositive repellent device must be secured to a gangion, adding labor and time to the fishing effort. Storing hundreds of powerful NIB or SmCo magnets in close proximity onboard of a metal fishing vessel is not practical. These magnetic materials produce fluxes in excess of 1,000 Gauss, readily attracting other nearby magnetic metals. A lower flux magnetic material that maintains shark repellency is required.
[0024] A demersal longline study was conducted by Coastal Carolina University during the summer of 2008 at Winyah Bay, S.C. using magnetized hooks ranging from 40 gauss to 80 gauss (much weaker than powerful rare earth magnets). The results of this study were compared to magnet-on-hook trials at the same location. A significantly lower number of sharks were captured using magnetized hooks than with the magnet-on-hook design ([chi]2=4.50, d.f.=1, p=0.0339). While magnet-on-hook trials significantly reduced the chances of capturing a shark by half ([chi]2=4.545, d.f.=1, p=0.0330), sharks were repelled from ALL hooks in the magnetized hook trials. The researchers recognized a temporal variation existed between longline studies, and therefore conducted tonic immobility trials with five juvenile lemon sharks (Negaprion brevirostris). Using magnetized hooks (54 gauss), all five subjects violently roused and terminated immobility when the magnetized hook was presented.
[0025] In summary, a fishing hook with magnetic flux ranging from 5 to 80 Gauss and an electropositive coating is commercially desirable, as this would reduce attraction to other metals and tackle while maintaining shark repellency and high selectivity towards target catch.
DETAILED DESCRIPTION OF THE INVENTION
[0026] "By-catch" is any kind of fish that is caught in a fishing operation wherein the catching of the fish is not the object of the fishing operation. For example, if the target fish of a longline fishing operation is tuna, an elasmobranch caught on a hook of the longline is by-catch.
[0027] "Elasmobranchs" in this specification means one or more elasmobranchii in the super-orders Galeomorphii and Squalomorphii and orders Squaliforms (dogfish), Carcharhiniformes (requiem sharks), Lamniformes (mackerel sharks), and Orectolobiformes (carpet sharks).
[0028] "Electropositive" in this specification means possessing a revised Pauling electronegativity of less than 1.3. Examples of an electropositive metal suitable for use in the present invention are a Lanthanide (also referred to as Lanthanoid) metal, a Group 1 metal, a Group II metal, a Group III metal, Magnesium metal, or an alloy of electropositive metals.
[0029] "Ferromagnetic" in this specification means capable of retaining a magnetic characteristic after exposure to another magnetic field. Alloys of iron, cobalt, and many steels possess this property. Within ferromagnetic materials, the spin of unpaired electrons are aligned in the same direction. Also, a greater-than-average number of magnetic domains containing these aligned electrons are also aligned in the same direction, creating a net moment. This moment creates the familiar "north" and "south" poles of a permanent magnet or a ferromagnetic material.
[0030] "Gauss" is a measure of magnetic field strength. Gauss is a unit of the density of a magnet's flux (or flux density) measured in centimeter-gram-second. A tesla is equal to 10,000 gauss. Gauss and tesla are common units for referring to the power of a magnet to attract (or repel) other magnets or magnetic materials. The Gauss unit describes both the coercivity of a magnet and its saturation magnetization. Gauss describes how strong the magnetic fields are extending from the magnet and how strong of a magnetic field it would take to de-magnetize the magnet.
[0031] "Grade" of a neodymium-iron-boride magnet specifies the quality of material used to construct the magnet. All else being equal, the higher the quality of materials used to construct the magnet, the greater the magnet's strength. In grading neodymium-iron-boride magnets, a lower grade, e.g., N35 does not have as much magnetic strength as a higher grade, e.g. N45.
[0032] "Hook" in this specification refers to a metal fishing hook for marine use. Fishing hooks are further divided into specialized shapes depending on the type of prey sought, such as circle hooks, J-hooks, and treble hooks. The metals used in the manufacture a fishing hook typically include steel or stainless steel, and optionally include cadium, tin, zinc, gold, or nickel platings.
[0033] "Pull force" is the attractiveness of a magnet to a mild steel flat surface in pounds. The formula for calculating pull force is provided in detail herein.
[0034] "Target fish" is any kind of fish, the catching of which is the object of a fishing operation. For example, the target fish of a longline fishing operation may be tuna. A fish that is caught on the longline that is not tuna would not be a target fish.
[0035] "Tonic immobility" is the state of paralysis that typically occurs when an elasmobranch is subject to inversion of its body along the longitudinal axis of the body, i.e., is belly up. An elasmobranch can remain in this state for up to 15 minutes.
[0036] While not wishing to be bound to a specific physiological mechanism, the inventor hypothesizes that weakly magnetized materials are capable of repelling elasmobranchs more efficiently than high pull force magnets. In recent experimentation with captive juvenile lemon sharks (N. brevirostris) and free-swimming blacktip sharks (C. limbatus) magnetic fluxes of 0.6 gauss to 100 gauss measured at the hook were effective in reducing shark captures when compared to nonmagnetized control hooks. The inventors hypothesize that very high pull force magnets, particularly grade N38 and higher neodymium-iron-boride magnets, may be too strong to achieve consistent repellency with elasmobranchs. For example, rare earth magnets are capable of producing thousands of gauss near their surfaces. This is thousands of times greater than the Earth's geomagnetic signature that is observed around 500 milligauss. The presence of an overly powerful permanent magnetic flux may be so "unnatural" to an elasmobranch's ampullary organ that the organ does not register the effect at all, or nullifies it rather than produce an aversion signal. In contrast, a weakly magnetized steel fishing hook may only produce 100 gauss at its surface, and this is only 200 times stronger than the Earth's geomagnetic signature. This effect was observed using the tonic immobility bioassay with juvenile lemon sharks (N. brevirostris). The sharks terminated tonic immobility more often when weakly magnetized hooks were presented versus powerful rare earth magnets.
[0037] The strength of the magnetic flux decreases with the inverse cube of the distance from the magnetized hooks surface. A shark would experience less than 10 gauss only a few inches from the magnetized hook.
[0038] Cobalt and Iron are examples of ferromagnetic elements at room temperature. Steel, low-austenitic stainless steels, Samarium-Cobalt, Sendust, Neodymium-Iron-Boride, Permalloy, Supermalloy, Alnico, Bismanol, CuNiFe, Heusler alloy, and Fernico are examples of room-temperature ferromagnetic alloys. Some ferromagnetic materials, are strong enough to be used directly as a fishing hook. Steel and 400-series stainless steels are examples of materials suitable for use as the entire fishing hook. Soft alloys, such as Bismanol, do not possess this structural integrity and therefore are more useful as a coating or external treatment on an existing fishing hook.
[0039] A nonmagnetized ferromagnetic hook is made magnetic by exposing the hook to another permanent magnet or an energized electromagnet. Preferably, the nonmagnetized hook is placed in physical contact with a permanent magnet, such as a Barium-ferrite ceramic magnet. A nonmagnetized ferromagnetic hook may also be magnetized by placing it in close proximity to an electrified coil, commonly found on electromagnets. The magnetization process is nearly instantaneous and is reversible by heating above the Curie temperature, repeated mechanical shock, or degaussing equipment.
[0040] Weakly magnetized hooks are also desirable to fishermen for four reasons. First, in many commercial fisheries, sharks comprise a significant portion of by-catch. More by-catch equates to less target fish and potential loss of income and tackle. For this reason, it is very desirable for fishermen to have a shark by-catch reduction device which does not affect the target fish. Permanent magnets fulfill this requirement. Secondly, there is no additional tackle in the form of permanent magnets to store and rig onboard a moving metallic vessel. The present invention saves storage space and reduces vessel weight. Third, since the hook is only weakly magnetized, the tendency for the hooks to entangle and attach to other metal surfaces is greatly reduced. This makes handling magnetized metals on a metal vessel much easier than having a plurality of permanent magnets to contend with.
[0041] Finally, if a ferromagnetic fishing hook, such as a steel circle hook, is used, there is no significant additional expense to the fishermen to magnetize the hook other than their time. This eliminates the expense of purchasing permanent magnets to achieve the same effect.
[0042] The second component of the magnetoelectropositive hook incorporates the use of an electropositive metal on or within the hook material. The pure metal (ground state) form of Praseodymium, Neodymium, Cerium, Samarium, Ytterbium, or Magnesium metal is particularly effective at inducing aversive behavioral responses in juvenile sharks. For reasons not yet fully understood, elasmobranchs, particularly those of the order Carcharhiniformes, exhibit aversive behavior within a 0.2 meter range of these electropositive metals.
[0043] We first observed the unusual repellent effects of Lanthanide metals on sharks when tonically-immobilized juvenile lemon sharks (N. brevirostris) exhibited violent rousing behavior in the presence of a 153 gram 99.95% Samarium metal ingot. As the Samarium metal was moved towards the immobilized shark, the shark terminated tonic immobility, in the direction away from the approaching metal. For experimental controls, pure Chromium, an antiferromagnetic metal, and pyrolytic graphite, a highly diamagnetic substance, failed to produce any behavioral responses in juvenile lemon sharks.
[0044] Next, a polystyrene white plastic blinder was used to remove any visual and motion cues from an approaching metal. This blinder was placed close to the shark's eye, sufficiently shielding its nares, eyes, gills, and head up to its pectoral fin. Again, Samarium metal terminated tonic immobility in all test subjects at a range of 2 to 50 cm from the blinder. Chromium metal and pyrolytic graphite did not produce any notable behavioral shifts.
[0045] In order to confirm that pressure waves were not affecting the test subjects, the tester's hand was moved underwater towards the shark's head both with and without blinders at varying speeds. This motion also did not disrupt the immobilized state.
[0046] The same series of experiments were repeated with juvenile nurse sharks (G. cirratum) and yielded the same behavioral results.
[0047] The same experimental protocol was repeated with a 73 gram ingot of 99.5% Gadolinium metal and yielded the same behavioral results in both juvenile lemon sharks and nurse sharks. It is noted that the rousing behavior was most violent when Samarium metal was used. Additionally, the Gadolinium metal corroded quickly after seawater exposure, and therefore would be appropriate for a one-time use application.
[0048] Next, in order to eliminate the possibility of galvanic cell effects, juvenile sharks were removed from their pens and brought at least 15 meters away from any submerged metal objects. All testers and witnesses removed watches, rings, and jewelry so that only the lanthanide metal was exposed to seawater. The same experimental method was repeated in lemon sharks and we report that tonic immobility was terminated with Samarium metal in all tests.
[0049] We report that waving Samarium or Gadolinium in air above immobilized or resting sharks does not effect behavior, even when the metal is very close to the water's surface. The metal must be in contact with seawater in order to produce the repellent effect. This is notably different from the effects of a rare-earth magnet, which will often terminate tonic immobility at close range in air. It is thus proposed that any electropositive metal or alloy must be in contact with the seawater to produce the desired repellency effect.
[0050] The effects of lanthanide metal on free-swimming sharks were also evaluated. Two juvenile nurse sharks (less than 150 cm total length) were allowed to rest in an open-water captive pen. The tester approached the nurse sharks and moved his hand near the pen wall. His hand contained no metal. Both nurse sharks remained at rest. Next, the tester presented the 153 gram ingot of Samarium metal underwater to the pen wall and we note that both nurse sharks awakened and rapidly swam away from the tester's locale.
[0051] Next, a highly-stimulated competitively-feeding population of six blacknose sharks (C. acronotus) (total length up to 120 cm) and six Carribean reef sharks (C. perezii) (total length up to 210 cm) was established using chum and fish meat. A diver entered the water near the population of sharks with the 153 gram of Samarium metal secured to one end of a 1.5 meter-long polyvinyl chloride pole. As free-swimming sharks swam close to the diver, the control end of the pole (without metal) was presented in a left-right waving motion. Approaching sharks would swim past, bump, or briefly bite the pole. The diver then turned the Samarium metal-end of the pole towards the approaching sharks. All blacknose sharks exhibited a "twitching" or "jerking" behavior as they came near the metal ingot and quickly swam away. Caribbean reef sharks generally avoided the metal, but did not exhibit the twitching behavior.
[0052] Some pure Lanthanide metals are extremely reactive to air and water, and therefore are not particularly well-suited for long time use in the marine environment. For example, pure Europium metal has been observed to appreciably oxidize in air in a matter of hours and degrades quickly in moist air. Other metals, such as Erbium and Samarium have a much higher resistance to oxidation in air and slowly react with cold seawater. Other reactive pure Lanthanide metals are acceptable for one-time use as long as they are kept protected prior to use.
[0053] Mixtures and alloys containing Lanthanide metals may serve as an economical alternative to pure Lanthanide metals. In particular, Cerium Misch metal, Lanthanum Misch metal, Neodymium-Praseodymium Misch metal and Samarium-Cobalt (SmCo) mixtures and alloys may be used in shark-repelling devices.
[0054] It is not yet fully understood why sharks are responding to Lanthanide metals. It would seem that some type of detection is occurring in the Ampullae of Lorenzini organ, but how electrical currents are being generated and detected with a solitary rare earth metal in seawater is not known at this time. We hypothesized that a magnetic or electrical field was being induced by the metal's movement through seawater. We attempted to measure minute magnetic fields being produced by the movement of Samarium metal through seawater in a closed system. A submersible calibrated milliGauss meter probe was secured in a plastic tank containing seawater with the same salinity, pH, and temperature of the water used in previous shark testing. After zeroing out the Earth's magnetic field, we did not detect any magnetic fields being produced by the movement of Samarium metal through the tank, within tenths of a milliGauss. Because there appears to be a lack of a magnetic field component, there cannot be an electrical field component. This is a difficult concept because the sharks are responding, at most times violently, only when the metal is in contact with seawater. The same phenomenon occurs when the sharks are far-removed from any other pure metals or alloys in seawater.
[0055] The effect is not limiting to the order of the shark, as both nurse sharks (Orectolobiformes) and lemon sharks (Carcarhiniformes) responded in a similar manner.
[0056] Another hypothesis is that water-soluble salts are being formed and driven towards the shark as the metal is moved through seawater. The shark, in turn, may be hypersensitive to the presence of rare-earth compounds or ions. The use of our blinder during the experiments should have steered any water containing rare earth salts around the shark's nose and mouth, limiting exposure, but the response was equal with or without blinders. In one test, an immobilized shark was moved towards a stationary Samarium ingot. The shark exhibited bending away from the ingot prior to terminating immobility. This movement would have pushed metal salts away from the shark.
[0057] Further experiments using solutions of the nitrates and chlorides of the early-Lanthanide metals showed no behavioral shifts (using seawater controls) when presented to immobilized sharks at doses up to 25 mL to the nares.
[0058] Captive Cobia, which are commercially valuable marine fish, were exposed to Lanthanide metals during feeding trials. We report that exposure to Holmium, Gadolinium, Dysprosium, and Samarium ingots did not disrupt normal feeding behavior. Cobia do not possess the Ampullae of Lorenzini organ found in sharks.
[0059] A close correlation was found between the revised Pauling electronegativity values for these metals, and behavioral response. As the revised Pauling electronegativity decreased, the violence of the response seemed to increase. A repellency threshold was found at an electronegativity of 1.3 or less-Metals with electronegativities greater than 1.3 did not produce the response. Highly reactive metals, such as Strontium and Calcium (electronegativities of 0.89 and 1.00 respectively) produced a rousing reaction as expected.
EMBODIMENTS
[0060] The present invention combines the repellent effects of ferromagnetism along with electropositivity to offer two shark repellents within a standard metal fishing hook. In one embodiment of the invention, an electropositive metal is incorporated onto the hook by wrapping a ribbon, foil, or sheet of the metal around a portion, portions, or the entire magnetized hook. In another embodiment of the invention, a coating of electropositive metal is deposited onto a portion, portions, or the entire magnetized exterior hook surface through sputtering, thermal evaporation, thick-film deposition, or chemical vapor deposition techniques. In a third non-limiting embodiment of the invention, an electropositive metal or an alloy of electropositive metals is combined with gallium metal to produce a low-melting point alloy. The gallium-electropositive metal alloy is warmed to its melting point and applied to a portion, portions, or the entire surface of a cleaned and magnetized hook. Upon cooling, an electropositive coating remains at the application site of the magnetized hook. In yet another non-limiting embodiment of the invention, a hook is made directly from a ferromagnetic alloy that also contains one or more electropositive metals. This alloy would ideally have a mechanical strength and machinability comparable to standard fishing hooks.
INDUSTRIAL APPLICATION
[0061] The present invention finds use in commercial fisheries where unintentional shark by-catch is a problem. The use of magneto-electropositive fishing hooks reduces the number of sharks captured on hook and therefore makes these hooks available for target fish. The magneto-electropositive hook is particularly useful in tuna and swordfish fisheries.
REFERENCES
[0062] NOAA Fisheries, National Marine Fisheries Service, 2004. Profile: The Atlantic pelagic longline fleet. Northeast Distant Fishery Sea Turtle Bycatch Reduction Fact Sheet. Available online: www.nmfs.noaa.gov/mediacenter/turtles/docs/pelagic_longlining.pdf
[0063] Gilman, E., Clarke, S., Brothers, N., Alfaro-Shigueto-J., Mandelman, J., Mangel, J., Petersen, S., Piovano, S., Thomson, N., Dalzell, P., Donoso, M., Goren, M., Werner, T. 2007. Shark depredation and unwanted bycatch in pelagic longline fisheries: Industry practices and attitudes, and shark avoidance strategies. Western Pacific Regional Fishery Management Council.
[0064] Kalmijn A. 1973. Electro-orientation in sharks and rays: Theory and experimental evidence. Scripps Institute of Oceanography, manuscript. 73-39.
[0065] Kalmijn, A. J. 1974. The detection of electric fields from inanimate and animate sources other than electric organs. Handbook of Sensory Physiology (ed. A. E. Fessard), 147-200.
[0066] Kalmijn A. 1982. Electric and magnetic field detection in elasmobranch fishes. Science. 218:916-918.
[0067] Kalmijn A. 1984. Theory of electromagnetic orientation: a further analysis. In: Bolis L, Keynes R D, Maddrell S H P, editors. Comparative physiology of sensory systems. Cambridge, UK: Cambridge Univ Press. p 525-560.
[0068] Phillips, J. B. 1996. Magnetic navigation. J. Theor. Biol 180:309-319.
[0069] Carey, F. G., Scharold, J. V. 1990. Movements of blue sharks (Prionace glauca) in depth and course. Mar. Biol. 106:329-342.
[0070] Klimley, A. P. 1993. Highly directional swimming by the scalloped hammerhead sharks, Sphyrna lewini, and subsurface irradiance, temperature, bathymetry, and geomagnetic field. Mar. Biol. 117: 1-22.
[0071] Wiltschko, R., Wiltschko, W. 1995a. Magnetic orientation in animals. Springer-Verlag, Frankfurt.
[0072] Holland, K. N; Wetherbee, B. M; Lowe, C. G; Meyer, C. G. 1999. Movements of tiger sharks (Galeocerdo cuvier) in coastal Hawaiian waters. Mar. Biol. 134:665-673.
[0073] Skiles, D. D. 1985. The geomagnetic field: Its nature, history, and biological relevance. In J. L. Kirschvink, D. S. Jones, and B. J. MacFadden (eds.), Magnetite biomineralization and magnetoreception in organisms: A new biomagnetism, pp. 4-102. Plenum Press, New York.
[0074] Kirschvink, J. L., Walker, M. M., Diebel, C. E. 2001. Magnetite-based magnetoreception. Curr Opin Neurobiol 11: 462-467.
[0075] Wiltschko, W., Munro, U., Wiltschko, R, Kirschvink, J. L. 2002. Magnetite-based magnetoreception in birds: The effect of a biasing field and a pulse on migratory behavior. J Exp Biol 205: 3031-3037.
[0076] Ritz, T., Adem S., Schulten, K. 2000. A model for photoreceptor-based magnetoreception in birds. Biophys. J 78:707-718.
[0077] Johnsen, S., Lohmann, K. J. 2005. The physics and neurobiology of magnetoreception. Nature Rev. Neurosci. 6,703 -712.
[0078] Kalmijn, A. J. 1966. Electro-perception in sharks and rays. Nature (Lond.) 212:1232-1233.
[0079] Kalmijn, A. J. 1971. The electric sense of sharks and rays. J. Exp. Biol. 55:371-383.
[0080] Meyer, C. G., Holland, K. M., Papastamatiou, Y. P. 2005. Sharks can detect changes in the geomagnetic field. J R Soc Interface. March 22; 2(2): 129-130.
[0081] Kajiura, S. M., Holland, K. N. 2002. Electroreception in juvenile scalloped hammerhead and sandbar sharks. J. Exp. Biol. 205:3609-3621.
[0082] Kajiura, S. M. 2003. Electroreception in neonatal bonnethead sharks, Sphyrna tiburo Mar. Biol. 143: 603-61.
[0083] Peters, R. C., Eeuwes, L. B., Bretschneider, F. 2007. On the electrodetection threshold of aquatic vertebrates with ampullary or mucous gland electroreceptor organs. Biological Reviews 82(3): 361-373.
[0084] O'Connell, C. P., Stroud, E. M., Herrmann, M., Rice, P. H., Gruber, S. 2007.Evaluation of barium-rerrite permanent magnets on the behavior of four Species of elasmobranchs. As presented to the American Elasmobranch Society, Jul. 15.
[0085] O'Connell, C. P. 2008. Shark Deterrent and Incidental Capture Workshop, Apr. 10-11, 2008. U.S. Dep. Commer., NOAA Tech. Memo. NOAA-TM-NMFS-TM-PIFSC-16, 48-50.
[0086] O'Connell, C. P., Rice, P. H., Stroud, E. M., Abel, D. C., Simuro, N. 2009. Effectiveness of barium ferrite permanent magnets on the feeding behavior of elasmobranchs. As presented to the American Fisheries Society, South Carolina chapter, Feb. 13.
[0087] Stroud, E. M. United States patent application 20070256623 (May 7, 2007).
[0088] Stroud, E. M. Canadian patent application 2,598,148 (May 7, 2007).
[0089] Stroud, E. M. U.S. provisional patent application 61/275,684 (Sep. 24, 2008).
[0090] A. J. Bard, R. Parsons, and J. Jordan, Standard Potentials in Aqueous Solutions, IUPAC (Marcel Dekker), New York, USA, 1985.
[0091] Swimmer, Y., Wang, J. H., McNaughton, L. 2008. Shark Deterrent and Incidental Capture Workshop, Apr. 10-11, 2008. U.S. Dep. Commer., NOAA Tech. Memo. NOAA-TMNMFS-TM-PIFSC-16, iii.
[0092] Wang, J. H., Swimmer, Y., McNaughton, L. 2008. Shark Deterrent and Incidental Capture Workshop, Apr. 10-11, 2008. U.S. Dep. Commer., NOAA Tech. Memo. NOAA-TMNMFS-TM-PIFSC-16, 28-32.
[0093] Stoner A. W., Kaimmer, S. M. 2008. Reducing elasmobranch bycatch: Laboratory investigation of rare earth metal and magnetic deterrents with spiny dogfish and Pacific halibut. Fisheries Research 92(2-3), 162-168.
[0094] Kaimmer, S. M., Stoner, A. W. 2008. Shark Deterrent and Incidental Capture Workshop, Apr. 10-11, 2008. U.S. Dep. Commer., NOAA Tech. Memo. NOAA-TM-NMFS-TMPIFSC-16, 64-66.
[0095] Stoner, A. W., Kaimmer, S. M. 2008. Shark Deterrent and Incidental Capture Workshop, Apr. 10-11, 2008. U.S. Dep. Commer., NOAA Tech. Memo. NOAA-TM-NMFS-TMPIFSC-16, 60-63.
[0096] Brill, R., Bushnell, P., Smith, L., Speaks, C., Sundaram, R., Stroud, E., Wang, J. 2009. The repulsive and feeding deterrent effects of electropositive metals on juvenile sandbar sharks (Carcharhinus plumbeus). In press, Fisheries Bulletin. FB-3298.
[0097] Tallack, S. M. L., Mandelman, J. W. 2009. Do rare-earth metals deter spiny dogfish? A feasibility study on the use of electropositive "mischmetal" to reduce the bycatch of Squalus acanthias by hook gear in the Gulf of Maine. ICES Journal of Marine Science. 66: 315-322.
[0098] O'Connell, C. P., Rice, P. H., Stroud, E. M., Abel, D. C., Simuro, N. C. The Effects of Permanent Magnets on the Southern Stingray (Dasyatis americana) and the Nurse Shark (Ginglymostoma cirratum). Marine and Freshwater Behavior and Physiology, April 2010.
PENDING PATENT REFERENCES
[0099] U.S. patent application Ser. No. 11/800,545, "ELASMOBRANCH-REPELLING ELECTROPOSITIVE METALS NAD METHODS OF USE"
[0100] U.S. patent application Ser. No. 11/886,109, "ELASMOBRANCH-REPELLING MAGNETS AND METHODS OF USE"
Elasmobranch-Repelling Compounds, Methods of Use and Devices
US2010203154
Elasmobranch-repelling compositions are prepared from elasmobranch carcasses. Extraction of these elasmobranch carcasses with polar solvent after a period of aerobic decay yields semiochemical repellents that induce a flight reaction in sharks when introduced into the sharks' oceanic proximity
[0001] This invention relates generally to elasmobranch repellents, methods of making and using such repellents and devices for administering such repellents. This invention also relates to a process for selecting elasmobranch carcasses and using polar solvents to extract semiochemicals that induce a flight reaction in elasmobranchs. Qualitative techniques are described, which allow for detection of the production of semiochemicals during the extraction process. Without being limited to a specific theory, it is believed that extracted semiochemicals are detected in elasmobranchs by olfaction because behavioral responses are achieved with very low concentrations of the inventive repellent.
BACKGROUND OF THE INVENTION
[0002] Shark attacks on humans have been recorded from ancient times. One such attack on an unlucky Mediterranean sponge diver is recorded from the third century B.C. (Thomas B. Allen, Shark Attacks: Their causes and avoidance 35 (2001)). And since the early part of the twentieth century, the populace of the United States has been riveted by sporadic stories of sensational and gruesome human encounters with sharks. As the twentieth century progressed and America's love for the seashore grew, so did its fascination with the remote but real possibility of a dangerous brush with one of these creatures. From at least 1916, shoreline municipalities began to develop physical structures to keep public bathing areas safe from the perceived danger of sharks. And through the present day, reports of shark attacks have frightened coastal communities and negatively impacted their economies as seashore revelers curtailed their beach excursions with each new and ever frightening story of voracious sharks in a particular town's waters.
[0003] As the number of humans spending time in the ocean has increased, so has the number of shark attacks and along with that increase in attacks so grows the urgent need for a repellent. Further, as the twenty-four hour news cycle continues its frenetic discussion of the threat of sharks to humans, each shark attack in the developed world appears to be reported with greater sensation and grander desperation. As such, the ever-pressing need to develop an effective shark repellent is even greater than before as the public seeks to provide itself some assurance that it will not be a victim of the next injurious encounter with a shark.
[0004] While fear of attack by sharks seems to have appeared in the United States in the early part of the twentieth century, the Second World War particularly amplified this fear when U.S. service personnel were called to combat in the dangerous and "shark infested" South Pacific. During that time, the U.S. Navy began a concerted effort to develop a chemical shark repellent to protect sailors and air personnel exposed to sharks when downed in shark-prone waters. Since then, government and private industry have worked to discover and develop a chemical shark repellent potent enough to protect humans. (Johnson and Baldridge (1985).
[0005] To establish clear criteria for government development of an effective chemical shark repellent, Johnson and Baldridge set forth a goal in 1985 of finding a chemical that would repel sharks in ocean water at 1 part per billion. While the goal was a good one, no previously-developed chemical repellent has even come close to achieving the standard.
[0006] An effective repellent would not only provide some assurance to humans bathing or adrift in waters frequented by sharks, an effective repellent would also significantly help the commercial fishing industry. Commercial longline fishing operations routinely target swordfish and tuna. However, the longline fishing hook is not selective, and it is not uncommon for more sharks to be caught than swordfish or tuna. Sharks that are caught as unintended targets are commonly called "by-catch." Often, the shark dies on the hook prior to retrieval. If a live shark is cut free during retrieval, the hook, snood and gangion are usually lost. This presents significant monetary loss as well as significant inadvertent death for millions of sharks. There has been a long-felt need to reduce by-catch losses in the fishing industry and to save the lives of many millions of sharks each year. Currently, as many as 80 species of shark are considered threatened with extinction and it is estimated that up to 100 million sharks are killed each year by humans. It is no surprise, then, that an effective repellent would satisfy a long-felt need in the commercial fishing industry.
[0007] It has been recognized for some time that development of a repellent effective against two particular orders of shark, Carcharhinoforme and Lamniformes, would provide considerable protection to humans and considerable assistance to commercial fishing. This is because nearly all of the known aggressive species of sharks and the predominant kinds of sharks that also interfere with commercial fishing are from those two orders. Orders Squaliformes and Orectolobiformes, on the other hand, represent sharks that have caused relatively few injuries throughout history and do not commonly harm commercial fishing interests.
[0008] Sharks and their close relatives, rays and skates, are classified in biological taxonomy within the class Chondrichthyes (fish) and the sub-class Elasmobranchii (fish without bones). Within the sub-class Elasmobranchii, sharks are classified in the sub-class Selachii, and rays and skates are classified within the sub-class Batoidei.
[0009] Of the more than 350 known species of shark, as many as 35 species have been recorded attacking humans. Repeated attacks, however, have been recorded with less than 15 of these species. The frequency of shark attacks worldwide is quite small compared to the number of humans who work and play in the ocean each day. Less than about 100 humans are attacked by sharks each year with fatalities from shark attack averaging around 30. Nevertheless, the real fact of shark attack and the constant possibility, though low probability, of shark attack makes the need for an effective shark repellent a pressing reality for millions of ocean-going people every day.
[0010] While fatal shark attacks have most likely occurred for millennia, recorded events have been rare until the twentieth century. One early recorded fatal shark attack occurred in 1580 when a man overboard on a Portuguese sailing vessel was reportedly "torn to pieces" while clinging to a life buoy. (Allen (2001) at 33). This was certainly not the earliest recorded shark attack. In fact, the danger of shark attacks on sponge divers in the Mediterranean was documented in the Natural History of Pliny the Elder in 77 A.D. and the above-noted fatal story of a sponge diver who lost part of his lower body to a shark was recorded in the third century B.C. (Allen (2001) at 35). Many shark attacks have been recorded ever since. There appears, however, to have been no consideration of methods of limiting shark attacks (at least in the United States) until 1916.
[0011] The summer of 1916 ushered in "the year of the shark" for the coastal regions around New York City. Over just 12 days in that summer, at least four people were killed by sharks along the New Jersey coastline. (Allen (2003) at 174). These attacks later inspired the movie Jaws (1975). (Thomas B. Allen, The Shark Almanac 174 (2003)). Beginning in 1916, the American public embraced a collective and long-enduring fear of sharks. This fear swelled to a point of concern for the U.S. government when it entered World War II against Japan in the South Pacific. (Allen (2001) at 207). To maintain morale among sailors and airmen (and their families) who faced the constant possibility of finding themselves adrift and exposed at sea, the U.S. government began research directed at protecting service personnel from shark attack. (Allen (2001) at 207). In this effort, the U.S. Navy began a program to develop a chemical shark repellent. The resulting product was known as "Shark Chaser."
[0012] In Chapter 17 of Dr. Perry W. Gilbert's 1975 printing of "Sharks and Survival", Richard L. Tuve of the U.S. Naval Research Laboratory describes the development of the U.S. Navy "Shark Chaser" chemical shark repellent. The program originated with the Office of Strategic Services in March 1942. Initial research was based on anecdotal evidence; Floridian fishermen contended that if a shark died on an unattended hook and line, further fishing in that area became undesirable. The researchers, therefore, hypothesized that some substance emitted by the decomposing body drove other sharks away from the vicinity.
[0013] As research continued, Woods Hole investigators and U.S. Navy scientists determined (erroneously it turns out) that the principal chemical material exuding from the decomposing shark was ammonium acetate. Scientists at Wood Hole also proposed the use of copper, which was known to reduce feeding in captive fishes and sharks. The ultimate combination of ammonium acetate and copper produced copper acetate, which was combined with nigrosine dye to provide a visual indication of the repellent dispersion.
[0014] The dye and copper acetate combination was molded into cakes and field testing began in 1944. Following a series of successful tests, a readjustment to 20% copper acetate and 80% nigrosine dye cake was sold as the "Shark Chaser." The military specifications for "Shark Chaser" were given under MIL-S-2785A as of Feb. 2, 1961.
[0015] As the Shark Chaser repellent found widespread use, continued research revealed that copper acetate was not effective in repelling sharks. In Chapter 2 of Bernard J. Zahuranec's 1983 printing of "Shark Repellents from the Sea: New Perspectives" the author gives insight into the inefficacy of the Shark Chaser. From tests in the shark pens at Bimini, Bahamas, Gilbert and Springer (1963) concluded that copper acetate fails to repel or inhibit the feeding activities of several species of sharks we have worked with at Bimini. Tester (1963) also reported the inefficacy of copper acetate against tiger sharks and other fish. Some theorized that the nigrosine dye itself was actually a visual deterrent. It was eventually concluded that copper acetate was not a practical deterrent for human use, and the military ultimately halted the issuance of the Shark Chaser. Recent research by the present inventors has confirmed these earlier findings that copper acetate is ineffective as a shark repellent and that ammonium acetate is not a principal component of decomposing shark tissue. See Tables 2 and 4 and FIG. 8.
[0016] While copper acetate was abandoned by the U.S. government in the 1960s, shark repellent research continued in the United States, with focus on marine organisms as sources of a repellent. Holothurins, anemones, urchins, and gorgonians were explored for a potential toxin but no shark repellent activity was detected. More research has been conducted on other naturally-occurring compounds. The inventors report that holotoxin from macerated sea apples, as well as seven types of potent hemolytic glycosides (saponins) from plants, were not effective as shark repellents.
[0017] Over the last 50 years antishark measures employed to protect humans from shark have included electrical repellent devices (Gilbert & Springer 1963, Gilbert & Gilbert 1973), acoustical playbacks (Myrberg et al. 1978, Klimley & Myrberg 1979), visual devices (Doak 1974) and chemical repellents (Tuve 1963, Clark 1974, Gruber & Zlotkin 1982). None of these procedures proved totally effective in preventing shark attacks. (Sisneros (2001)).
[0018] Following World War II, when reports of Shark Chaser's ineffectiveness began to appear, the Office of Naval Research began to reconsider the matter of chemical shark repellents and renewed the screening and testing of possible candidates (Zahuranec & Baldridge 1983). Hundreds of chemical substances were tested on sharks in an effort to find a chemical that would produce a quick and effective repellent response (Springer 1954, Gilbert & Springer 1963, Tester 1963). These chemicals included powerful toxins that could (and did) kill a shark after brief exposure; but none elicited the desired repellent response. Support for the research eventually ended after many attempts had provided no effective shark repellent. (Sisneros (2001)).
[0019] As described in the ReefQuest Centre for Shark Research:
In 1974, ichthyologist Eugenie Clark noticed that the delicate Moses Sole (Pardachirus marmoratus) was easy to catch and appeared to secrete a milky, astringent substance from the base of its dorsal and anal fin spines. Suspecting that the little fish was protected by a toxin of some kind, Clark collected several specimens for study. She found that the Moses Sole did indeed secrete a toxin she named "pardaxin," which caused red blood cells to rupture and-most intriguingly-repelled sharks. Tests by Clark in the laboratory and open sea revealed that at least four species of sharks were repelled by pardaxin for 10 hours or longer.
[0021] While fresh pardaxin repelled sharks, it presented serious stability problems because it was not stable for room temperature storage, and was heat-sensitive. Pardaxin could be freeze-dried, but this form was only 30% as effective as the fresh secretion, as reported by Zlotkin (1976). Chemical analysis yielded that pardaxin was an acid protein of 162 amino acids with a MW of 17,000 Daltons. The acid protein had a difficult synthesis pathway making commercial production not commercially practical. Sigma-Aldrich currently offers pardaxin for sale in the U.S. at $487.00 US for 1 milligram (product #P0435-1MG). Similar compounds such as mosesin and pavoninin present the same difficulties. There has been and remains a long-felt need for a shark repellent that can be produced and stored at room temperature with high yields of repellent. Further, it is believed that pardaxin, mosesin, and pavoninin act on the shark's respiratory system, requiring a minimum concentration of repellent to enter the mouth and contact the gill rakes of the shark, i.e., repellent had to be squirted directly into the shark's mouth.
[0022] Zlotkin noted that pardaxin possessed surfactant properties, reducing surface tension by as much as 60%. As described at the ReefQuest Centre for Shark Research:
Zlotkin teamed with shark biologist Samuel Gruber to test a hunch: could commercially available soaps repel sharks? Zlotkin and Gruber tested two inexpensive commercial soap components, sodium and lithium lauryl sulfate (SLS and LLS, respectively-SLS, incidentally, is a common ingredient in shampoos), on young Lemon Sharks (Negaprion brevirostris). They found that both compounds were even more effective than pardaxin at repelling captive Lemon Sharks.
Further tests by Nelson et al. found that SLS was an effective repellent against blue sharks and even great white sharks. As described in "The Behavior and Sensory Biology of Elasmobranch Fishes: An Anthology in Memory of Donald Richard Nelson" (Tricas, T. C. & S. H. Gruber (ed.) (2001)), as well as "Surfactants as chemical shark repellents: past, present, and future" (J. A. Sisneros (2000))," the greatest limitation of SLS is that it is required to be squirted into the mouth of an approaching shark. It is not effective in surrounding-cloud-mode dispersions. Therefore, SLS is only useful when the user can clearly see an approaching shark and orchestrate the delivery of SLS into the animal's mouth. There has been a long-felt need for a repellent administered in surrounding cloud dispersions, thereby avoiding the impracticable need for direct-oral delivery.
[0024] In 2001, Sisneros reported further research on compounds related to pardaxin. Sisneros confirmed that dodecyl sulfate was the most effective surfactant shark repellent available at the time and that even the best repellent did not meet the Navy's potency requirement for a nondirectional surrounding-cloud type repellent of 100 parts per billion (0.1 ppm or 100 micrograms/Liter). Sisneros further concluded that dodecyl sulfate would only be practical as a directional repellent such as in a squirt application. Sisneros suggested that future research should test the action of alkyl sulfates on cell membranes, the potential of other biotoxic agents, and semiochemicals in the search for an effective chemical shark repellent. (Id.)
[0025] The existence of semiochemical repellents were first considered by Rasmussen & Schmidt in 1992. They suggested that sharks may be chemically aware of the presence of potential danger by sensing the bodily secretions from potential predators. Rasmussen & Schmidt hypothesized that lemon sharks, especially juveniles, inherently recognize chemical exudates produced by the American crocodile, Crocodylus acutus, a known predator of sharks. The concentrations needed to produce aversive responses in lemon sharks ranged from 10-7 to 10-9 M, which was near the functional limit of shark chemoreceptors (Hodgson & Mathewson 1978).
[0026] Sisneros also noted that another proposed potential source for shark repellent semiochemicals might perhaps be found in decomposing shark flesh (Baldridge 1990, Rasmussen & Schmidt 1992) because anecdotal information from fishermen claimed that sharks avoid areas containing decomposing carcasses of previously caught dead sharks. Sisneros postulated that perhaps there are semiochemicals found in extremely low concentrations in decaying shark flesh that act as alarm pheromones and provide warning signals to nearby sharks. None of those postulated compounds were known or have since been found and there have been no commercially available effective chemical shark repellents. As such, the long felt need for an effective repellent had not been satisfied until the present invention.
[0027] U.S. Pat. Nos. 4,490,360 and 4,340,587 describe the use of lucibufagins from fireflies and extractions of fireflies as a shark repellent. While the specifications suggest that behavioral changes were occurring in numerous species of animals, no effects were observed on larger inshore and pelagic sharks. Further, while one specification describes the "very extensive practical use in protecting bathing zones from the invasion of objectionable sea life such as sharks," the Atlantic Sharpnose species represents a very small-sized inshore species which has no reported aggressiveness nor represents a bycatch problem. Additionally, no practical synthesis is described for lucibufagins, therefore tremendous quantities of fireflies are required to produce drum-quantities of a repellent.
[0028] Data on the use of firefly-derived repellents were also reported against the Atlantic Sharpnosed Shark (Rhizoprinodon terraenovae), the smooth dogfish (Mustelus canis), the pinfish (Lagadon rhomboides), and killifish (Fundulus heteroclitus) in a paper presented at a symposium in 1981. (Bonaventura et al., Problems and Possibilities: The Development of an Effective Shark Repellent for Naturally Occurring Biologically Active Substances, Jan. 5, 1981, Annual Meeting of the American Association for the Advancement of Science, Toronto, Canada). These data additionally provide no support for a repellent of inshore and pelagic sharks that would be useful as an effective shark repellent.
[0029] U.S. Pat. No. 6,606,963 describes an acoustical system which produces shark-repelling waveforms. This invention affects the shark's hearing and lateral line sensory systems. However, as described by Klimley, Myrberg et al., sharks rapidly habituate to a sound source unless output power is very high. The present invention overcomes these limitations by, in theory, affecting the olfactory system. There has been a long-felt need for a repellent that is effective such that competitively feeding populations of sharks will stop feeding and will avoid all food stimuli in the presence of the repellent, wherein no habituation is observed after exposure.
[0030] Researchers have historically used several bio-assays to determine if a repellent evokes a flight response in shark. One such bio-assay introduces repellent of a certain concentration and volume to a position in a tank and measures avoidance in sharks of that portion of a tank or other aversive swimming behavior.
[0031] Another such bio-assay introduces repellent of a certain concentration and volume into the feeding zone of sharks and measures whether sharks flee the feeding zone and/or cease feeding behavior.
[0032] Another preliminary bio-assay measures the effect of a repellent on a shark that is immobilized in "tonic immobility." Tonic immobility is a state of paralysis that typically occurs when a shark is subject to inversion of its body along the longitudinal axis. This state is called "tonic," and the shark can remain in this state for up to 15 minutes thereby allowing researchers to observe effects of chemical repellents. After behavioral controls are established, an effective chemical repellent will awaken a shark from a tonic state. Researches can quantify dose sizes, concentrations, and time to awaken from these studies. A microliter autopipettor is used to observe effects at the 10-100 uL level. A 60 cc syringe is used as a baseline, looking for a preliminary response.
[0033] Another bioassay is known as the Johnson-Baldridge test. The test is defined as the delivery of 100 mg of chemical repellent into a 6 cubic meter boundary of water over a 3.5 hour period under steady-state conditions. This level of repellent delivery from a point source is considered to represent a concentration of 0.1 ppm. This is a proposed criterion in the art for an "effective" repellent. If sharks demonstrate aversive behavior under these conditions, then the criteria is satisfied. The inventors have designed and constructed an experiment to test if semiochemicals meet the Johnson-Baldridge criteria. A PVC tripod was situated in the ocean. The tripod supported a peristaltic metering pump, set to meter out exactly 100 mL of repellent per hour. The tripod also supported a video camera and transmitter, which observed the area under the tripod, marked off for 6 cubic meters and compensated for tidal changes. The video was monitored and recorded on shore. A fish head was secured under the tripod, within view of the camera. Once a population of sharks was established near the tripod, a control was performed. A second fish head was secured, the pump was started, and behavior was observed. If the fish head was protected for the 3.5 hour period, the criteria were met.
BRIEF SUMMARY OF THE INVENTION
[0034] Applicants have discovered an effective elasmobranch repellent. According to a non-limiting embodiment of the present invention, a repellent is provided comprising a semiochemical from a carcass of an elasmobranch. The inventive semiochemical terminated tonic immobility and evoked a flight response in an elasmobranch. It was also noticed that the inventive semiochemical did not evoke a flight response in fish having a bony skeleton. In another non-limiting embodiment of the present invention, the repellent comprises a semiochemical and a polar solvent. In another non-limiting embodiment of the present invention, the repellent comprises a carcass of an elasmobranch treated with a polar solvent for between about one month to about six months. In another non-limiting embodiment of the invention, the repellent is filtered from the polar solvent treated elasmobranch carcass. In another non-limiting embodiment of the present invention, the repellent comprises a mixture of semiochemicals from more than one carcass of more than one elasmobranch species.
[0035] According to a second embodiment of the present invention, a method of repelling an elasmobranch is provided comprising administering a semiochemical in the expected proximity of an elasmobranch. In another non-limiting embodiment of the present invention, the semiochemical is from an elasmobranch carcass treated with a polar solvent.
[0036] According to a third non-limiting embodiment of the present invention, a repellent is obtained by a process comprising the steps of exposing a carcass of an elasmobranch to a polar solvent, and filtering the repellent from the carcass. In another non-limiting embodiment of the present invention, the repellent is obtained by a process wherein an elasmobranch carcass is aerobically decayed prior to exposure to a polar solvent and a portion or the entirety of the pre-treated carcass is then exposed to a polar solvent. In a non-limiting preferred embodiment, the elasmobranch carcass is aerobically decayed to a degree of decomposition between the onset of rigor mortis and the completion of putrefaction prior to exposure to the polar solvent. In another non-limiting preferred embodiment, the pre-treated carcass is completely immersed in a polar solvent.
[0037] In a non-limiting embodiment of the present invention, the inventive repellent is characterized on an HPLC chromatogram with three characteristic peaks with relative peaks detected in the range between approximately 240 nm to approximately 340 nm at about 5, about 6 and about 7 minutes and the relative peak at about 7 minutes is greater than the relative peaks at about 5 minutes and about 6 minutes. In a preferred embodiment, the repellent HPLC chromatogram has the following characteristics
[0000]
Column: Novapak 0.5u C18 reversed phase
Flow rate: 0.5 ml/min
Mobile phase: A: Methanol, 0.1% acetic acid
B: Water, 0.1% acetic acid
Gradient: 0-10 minutes 100% A
10-12 minutes, 0% A, 100% B, linear
12-20 minutes 100% B
20-22 minutes 0% B, 100% A, linear
22-60 minutes, 100% A
Injection: 50 ul into a 50 ul loop
Column temperature: 25[deg.] C.
In a preferred non-limiting embodiment, the repellent has the following ultraviolet absorbances: 300 nm, greater than 1 AU; 312 nm, greater than 2 AU; and 322 nm, greater than 2 AU.
[0038] According to a fourth non-limiting embodiment of the present invention, a process for making an elasmobranch repellent is provided comprising the step of extracting a semiochemical from a carcass of an elasmobranch by exposing said carcass to a polar solvent and filtering said repellent from said carcass. In a preferred non-limiting embodiment, the method of manufacture of the inventive repellent comprises (1) placing a carcass of an elasmobranch in an extraction vessel, (2) exposing the carcass to aerobic decomposition, (3) treating said carcass and the decomposition fluids of said carcass with a polar solvent preferably in 50% water, 40% methanol, 6.5% ethanol, and 3.5% methyl isobutyl ketone, by weight, (4) monitoring for detectable semiochemicals, and (5) filtering the repellent from the carcass.
[0039] According to a fifth non-limiting embodiment of the present invention, a compound for repelling elasmobranch is provided wherein the compound is characterized by a uv-visible spectrum having an absorbance peak between about 280 nm and about 340 nm.
[0040] According to a sixth non-limiting embodiment of the present invention, a specially designed container is provided for administering an elasmobranch repellent comprising a pressurized container and an actuator for release of the repellent when activated. In a preferred non-limiting embodiment, the container is an aerosol container comprises an actuator that triggers a continuous release of repellent when activated. In another non-limiting preferred embodiment, the aerosol container is weighted in the vicinity of the actuator to provide an erratic motion in the water when the container is administered, the actuator is activated and the repellent is discharged from the container.
[0041] According to a seventh non-limiting embodiment of the present invention, a method of repelling an elasmobranch is provided comprising administering a semiochemical from a raft, buoy or piling in the expected vicinity of an elasmobranch. In a preferred non-limiting embodiment of the present invention, the semiochemical is administered from the raft, buoy or piling from a pressurized diptube that discharges the semiochemical above the surface of the water.
[0042] According to an eighth non-limiting embodiment of the invention, a method of repelling an elasmobranch is provided comprising attaching to a fishing longline a mass of carcass of an elasmobranch that has been treated with polar solvent.
[0043] According to a ninth non-limiting embodiment of the present invention, a kit is provided comprising a semiochemical repellent and a vehicle for administering the semiochemical repellent. Such vehicle of administration may include known devices and novel devices disclosed herein.
BRIEF DESCRIPTION OF THE DRAWINGS
[0044] The invention will now be described by way of example with reference to the accompanying drawings wherein:
[0045] FIGS. 1A and 1B illustrate process flow charts for extraction of semiochemical repellents in accordance with the present invention.
[0046] FIG. 2 illustrates comparison of uv-visible spectra of eight exemplary semiochemicals in accordance with the present invention. The composite spectra represent eight exemplary repellents in accordance with the present invention wherein uv-visible spectral maxima reside in the region of around 280 nm to around 340 nm. A distinct signature peak is notable in each of these exemplary repellents at around 300 nm with a semiochemical of a Great White shark head having the highest relative detected absorbance at the signature 300 nm peak.
[0047] FIG. 3 illustrates HPLC chromatograms of the early-eluting components of semiochemicals GWH and A1 in accordance with the present invention. Notable are peaks at around 5, around 6 and around 7 minutes. The 7 minute peak is stronger than the others.
[0048] FIG. 4 illustrates HPLC chromatograms of late-eluting components of exemplary semiochemicals GWH and A1 in accordance with the present invention. Noted are peaks of the semiochemicals at around 31 minutes, around 34 minutes, around 36 minutes and around 42 minutes with a signature sharp peak in the range of about 30 to about 40 minutes followed by a broad, double-maxima peak about two minutes later.
[0049] FIG. 5 illustrates HPLC chromatograms of primary amines in exemplary semiochemicals GWH and A1 in accordance with the invention at 570 nm after treatment with ninhydrin. Noted are peaks indicative of primary amines. Further, the chromatograms contain peaks at around 5, around 6 and around 7 minutes with the 7-minute peak much stronger than the 5-minute and 6-minute peaks.
[0050] FIG. 6 illustrates HPLC chromatograms of secondary amines in exemplary semiochemicals GWH and A1 in accordance with the invention at 440 nm after treatment with ninhydrin. Peaks detected at 440 nm are indicative of secondary amines. Further, the chromatograms contain a first strong and sharp peak around 34 minutes and a strong broad peak with two components eluting about 2 minutes later.
[0051] FIG. 7 illustrates a GC-MS spectrograph of an exemplary semiochemical GWH in accordance with the present invention on Hewlett Packard model 6890 GC with 5973 MSD having a column of DB-5 40 m*0.18 mm*0.40 mm film, a carrier of helium at 1 mL/min; an injection of 1 microliters, splitless at 280[deg.] C.; heated to 40[deg.] C. and held for 5 minutes then to 300[deg.] C. at 10[deg.] C./min and held for 5 minutes; with the transfer line heated to 300[deg.] C.; and an MSD scan at 20-700 m/z. The mass spectral data in combination with the chromatogram was analyzed using quality of NIST 98.1 library match.
[0052] FIG. 8 illustrates a uv-vis absorbance spectrum of an exemplary semiochemical CP in accordance with the present invention. Noted are peaks at around 440 nm and around 570 nm.
[0053] FIG. 9 illustrates a uv-vis absorbance spectrum of 50% w/w ammonium acetate (a proposed and discredited shark repellent) in water, derivatized with 0.1 g ninhydrin at 40[deg.] C. for 15 minutes. Noted are no maxima at around 440 nm or around 570 nm.
[0054] FIG. 10 illustrates a GC-MS chromatogram of an exemplary semiochemical CP in accordance with the invention on Hewlett Packard model 6890 GC with 5973 MSD having a column of DB-5 40 m*0.18 mm*0.40 mm film, a carrier of helium at 1 mL/min; an injection of 1 microliters, splitless at 280[deg.] C.; heated to 40[deg.] C. and held for 5 minutes then to 300[deg.] C. at 10[deg.] C./min and held for 5 minutes; with the transfer line heated to 300[deg.] C.; and an MSD scan at 20-700 m/z. The mass spectral data in combination with the chromatogram was analyzed using quality of NIST 98.1 library match.
[0055] FIG. 11 illustrates a comparison of uv-vis spectra of one-year-old semiochemicals A2, A13N and SQ1. The uv-visible spectra of each semiochemical was taken using a Perkin Elmer Lambda 12 dual-beam scanning spectrophotometer, neat semiochemical solution was micron filtered and loaded into quartz cuvettes, representative uncontaminated solvents used in the extraction process (at the same ratios used to perform the extractions) were used as a reference sample or "blank," and a peak at around 300 nm is seen for each semiochemical in accordance with the invention.
[0056] FIG. 12 illustrates a comparison of uv-visible spectra of semiochemical CL at 0, 7, 21 and 40 days during the extraction process. A 300 nm peak is shown to increase in absorbance over time.
[0057] FIGS. 13-15 illustrate HPLC chromatograms of exemplary semiochemical A2 in accordance with the invention derivatized with ninhydrin using a variety of solvents and injection volumes.
[0058] FIG. 16 illustrates an FTIR spectrum of exemplary semiochemical A2 in accordance with the invention. The resulting proposed stretches corresponding to the spectral peaks are provided in Example 4G, below.
[0059] FIG. 17 illustrates a Head Space Total Ion GC-MS chromatograph of an exemplary semiochemical A2 in accordance with the present invention.
[0060] FIG. 18 illustrates a Direct Injection GC-MS Total Ion chromatograph of an exemplary semiochemical A2 in accordance with the present invention.
[0061] FIG. 19 illustrates a total ion LC-MS chromatograph of exemplary semiochemical A2 in accordance with the present invention. Mass to charge ratios are noted in the chromatogram.
[0062] FIG. 20 illustrates a GC-MS chromatograph of exemplary semiochemical CF-Composite in accordance with the present invention on Hewlett Packard model 6890 GC with 5973 MSD having a column of DB-5 40 m*0.18 mm*0.40 mm film, a carrier of helium at 1 mL/min; an injection of 1 microliters, splitless at 280[deg.] C.; heated to 40[deg.] C. and held for 5 minutes then to 300[deg.] C. at 10[deg.] C./min and held for 5 minutes; with the transfer line heated to 300[deg.] C.; and an MSD scan at 20-700 m/z. The mass spectral data in combination with the chromatogram was analyzed using quality of NIST 98.1 library match.
[0063] FIG. 21 illustrates a GC-MS chromatograph of an exemplary semiochemical B-Composite in accordance with the present invention on Hewlett Packard model 6890 GC with 5973 MSD having a column of DB-5 40 m*0.18 mm*0.40 mm film, a carrier of helium at 1 mL/min; an injection of 1 microliters, splitless at 280[deg.] C.; heated to 40[deg.] C. and held for 5 minutes then to 300[deg.] C. at 10[deg.] C./min and held for 5 minutes; with the transfer line heated to 300[deg.] C.; and an MSD scan at 20-700 m/z. The mass spectral data in combination with the chromatogram was analyzed using quality of NIST 98.1 library match.
[0064] FIG. 22 illustrates HPLC chromatographs of early-eluting components of one-and-a-half-year-old degraded semiochemical A2 and more-than-one-year-old degraded semiochemical N2. Noted is the absence of a strong peak at around 7 minutes.
[0065] FIG. 23 illustrates HPLC chromatographs of late-eluting components of one-and-a-half-year-old degraded semiochemical A2 and more-than-one-year-old degraded semiochemical N2. Noted are one sharp peak and one sharp double peak at about 3 and about 2 minutes before a weak, broad double-peak at around 35 minutes.
[0066] FIG. 24 illustrates prior known solution delivery devices modified to contain semiochemical repellent in accordance with the present invention. FIG. 24A illustrates a pressurized delivery pole apparatus. FIG. 24B illustrates a delivery device syringe. FIG. 24C illustrates a cattle-treatment "drench" gun.
[0067] FIG. 25 illustrates a novel exemplary semiochemical delivery device in accordance with the present invention. FIG. 25A illustrates an aerosol canister for administration of semiochemical repellent to a shark environment. FIG. 25B illustrates the various axes of rotation of the exemplary canister. FIG. 25C illustrates directional discharge of repellent by the novel exemplary device in all directions yet having a preference for discharge in the water due to continuous discharge of repellent and being weighted in the vicinity of the actuator. It is noted that repellent is discharged in the water and into the air above the water, creating a concentration of repellent in the immediate vicinity of the container and creating a wider dispersion of repellent as it settles out of the air onto the surface of the water.
[0068] FIG. 26 illustrates a mortar-launched aerosol "bomb" canister for administration of semiochemicals in accordance with the present invention from a distance.
[0069] FIG. 27 illustrates an automated repellent dispenser in accordance with the present invention comprising a raft or other floating or fixed device that delivers repellent by discharging repellent above the surface of the water.
[0070] FIG. 28 illustrates a duel barreled semiochemical repellent discharger in accordance with the present invention.
[0071] FIG. 29 illustrates semiochemical repellent pouches in accordance with the invention.
[0072] FIG. 30 illustrates an apparatus for administering repellent along fishing longline in accordance with the present invention.
[0073] FIG. 31 illustrates a semiochemical repellent backpack discharger in accordance with the present invention for use, for example, by scuba divers and those who snorkel.
[0074] FIG. 32 illustrates a spear gun fitted with a repellent discharge device in accordance with the present invention.
[0075] FIG. 33 illustrates a repellent delivery device adapted to a surfboard in accordance with the present invention. FIG. 33A illustrates a surfboard with a pressurized chamber that is discharged by a surfer in an emergency. FIG. 33B illustrates a surfboard with a chamber for containing repellent and a drip valve and vent for continuous discharge during surfing.
[0076] FIG. 34 illustrates a wristwatch comprising a repellent canister in accordance with the present invention.
[0077] FIG. 35 illustrates a belt (FIG. 35A) or bracelet (FIG. 35B) comprising pressurized repellent in accordance with the present invention.
[0078] The novel features, which are believed to be characteristic of the present invention, will be further understood from the following discussion.












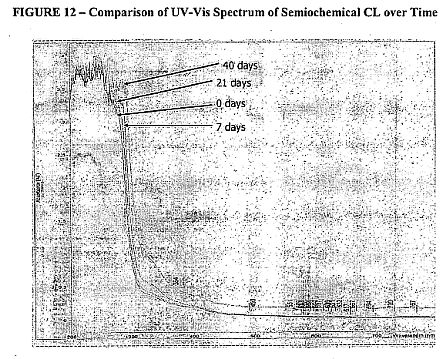


&c...
DETAILED DESCRIPTION OF THE INVENTION
[0079] "Acoustical stimulation" is the arousal or activation of a subject related to sound or sense of hearing. Elasmobranchs are attracted to low-frequency pulsed sounds, similar to those emitted by wounded prey. Acoustical stimulation of a subject is generally accomplished by the pulsing of sound waves in the frequency of 25 to 100 Hz. Some elasmobranchs are attracted to sound sources from distances as great as 250 m.
[0080] "Canister" is a large or small container or vessel of any shape. An "aerosol canister" is a large or small container or vessel of any shape the contents of which are held under pressure and may contain a propellant gas or material to discharge a desired substance in a spray, liquid, foam or mist.
[0081] "Carcass" is the dead body of an animal or any portion thereof. For use in this application and unless otherwise indicated, a carcass refers to the dead body of an elasmobranch whole or in part and cleaned or uncleaned of stomach contents. Carcass may include the head, tail and/or muscle tissue.
[0082] "Complete putrefaction" is the degree of decomposition where muscular tissue has substantially liquefied. Typically, the muscular tissue falls away, as a slime, from the skin, not retaining any shape. This period roughly coincides with the "black putrefaction" or "butyric putrefaction" periods of mammalian decay, approximately 20-50 days after death.
[0083] "Conspecific" means of the same species.
[0084] "Conspecific repellents" are repellents that are made from a species of elasmobranch that repels the same species or family or order of elasmobranch.
[0085] "Elasmobranchii" represents the subclass of class Chondrichthyes (cartilaginous fish), which includes the sharks and rays. In this specification, "elasmobranchs" represent the super-orders and orders of elasmobranchs that are of interest for producing a repellent based on availability and conservation, and also those that present a potential threat to humans or represent a bycatch problem in commercial fisheries. As such, "elasmobranchs" in this specification means one or more elasmobranchii in the super-orders Galeomorphii and Squalomorphii and orders Squaliforms (dogfish), Carcharhiniformes (requiem sharks), Lamniformes (mackerel sharks), and Orectolobiformes (carpet sharks).
[0086] "Feeding zone" is the area in which sharks have been stimulated and demonstrate aggressive feeding behavior.
[0087] "Heterospecific" means from a different species.
[0088] "Heterospecific repellents" are repellents that are from a species of elasmobranchs that repels a different species or family or order of elasmobranch.
[0089] "Polar solvent" is a first substance capable of dissolving another substance wherein the first substance comprises molecules with electric charges unequally distributed, leaving one end of each molecule more positive than the other.
[0090] "Putrefaction" is the degree of decomposition at which most of a carcass is decomposed.
[0091] "Rigor mortis" is the degree of decomposition at which a carcass becomes stiff.
[0092] "Semiochemical" is a compound or mixture of compounds derived from the carcass of an elasmobranch that can terminate tonic immobility of an elasmobranch with a dosage of less than 500 microliters, that can evoke a flight reaction in an elasmobranch that has been stimulated to feed and that does not evoke a flight response in telios fish.
[0093] "Tonic immobility" is the state of paralysis that typically occurs when an elasmobranch is subject to inversion of its body along the longitudinal axis of the body, i.e., is belly up. The elasmobranch can remain in this state for up to 15 minutes.
I. SEMIOCHEMICAL EXTRACTION PROCESS
[0094] Semiochemical repellents in accordance with the present invention are prepared from an elasmobranch carcass as illustrated in FIG. 1A. An elasmobranch carcass is collected (whole or in part). The carcass or carcasses are aerobically decayed preferably beyond the degree of decomposition of rigor mortis but before complete putrefaction. Some semiochemical compounds are eluted during the controlled aerobic decay.
[0095] The elasmobranch carcass can be of a single variety or of multiple varieties of elasmobranch and may represent whole carcasses or a part or parts of different carcasses. The carcass sample preferably contains at least a portion of muscle tissue. A whole carcass, dressed tube of carcass, steak of carcass or section or sections of carcass may be used, cleaned or uncleaned of entrails or stomach contents.
[0096] The carcass sample is allowed to aerobically decay beyond rigor mortis but before putrefaction, which period of time may be from about one day to about one month. The aerobically decayed tissue is transferred to a bath of polar solvent, preferably along with any semiochemicals that may have been released. The decayed tissue is kept in a bath of polar solvent in an extraction vessel for from about 1 week to about 6 months and up to about one year. The polar-solvent-carcass vessel contents are sampled from time to time to determine the stage of extraction of semiochemical(s). When the presence of semiochemicals is detectable at a determined end-point, the contents of the extraction tank may be filtered for use as an elasmobranch repellent or the pre-filtered contents may be formed into a mass for use as an elasmobranch repellent.
[0097] A preferred extraction process is described in FIGS. 1A-1B. For example, muscular tissue from one or more sharks of the Order of Carcharihiniform, Orectobolobiform, Lamniform or Squaliform are obtained and allowed to aerobically decay in an environment free from insects and other flesh eating organisms (1). In a preferred embodiment, the shark tissue is aerobically decayed in an extraction vessel of polypropylene, high density polyethylene (HDPE) or glass for about ten days (2). Polar solvent is then introduced to the extraction vessel of sufficient volume to cover the decayed shark tissue (3). After an amount of time, preferably one month, the extraction is sampled for instrumental analysis to determine whether or not semiochemicals have begun to be produced.
[0098] If semiochemical production is not yet sufficient to achieve a desired end-point (as set forth in this specification by standard signatures on analytical instruments or by a demonstration by testing the repellent activity against a shark), the extraction process is allowed to proceed for another period of time. The following period of time for further incubation of the extraction may be any amount of time, ordinarily less than about six months. Preferably, the waiting period is from about 1 day to about one month, depending on the expected production time for semiochemicals from the particular shark tissue in the particular polar solvent. The skilled artisan will easily determine optimal waiting periods between instrumental analyses of the extraction. The waiting period and instrumental analysis should be repeated until there has been sufficient production of semiochemicals to achieve a desired end-point. The desired end-point is chosen by detection of sufficient semiochemicals using instrumental analysis or by the demonstration of a flight reaction in shark when applied to the shark's environment. Semiochemical repellent may be recovered at various stages (2A, 3A, 4A) in the process.
[0099] When the extraction process has produced sufficient semiochemicals to achieve a desired end-point, the contents of the extraction vessel is preferably filtered (4). The filtrate is containerized and prepared for administration as an elasmobranch repellent (5). The methods of the present invention are able to produce more than 15 liters of repellent solution from a 2.2 kg shark specimen. A 6-foot carcass is able to produce approximately 50 gallons of repellent solution according to the extraction process described in the present invention.
[0100] When semiochemicals in sufficient abundance are detected, the decay process may likewise be halted by lowering temperature or immersion in solvents for preservation until use. In an alternative, once semiochemicals in sufficient abundance are detected the contents of the extraction vessel is formed into a mass or masses for administration as an elasmobranch repellent.
[0101] In the extraction process, whole carcasses are preferable over sectioned carcasses. In lieu of an entire carcass, successful semiochemical repellents have been prepared using the entire head, the entire tail, or the liver from a specimen. Blood alone is not preferred for semiochemical derivation
[0102] At the outset, carcasses should be allowed to decay aerobically, past the stage of rigor mortis, but before complete putrefaction. This may be accomplished by leaving the carcass in open air or a cooler for a period of time, taking care to not allow insects and scavengers to manifest. Decomposition fluids are preferably retained. A freshly killed carcass is unsuitable for deriving semiochemicals because specific catabolites have not yet been produced. A fully decayed carcass is unsuitable for deriving semiochemicals because specific catabolites are fully depleted or metabolized. Anaerobic decay is an unacceptable method, and produces high yields of organic sulfur compounds and low yields of semiochemicals.
[0103] Carcass selection may be made based on what species of elasmobranch are desired to be repelled. For example, it is not preferred to utilize stingray carcasses when trying to develop a hammerhead-specific repellent. It is preferred, however, to use a stingray carcass to develop a stingray repellent. Most genus Carcarhinus specimens are very suitable for preparing broad-spectrum shark repellents. It is demonstrated herein that lemon shark carcasses not only produced semiochemical solutions which repelled lemon sharks, but also repelled blacknose, reef, and bull sharks.
[0104] Carcasses from multiple species are also suitable. For example, a vessel containing two lemon shark carcasses, one nurse shark carcass, and one smooth dogfish carcass produced a high yield of semiochemicals after 6 months of extraction time.
[0105] After the initial decay period and before total putrefaction, the decomposition fluids, blood, and the carcass mass are preferably placed in an extraction vessel. In a preferred embodiment, the carcass is not cleaned, gutted, or rinsed prior to transfer.
[0106] The extraction vessel is preferably a container which is impervious to organic solvents and acids, and which seals air-tight to prevent escape of solvent vapors. The vessel is ideally polypropylene plastic or glass. This vessel should possess access points for solvent addition, draining, circulation/stirring, and viewing.
[0107] The optimal positioning of the carcass in the vessel and solvent is with the carcass positioned vertically, head down, in the vessel. More than one carcass may be positioned in the vessel to increase yield.
[0108] A solvent for the extraction process is any polar solvent that is less than 100% water. A preferred extraction solvent is a water:solvent mixture at a 50:50 mix ratio by weight of water to another polar solvent. The skilled extraction chemist will understand that adjustments may be made to improve yields. A preferred water to other polar solvent ratio is 50:50 water:solvent, by weight.
[0109] Any single, binary, ternary, or multiple solvent system is suitable for the 50:50 mixture. For example, n-propanol, iso-propanol, glycol ethers, methanol/ethanol systems, acetic acid, hydrochloric acid solutions, butanol, dimethylsulfoxide, and short-chain aldehydes and ketones are acceptable solvents. A preferred polar solvent is 80% methanol, 17% ethanol, and 3% methyl isobutyl ketone by weight. Water in combination with the aforementioned solvents is also suitable, as long as anaerobic decay is minimized. Leaving the carcass in pure water is not a preferred solvent system. It is preferred that the solvent cover the entire carcass mass.
[0110] The extraction process should be carried out at about room temperature. Elevated temperatures speed the extraction process, but produce lower-efficacy semiochemical solutions. Soxhlet extraction similarly produces low-efficacy semiochemical solutions. The most effective process is simply to leave the vessel at room temperature and slow circulation for 3 to 6 months, depending on the solvent strength.
[0111] The solvent should be sampled periodically to monitor the presence of semiochemicals and to determine a desired end-point for the extraction process. End-point may be determined with uv-visible spectrophotometry, high performance liquid chromatography (HPLC), mass-spectrometry, infrared detection, visible detection of a yellow color or testing of samples on shark to determine if a flight reaction is induced.
[0112] Spectrophotometry is a simple method for determining the "ripening" state of the solvent mixture. Over time, peak absorbances will be observed between about 290 nm and about 320 nm, with some maxima being extremely strong when the extraction process is operating efficiently. When clear solvents are employed, the solvent/semiochemical mixture develops a characteristic pale-yellow coloration after 3 months, indicating the presence of the semiochemicals.
[0113] Preferred end-points for the extraction process as detected by different instrumental analyses are set forth in the following section.
[0114] At the end of the extraction process, the semiochemical solution may be filtered, but not distilled. Rotary evaporation and fractional distillation has been observed to ruin the efficacy of the semiochemical solution. Fritted glass filters and micron filters are very suitable for removing skin and biomass particles, as well as improving visual clarity. Vacuum may be employed in the filtration process, but heat is not preferred. Preferably, the solution is used at full strength for maximum repellency on wild sharks and rays.
[0115] A. Instrumental Analysis for Determining Desired End-Point of Extraction Process
[0116] The end point of the extraction process may be determined by instrumental analysis. End-point is reached when a semiochemical has been produced in the extraction process to a point where it is detectable in sufficient amounts by instrumental analysis or where the extraction has developed to the point of evoking a flight response, evoking aversive swimming behavior, evoking termination of tonic immobility, or providing protection sufficient to satisfy the Johnson-Baldridge test in elasmobranchs. Liquid chromatography, spectrophotometry, gas chromatography and qualitative analytical techniques are preferably used to identify the point in time where semiochemical concentration reaches a maximum. Semiochemicals possess characteristic absorbance maxima, fragmentation, retention times, and physical properties, such as odor, color and pH.
[0117] A desirable end-point may be determined by testing a filtered sample from the extraction vessel on HPLC according the following gradient elution configuration:
[0000]
Column: Novapak 0.5u C18 reversed phase
Flow rate: 0.5 ml/min
Mobile phase: A: Methanol, 0.1% acetic acid
B: Water, 0.1% acetic acid
Gradient: 0-10 minutes 100% A
10-12 minutes, 0% A, 100% B, linear
12-20 minutes 100% B
20-22 minutes 0% B, 100% A, linear
22-60 minutes, 100% A
Injection: 50 ul into a 50 ul loop
Detection: 240 nm, range 1 AUFS
Column temperature: 25[deg.] C.
In this setup, 6 characteristic compounds elute within the first 8 minutes, producing 6 peaks. Of these characteristic peaks, 3 are of particular interest. A distinctive strong peak at about 7 minutes and two moderate peaks at about 5 and about 6 minutes demonstrate well developed semiochemical extract. A second group of compounds elute after 23 minutes, indicating up to 25 additional compounds, with weak to moderate absorbances.
[0118] A desirable end-point may also be determined by testing a filtered sample of the extraction on HPLC after amines in the semiochemical repellent samples have been derivatized using ninhydrin to produce strong chromophores. Derivatization with ninhydrin yields two colored products, Rhuemann's purple at 570 nm for primary amines, and a colored product with an absorbance maximum at 440 nm for secondary amines. These colored products are detected using an HPLC and an ultraviolet-visible detector. Derivatization may be performed pre- or post-column, but post-column work must employ additional pumps, flow combiners, and elevated temperatures ahead of the detector. Preferably the derivatization is performed pre-column. Samples are prepared by combining 50% w/w of a 1% ninhydrin in 2-propanol solution with 50% w/w of a semiochemical sample. Samples are allowed to derivatize for 2 hours at 40[deg.] C. prior to analysis. The following system configuration is used:
[0000]
Column: C18, reversed phase
Flow rate: 1 ml/min
Mobile phase: 80% water, 20% acetonitrile
Injection: 10 uL
Detection: 570 nm for primary amines,
440 nm for secondary amines
Column temperature: 35[deg.] C.
[0119] This method produces up to 5 characteristic peaks between 1 and 2 minutes for primary amines at 570 nm. The method also produces up to 5 characteristic peaks between 1 and 2 minutes for secondary amines at 440 nm. An entity at both detection wavelengths is observed at 4.8 minutes with a trace concentration. If, in an alternative method of HPLC analysis, the gradient elution configuration set forth above is employed at 570 nm, three characteristic peaks of particular interest elute at around 5, around 6 and around 7 minutes, with the strongest peak at 7 minutes. If the same gradient elution method is employed at 440 nm, a characteristic broad double peak is expected to elute at around 30 to around 40 minutes preceded by about two minute by an earlier sharp peak.
[0120] End-point may also be determined using uv spectral analysis. The ultraviolet spectra of an extracted semiochemical repellent solution may be considered to contain sufficient semiochemical products when they yield the following generally characteristic absorbances:
240 nm, greater than 2 AU
266 nm, greater than 1 AU
273 nm, greater than 1 AU
280 nm, greater than 1.5 AU
289 nm, greater than 1.5 AU
294 nm, greater than 2 AU
300 nm, greater than 2.5 AU
312 nm, greater than 3 AU
322 nm, greater than 3 AU.
[0130] The visible spectrum of a semiochemical repellent solution yields a weak but likewise characteristic absorbance maximum in the red region, at 657 nm (less than 0.5 AU). A salient peak to determine sufficient development of semiochemicals using uv-visible spectrophotometry is often a signature peak beginning around 300 nm and reaching a maximum near 310 or 320 nm.
[0131] For example, semiochemical CL (from C. limbatus) was sampled at 0, 7, 21 and 40 days to determine development of semiochemical uv-vis signature. (See FIG. 11.) Signature absorbance at around 300 nm increased as extraction proceeded. A 300 nm shoulder was barely perceptible at 0 days but increased throughout 7, 21 and 40 days of the extraction process to become a distinct peak at 40 days around 3.5 AU.
[0132] B. Instrumental Analysis of Semiochemicals-Composition of Matter
[0133] Semiochemical extractions may be qualitatively tested for the presence of sufficient semiochemicals to act as an elasmobranch repellent using a range of instrumental analytical techniques. These qualitative techniques include HPLC, uv-visible spectroscopy, infrared spectroscopy and mass spectrometry coupled with other separation techniques.
[0134] 1. UV-Visible Spectrophotometry
[0135] To test an extraction for sufficient presence or development of semiochemicals using uv-visible spectrophotometric analysis a uv-visible spectrophotometer may be employed. A dual-beam scanning spectrophotometer, such as the Perkin Elmer Lambda 12 model, is preferable. Neat semiochemical solutions should be micron-filtered and loaded into quartz cuvettes. Representative uncontaminated solvents used in the extraction process, at the same ratios used to perform the extraction, are used as a reference sample or "blank."
[0136] An extracted semiochemical repellent solution may be considered to contain sufficient semiochemical products when its ultraviolet spectrum yields the following generally characteristic absorbances:
240 nm, greater than 2 AU
266 nm, greater than 1 AU
273 nm, greater than 1 AU
280 nm, greater than 1.5 AU
289 nm, greater than 1.5 AU
294 nm, greater than 2 AU
300 nm, greater than 2.5 AU
312 nm, greater than 3 AU
322 nm, greater than 3 AU.
[0146] The visible spectra of a semiochemical repellent solution yields a weak but characteristic absorbance maxima in the red region, at 657 nm, less than 0.5 AU.
[0147] 2. Fourier-Transform Infrared Spectrophotometry
[0148] Fourier-Transform Infrared Spectrophotometry provides confirmation of certain functional groups in a semiochemical solution. Since the extraction solution contains water, another extraction must be performed to remove the semiochemicals from the water. FTIR cannot be accomplished in the presence of water. A simple extraction using a separatory funnel, with a 50:50 weight ratio mixture of a water-insoluble solvent to the semiochemical solution is very adequate. Strong water-insoluble solvents include diethyl ether, methylene chloride, and chloroform. The water-insoluble phase of this extraction may be further dried using magnesium or sodium sulfate, to remove all traces of water.
[0149] In an FTIR analysis, a waterless sample from the water-insoluble phase described above is set on a KBr crystal. A scan from 1100 nm to 3500 nm of a semiochemical extraction may indicate the following groups:
[0000]
2800-3000 nm Asymmetric and symmetric CH3 groups
1300-1400 nm Scissor, asymmetric, and symmetric CH3 groups
1126.00 nm C-O bond stretching
1434.56 nm C-O bond stretching
1637.28 nm C-C bond stretching
2846.60 nm C-H bond stretching
2916.50 nm C-H bond stretching
2951.46 nm C-H bond stretching
3321.94 nm OH bond stretching, indicating alcohols along with the
above three preceding stretches.
(FIG. 16.)
[0150] 3. High Pressure Liquid Chromatography, HPLC
[0151] High Pressure Liquid Chromatography, HPLC is also used to detect the presence of semiochemicals in the extraction solution. A gradient HPLC system shows the presence of semiochemicals in two groupings, according to the following method:
[0000]
Column: Novapak 0.5u C18 reversed phase
Flow rate: 0.5 ml/min
Mobile phase: A: Methanol, 0.1% acetic acid
B: Water, 0.1% acetic acid
Gradient: 0-10 minutes 100% A
10-12 minutes, 0% A, 100% B, linear
12-20 minutes 100% B
20-22 minutes 0% B, 100% A, linear
22-60 minutes, 100% A
Injection: 50 ul into a 50 ul loop
Detection: 240 nm, range 1 AUFS
Column temperature: 25[deg.] C.
[0152] In this setup, three particularly distinctive peaks may be observed within the first about 8 minutes with the peaks spaced about one minute apart. The strongest peak is generally the final peak of the three. Most often peaks elute at about 5 minutes, about 6 minutes and about 7 minutes with the peak at 7 minutes relatively stronger than the peaks at 5 and 6 minutes. A second group of compounds elute after 23 minutes, indicating up to 25 additional compounds, with weak to moderate absorbances.
[0153] In another analysis, components with absorbances at 622 and 624 nm elute at approximately 1.21 minutes using the following configuration:
[0000]
Column: 0.5u C18 reversed phase
Flow rate: 1 ml/min
Mobile phase: 80% w/w water, 20% w/w acetonitrile
Injection: 50ul into a 50ul loop
Detection: 622-625 nm
Column temperature: 35[deg.] C.
HPLC coupled to fluorescence is also used to detect amino acids in the semiochemical mixture. Amino acids were derivatized with an active ortho-pthalaldehyde (OPA) reagent, which is prepared by treating OPA with an excess of a thiol compound, namely 2-mercaptoethanol, to form an OPA-2-mercaptoethanol adduct. This adduct reacts with primary amines to form fluorescent isoindoles, which are readily detected by a fluorescence detector post-column.
[0154] HPLC resolution can be improved by deproteinization. Membrane-filtered semiochemical samples are treated with perchloric acid, and then neutralized with potassium hydroxide, producing insoluble potassium perchlorate. The neutralized sample is centrifuged for 15 minutes, and the supernatant is analyzed by HPLC. Deproteinized samples generally produce better peak resolution and symmetry.
[0155] 4. Ninhydrin Derivatization
[0156] The amine functions in semiochemical repellent samples can be derivatized using ninhydrin to produce strong chromophores. Ninhydrin is a selective oxidizing agent which causes oxidative decarboxylation of amino acids producing CO2, NH3, and an aldehyde with one less carbon atom than the parent amino acid. The reduced ninhydrin then reacts with the liberated ammonia to form Ruhemann's Purple, a complex which maximally absorbs light at 570 nm. Secondary amines, e.g., Proline and 4-Hydroxyproline, react via a different path and form a yellow derivative with an optimal absorbance at 440 nm.
[0157] Since the reaction with amines is highly specific and the absorption characteristics of the formed chromophores follow Beer's Law, reagents based on Ninhydrin have long been the most popular choice for detection and quantitation of amines and amino acids.
[0000]
[0000] Ninhydrin reacts slowly at room temperature. Consequently, in automated amino acid analysis, elevated temperatures of up to 130[deg.] C. are employed to reduce the conversion time to about one minute.
[0158] Ninhydrin derivatization often yields two absorbances in a semiochemical shark repellent, one at 570 nm and one at 440 nm, corresponding to primary and secondary amine functions respectively. For example, the absorbance spectra of a semiochemical extraction from the head of C. perezii (using 50% water, 40% methanol, 6.5% ethanol, and 3.5% methyl isobutyl ketone) derivatized with 0.1 g ninhydrin at 40[deg.] C. for 15 minutes is found in FIG. 8. Clear maxima are observable at 440 nm (around 4 AU) and 570 nm (2.9 AU). When primary and secondary amines are not present, and the sample is derivatized with ninhydrin, absorbances at 440 nm and 570 nm are not observed. A uv-visible spectrum of 50% w/w ammonium acetate (a discredited shark repellent) in water, derivatized with 0.1 g ninhydrin at 40[deg.] C. for 15 minute showed no maxima at 440 or 570 nm. (See FIGS. 8 and 9.)
[0159] Products absorbing at 440 nm and 570 nm may additionally be resolved and detected using an HPLC and an ultraviolet-visible detector as described for end-point determination above. When ninhydrin-derivatized semiochemical extracts are run on HPLC with the following parameters a distinctive chromatograph is produced:
[0000]
Column: Novapak 0.5u C18 reversed phase
Flow rate: 0.5 ml/min
Mobile phase: A: Methanol, 0.1% acetic acid
B: Water, 0.1% acetic acid
Gradient: 0-10 minutes 100% A
10-12 minutes, 0% A, 100% B, linear
12-20 minutes 100% B
20-22 minutes 0% B, 100% A, linear
22-60 minutes, 100% A
Injection: 50 ul into a 50 ul loop
Detection: 240 nm, range 1 AUFS
Column temperature: 25[deg.] C.
For primary amines, three distinct 570 nm absorbing peaks elute at about 5, about 6 and about 7 minutes. (See FIG. 5.) For secondary amines, a distinctive pattern of 440 nm absorbing peaks elute. A sharp peak in the middle to later thirty minute range elutes followed about two minutes later by a broad double peaked elution. (See FIG. 6.)
[0160] 5. Thin-Layer Chromatography
[0161] Flash chromatography and thin-layer chromatography may be performed, in order to observe amine components in ninhydrin derivatized samples. Using the following system, the primary and secondary amines can be well separated via flash chromatography:
[0000]
Stationary phase: Silica gel, 230-400 mesh
Mobile phase: 66% n-butanol, 33% methyl formate, 1% glacial acetic
acid
Column height: 6''-30'', packed height, or 8'' * 8'' plates
Sample: 0.2 micron-filtered, derivatized with 1% ninhydrin
in 2-propanol.
[0162] In thin-layer chromatography, the underivatized sample is spotted. After the endpoint is reached, the plate is developed with either 1% ninhydrin in 2-propanol solution, or the OPA-2-mercaptoethanol solution described earlier. The mobile phase should be optimized for optimal retention factors (Rf's).
II. CONSPECIFIC AND HETEROSPECIFIC EFFECTIVE ELASMOBRANCH REPELLENT
[0163] The biological activity of elasmobranch-repelling semiochemicals extracted from various orders of elasmobranchs, particularly, Orders Orectolobiformes, Lamniformes, Carcharhiniformes and Squaliformes, has been demonstrated in elasmobranchs of the Order Carcharhiniformes and Order Lamniformes. Repellent activity has also been observed in conspecific species interactions and heterospecific species interactions. ((See Table 1)).
[0164] Semiochemical extractions produced from pelagic Lamniforms have demonstrated repellency on inshore Carcharhiniformes (e.g., ML1, ML2, B, GWH). Semiochemical extractions produced from inshore Carcharhiniformes have demonstrated repellency on highly migratory (pelagic) Carcharhiniformes (e.g., GCC). Semiochemical extractions produced from a Squaliform have demonstrated repellency on Carcharhiniformes (e.g., SQ1). Semiochemical extractions produced from one or more species of Carcharhiniformes have demonstrated repellency on entirely different species of Carcharhiniformes (e.g., CPP, GCC, CP). Semiochemical extractions produced from one species of Carcharhiniformes have demonstrated repellency on conspecific species (e.g., CP). Semiochemical extractions produced from one or more species of Orectolobiformes have demonstrated repellency on species of Carcharhiniformes (e.g., N2, BB1). (See Table 1).
[0165] Repellency activity may be demonstrated in any method described above or known to one of skill in the art. For the investigations undertaken herein two common methods of testing repellent activity were most often used.
[0166] A pressurized fluid delivery system was designed to deliver repellent into large feeding populations of sharks. The repellent was released as a subsurface cloud, which follows the current. A 1 L plastic container containing the semiochemical solution was pressurized to approximately 20 psig with a battery compressor or hand pump. A globe valve was used to hold back the fluid. The fluid was delivered to the end of a long PVC pole using Teflon tubing. This allowed the operator to place the tip of the pole well into the population of feeding sharks. By actuating the small globe valve, a cloud of the solution was released quickly and reliably into the feeding population. Controls were established using FD&C Red 40 dye and seawater, uncolored seawater, and air. These controls established that sharks were not afraid to approach the delivery pole, nor were sharks deterred from feeding by the jet of control fluid or air. During field tests with feeding populations of up to 12 Carcharhinus perezi with Carcharhinus acronatus, we consistently observed that as little as 4 fl. oz (approx 129 ml) of semiochemical "A2" reduced the feeding population to zero within 2 minutes when administered with the above-described testing apparatus.
[0167] Another method is a "tonic immobility" study. During tonic immobility studies, semiochemical is delivered using a plastic syringe, which is not in contact with the specimen. The test solution is released within 3 inches of the specimen's nose. Controls are established using separate syringes with seawater. Some controls were released with a high flow rate (30 mL/sec) in order to establish that sharks were not awakened by the jet of fluid over their noses.
[0168] Using the above-described tests, the repellent characteristics of a wide range of semiochemicals prepared in accordance with the invention has been established. For example, semiochemical extractions produced from pelagic Lamniforms (e.g., I. oxyrhincus) have demonstrated repellency on inshore Carcharhiniformes. In three tests, 450 ml to 700 ml doses of semiochemical composition GWH, derived from the head of a great white shark, repelled competitively-feeding blacknose and Caribbean reef sharks. (See Table 1).
[0169] Semiochemical extractions produced from inshore Carcharhiniformes have demonstrated repellency on highly migratory (pelagic) Carcharhiniformes. A 500 mL dose of semiochemical composition A13N, derived from lemon sharks, nurse sharks, and spiny dogfish; repelled two adult blue sharks which were previously stimulated by acoustical and olfactory attractants. Similarly, a 500 mL dose of semiochemical composition GCC, derived from a tiger shark carcass, was observed to repel a large adult blue shark stimulated by acoustical and olfactory attractants.
[0170] Semiochemical extractions produced from a Squaliform repelled species of Carcharhiniformes. A 250 mL dose of Composition SQ1, derived from the Cuban Dogfish, repelled competitively-feeding blacknose and Caribbean reef sharks. (See Table 1).
[0171] Semiochemical extractions produced from one or more species of Carcharhiniformes repelled entirely different species of Carcharhiniformes. A 500 mL dose of semiochemical composition CPP, derived from the head of a sandbar shark, repelled competitively-feeding blacknose and Caribbean reef sharks. Similarly, a 500 mL dose of composition A2, derived from lemon, nurse, and dogfish carcasses, repelled two adult bull sharks stimulated with olfactory attractants.
[0172] Semiochemical extractions produced from one species of Carcharhiniformes repelled a conspecific species of Carcharhiniformes (e.g., CP). In four tests using an aerosol delivery canister, semiochemical composition CP, derived from the head of a Caribbean Reef Shark, repelled competitively-feeding blacknose and Caribbean reef sharks. (See Table 1).
[0173] Semiochemical extractions produced from one or more species of Orectolobiformes repelled species of Carcharhiniformes. In tests using captive juvenile lemon sharks, aversive swimming responses were observed with a 10 mL dose of semiochemical extraction from nurse shark carcasses. Similarly, a 10 mL dose of semiochemical from a bamboo shark carcass produced aversive swimming responses in captive juvenile lemon sharks. (See Table 1).
[0000]
TABLE 1
REPELLENT SOURCE
Blind Code Order Family G. species Section Polar Solvent System Decay Process
A Carcharhiniformes Carcarhinidae N. brevirostris whole 50% water aerobic in
carcass, 40% methanol polypropylene
Orectolobiformes Ginglymostomatidae G. cirratum whole 8.5% ethanol at 25[deg.] C.
carcass, 1.5% methylisobutyl (RT)
Squaliforms Squalidae S. acanthias whole ketone
carcass
A2 Carcharhiniformes Carcarhinidae N. brevirostris whole 50% water aerobic in
carcass, 40% methanol polypropylene
Orectolobiformes Ginglymostomatidae G. cirratum whole 8.5% ethanol at 25[deg.] C.
carcass, 1.5% methylisobutyl (RT)
Squaliforms Squalidae S. acanthias whole ketone
carcass
A2 Carcharhiniformes Carcarhinidae N. brevirostris whole 50% water aerobic in
carcass, 40% methanol polypropylene
Orectolobiformes Ginglymostomatidae G. cirratum whole 8.5% ethanol at 25[deg.] C.
carcass, 1.5% methylisobutyl (RT)
Squaliforms Squalidae S. acanthias whole ketone
carcass
A2 Carcharhiniformes Carcarhinidae N. brevirostris whole 50% water aerobic in
carcass, 40% methanol polypropylene
Orectolobiformes Ginglymostomatidae G. cirratum whole 8.5% ethanol at 25[deg.] C.
carcass, 1.5% methylisobutyl (RT)
Squaliforms Squalidae S. acanthias whole ketone
carcass
A2 Carcharhiniformes Carcarhinidae N. brevirostris whole 50% water aerobic in
carcass, 40% methanol polypropylene
Orectolobiformes Ginglymostomatidae G. cirratum whole 8.5% ethanol at 25[deg.] C.
carcass, 1.5% methylisobutyl (RT)
Squaliforms Squalidae S. acanthias whole ketone
carcass
B Lamniformes Lamnidae I. oxyrhincus cross- 50% water aerobic in
section 50% acetone glass at
behind 25[deg.] C. (RT)
first
dorsal
B2 Lamniformes Lamnidae I. oxyrhincus cross- 100% water anaerobic in
section polypropylene
behind at 25[deg.] C.
first (RT)
dorsal
A13N Carcharhiniformes Carcarhinidae N. brevirostris whole 50% water aerobic in
carcass, 40% methanol polypropylene
Orectolobiformes Ginglymostomatidae G. cirratum whole 8.5% ethanol at 25[deg.] C.
carcass, 1.5% methylisobutyl (RT)
Squaliforms Squalidae S. acanthias whole ketone
carcass
ML1 Lamniformes Lamnidae I. oxyrhincus liver 50% water aerobic in
50% acetone polypropylene
at 25[deg.] C.
(RT)
ML2 Lamniformes Lamnidae I. oxyrhincus liver 50% water aerobic in
50% acetone polypropylene
at 25[deg.] C.
(RT)
SQ1 Squaliforms Squalidae S. cubensis whole 50% water aerobic in
carcass 40% methanol polypropylene
8.5% ethanol at 25[deg.] C.
1.5% methylisobutyl (RT)
ketone
CPP Carcharhiniformes Carcarhinidae C. plumbeus head 50% water aerobic in
40% methanol polypropylene
8.5% ethanol at 25[deg.] C.
1.5% methylisobutyl (RT)
ketone
GWH Lamniformes Lamnidae C. carcharias head 50% water aerobic in
40% methanol polypropylene
8.5% ethanol at 25[deg.] C.
1.5% methylisobutyl (RT)
ketone
GCC Carcharhiniformes Carcarhinidae G. cuvieri cross 50% water aerobic in
section 40% methanol polypropylene
behind 8.5% ethanol at 25[deg.] C.
pectoral 1.5% methylisobutyl (RT)
fins ketone
CP Carcharhiniformes Carcarhinidae C. perezii head 50% water aerobic in
40% methanol polypropylene
8.5% ethanol at 25[deg.] C.
1.5% methylisobutyl (RT)
ketone
N2 Orectolobiformes Ginglymostomatidae G. cirratum whole 50% water aerobic in
carcass 40% methanol polypropylene
8.5% ethanol at 25[deg.] C.
1.5% methylisobutyl (RT) ketone
BB1 Orectolobiformes Hemiscyllidae C. punctatum whole 50% water aerobic in
carcass 40% methanol polypropylene
8.5% ethanol at 25[deg.] C.
1.5% methylisobutyl (RT)
ketone
TARGET TEST
Blind Code Order Family G. species Dose Method Population Response
A Carcharhiniformes Carcarhinidae C. acronotus 500 ml cloud 15 repelled while
C. perezii stimulated with
bait
A2 Carcharhiniformes Carcarhinidae C. acronotus 500 ml cloud 12 repelled while
C. perezii stimulated with
bait
A2 Carcharhiniformes Carcarhinidae N. brevirostris range TI 1 terminated tonic
7 ml to immobility
30 ml
A2 Carcharhiniformes Carcarhinidae C. leucas 500 ml cloud 2 repelled while
stimulated with
bait
A2 Carcharhiniformes Carcarhinidae C. limbatus 1 ml/min johnson- 1 protected bait at
point baldridge point source for
source 1 hour until
pump battery
died
B Carcharhiniformes Carcarhinidae C. acronotus 200 ml cloud 12 repelled while
C. perezii stimulated with
bait
B2 Carcharhiniformes Carcarhinidae C. acronotus 1 qt cloud 6 no behavioral
C. perezii change, feeding
continued
A13N Carcharhiniformes Carcarhinidae P. glauca 500 ml cloud 2 repelled while
stimulated with
bait and
acoustics
ML1 Carcharhiniformes Carcarhinidae C. perezii 700 ml cloud 8 repelled while
C. acronotus stimulated with
bait
ML2 Carcharhiniformes Carcarhinidae C. perezii 700 ml cloud 8 repelled while
C. acronotus stimulated with
bait
SQ1 Carcharhiniformes Carcarhinidae C. perezii 250 ml cloud 12 repelled while
C. acronotus stimulated with
bait
CPP Carcharhiniformes Carcarhinidae C. perezii 500 ml cloud 7 repelled while
C. acronotus stimulated with
bait
GWH Carcharhiniformes Carcarhinidae C. perezii 500 ml cloud 9 repelled while
C. acronotus stimulated with
bait
GCC Carcharhiniformes Carcarhinidae P. glauca 500 ml cloud 2 repelled while
stimulated with
bait and
acoustics
CP Carcharhiniformes Carcarhinidae C. perezii 6 fl oz Aerosol 12 repelled while
C. acronotus stimulated with
bait
N2 Carcharhiniformes Carcarhinidae N. brevirostris 10 ml syringe 1 aversive
swimming
behavior
observed after
dose in captive
tank
BB1 Carcharhiniformes Carcarhinidae N. brevirostris 10 ml syringe 1 aversive
swimming
behavior
observed after
dose in captive
tank
Notes for Table 1:
The solvent system usually represents 50% w/w water with 50% of the mixture of 80% MeOH, 17% EtoH, and 3% methyl isobutyl ketone
P. glauca = blue shark, a highly migratory (pelagic) shark, not an inshore species
C. leucas = considered the most dangerous inshore shark species
C. punctatum = brownbanded bamboo shark, a harmless hand-sized shark
I. oxyrhincus = shortfin mako shark, obtained as steaks or liver
C. carcharias = great white shark, considered the most dangerous epipelagic shark species, 2nd most dangerous inshore
G. cuvieri = tiger shark
C. plumbeus = sandbar shark
C. perezii = Caribbean reef shark
C. acronotus = blacknose shark (not blacktip, C. limbatus)
S. cubensis = (deepwater) Cuban dogfish
S. acanthias = spiny dogfish
G. cirratum = nurse shark
N. brevirostirs = lemon shark
The following compounds have been established as effective controls in stimulated, tonically immobilized, and non-stimulated free-swimming sharks under chemical repellent evaluation:
seawater, dose ranges 100ul to 1000 ml
HPLC grade micron-filtered water, dose ranges 1 ml to 10 ml
methanol/ethanol/mibk/water solution dos at approx 500 ml
methanol/ethanol/mibk solution dose ranges 1 ml to 6 ml
diethylene glycol monoethyl ether dose ranges 1 ml to 6 ml
acetone/water solution dose at approx 500 ml
[0174] Semiochemical extractions produced from a tiger shark (Order Carcharhiniformes) repelled a juvenile Mako shark (Order Lamniformes, Family Lamidae, Genus Isurus) discussed in Example 12 below.
[0175] In sum, it has been demonstrated that a semiochemical extraction of the order Lamniformes repels a Carcharhiniform, a semiochemical extraction of the order Carcharhiniformes repels a Lamniforme, a semiochemical extraction of the order Orectolobiform repels a Carcharhiniform and a semiochemical extraction of the order Squaliform repels a Carcharhiniform. Likewise, a semiochemical extraction of the order Carcharhinoform conspecifically repels a Carcarhiniform. (See Table 1).
[0176] The repellents and methods describe herein provide the artisan with chemicals that have been demonstrated to repel, at very low concentrations, families of shark known to migrate in shallow coastal waters and species known to attack humans. In contrast to earlier ineffective chemicals, the inventors have discovered and herein disclose an effective semiochemical repellent shown to result from decomposing shark tissue. Controlled studies of these substances have shown that feeding is halted in a variety of species of sharks at low concentration. The present invention overcomes the hazards of earlier-tested noxious chemicals such as the nerve toxin VX by producing a safe-to-handle extract that does not to harm or kill sharks, does not harm humans and does not harm other marine organisms. Further, it is reported herein that teleost fishes, such as the Yellowfin Tuna, demonstrate no aversive response in the presence of repellents of the invention. Only sharks appear to be deterred by the compounds.
III. METHODS AND DEVICES OF DELIVERY OF REPELLENT
[0177] Once prepared using the methods of the invention, a semiochemical repellent may be delivered to the environment of an elasmobranch through a variety of methods and devices of delivery. Alternative non-limiting embodiments of methods of and devices for delivering a semiochemical repellent into an elasmobranch environment include an extendable pressurized delivery device such as a pole with a pressurized discharge tube for safe delivery to stimulated sharks during scientific inquiry, a pressurized repellent gun, a miniature pressurized repellent gun to be worn on the wrist or ankle, a spear fishing gun with an adjacent repellent cylinder, a time release sponge-material attached to a surfboard or otherwise placed near a diver, swimmer or in some other place of interest, a hollow surfboard with a calibrated drip to emit repellent, a pump delivery system affixed to a surfboard, a pressurized delivery device affixed to a surfboard wherein discharge of repellent may be triggered by the surfer, a floatation device, a wristwatch filled with repellent (pressurized or unpressurized), a carbon dioxide activated pressurized syringe, an aerosol bomb, a mortar-launched aerosol bomb, a remote-controlled buoy with a repellent tank that may be fired by a lifeguard or other person or mechanized system, a buoy with a metering pump that runs during swim time (daylight), a repellent pouch attached to longlines (muslin/burlap bags) or to clothing or surfboard or other water device, jellied repellent (glycol ether/hydroxypropylcelluose gels which time-dissolve in water), sunscreen/sun care formulations containing repellent, lotions containing repellent, porous fabric impregnated with repellent, rechargeable porous fabric impregnated with repellent, a kite- or balloon-deployed repellent bomb (remote control), a submerged repellent mine (remote control) for deeper water, a cattle-treatment drench gun converted to shark repellent gun (http://www.dr-register.com/drenchgun.htm), repellent-impregnated cable insulation and cable jackets for undersea lines.
[0178] Exemplary, non-limiting devices for and methods of administering elasmobranch repellents are discussed in detail in the following section.
[0179] A variety of delivery devices known in the art are illustrated in FIG. 24. For example, semiochemical repellent may be discharged through a pressurized tube that runs alongside an extended or extendable poll. (FIG. 24A.) The pressurized delivery pole apparatus may be useful for administering repellent to feeding or otherwise stimulated sharks. The apparatus may comprise a delivery device housing (pole) (310) with a repellent discharge tube (320) housed along or within the pole. The repellent discharge tube may be connected to a pressurized chamber or chambers (340) containing repellent (360). The delivery device may contain a check valve (370) to facilitate the maintenance of pressure. A trigger (350) may allow the pressurized repellent to discharge through the tube (320) and away from the pole (310). The pole may also contain a hook (330) or other device for presenting bait or other stimulant to the sharks at the end of the pole (310). During experimentation, the tube may be connected to more than one chamber (340) containing more than one experimental repellent solution (360). An alternative delivery device may be a pressurized syringe. (FIG. 24B.) Such a syringe (410) may be filled with repellent (450). It may have a plunger (420) to provide pressure and optionally to expel the solution from the syringe. It may also comprise a trigger (450), a check valve (470), a pressure release cap (490) and a nozzle (425). When the plunger is pressed, the cap (490) pops off the syringe and the pre-pressurized repellent is expelled in a pressurized stream. A commercially available delivery device is a cattle-treatment "drench" gun converted into a shark repellent gun comprising a reservoir (401) for repellent (402) and plunger (404) with reservoir filling handle (407), a trigger (405) and a discharge tube (406). (FIG. 24C.) The drench gun may be obtained from Dr. Register & Associates, 1513 5th Ave., East Menomonie, Wis. A cattle-treatment drench gun may be used to deliver a pressurized stream of semiochemical repellent in accordance with the present invention.
[0180] A. Pressurized Container Delivery Device
[0181] An exemplary and non-limiting semiochemical delivery device in accordance with the invention is a pressurized container comprising semiochemical. The container preferably may be of sufficient size to contain, and likewise comprise, sufficient repellent for at least one delivery of semiochemical sufficient to evoke a flight response in a shark, e.g., an aerosol can filled with repellent. (See FIG. 25.) The container may be constructed of degradable material. A non-limiting pressurized aerosol container (10) for administration of semiochemical repellent to a shark environment in accordance with the present invention may comprise a pressurized container (11) with sufficient tensile strength for pressurization and preferably sufficient capacity to hold a sufficient amount of semiochemical repellent (50) to repel at least one elasmobranch upon administration to water. The container may preferably be of sufficient size to be held comfortably in the human hand such that it could be thrown or released into water from the human grasp. The pressurized delivery device (10) may further comprise compressed gas (40) sufficient to expel the semiochemical repellent (50) contained therein. The container is preferably asymmetrically weighted having a weight (13) at the top (12) or base (14) portion of the container. The position of the weightedness of the container may be varied throughout the shape of the delivery device. The device may further comprise a nozzle (22) that is preferably a directional discharge nozzle. The device may further comprise an actuator (21) that when engaged allows the compressed contents of the aerosol container to be expelled. The device further preferably comprises a continuous discharge apparatus (20) to allow the contents of the can to be expelled with a single activation of the discharge apparatus (20). Preferably, when the actuator (21) is engaged, the nozzle (22) remains open to allow the can to be continuously and fully evacuated. The actuator preferably cannot be casually disengaged once engaged. The device (10) preferably floats. When the actuator (20) is engaged and the container (10) is disposed in water (60) the combination of continuous discharge, asymmetrical weight and motion of water allows the container (10) to move erratically on the surface of the water while spraying a cloud (61) of repellent into the water and placing a mist of repellent in the air (62) just above the surface of the water (60). Cloud dispersion, as used in this specification, includes dispersion in the air or water wherein the repellent is delivered as a liquid, mist, spray or foam. The directional movement of the device (10) may be alternatively manipulated by moving the relative positions of a weighted portion (13) of the container. As illustrated, the container (10) should discharge repellant proportionately more in the water than in the air since the weight (13) is in vicinity of the actuator (20). Movement may also be altered by altering the shape (15) of the container or by altering the direction of the discharge of the nozzle (22). For example, the canister may be in the shape of a ball, thereby limiting the impact of axial rotation, and the direction of discharge may be positioned to discharge along the canister axis, thereby limiting the impact of medial rotation.
[0182] Erratic motion may be created by several characteristics of a pressurized container, each characteristic representing a non-limiting alternative of a delivery device in accordance with the invention. In a non-limiting preferred alternative, an aerosol container of the invention floats. This allows erratic movement on the surface of the water as the repellent is expelled at or near the surface of the water. In another non-limiting alternative, the container does not float. It sinks into the water and repellent is discharged directly into the water where it provides a high concentration of the repellent in a desired place.
[0183] In a non-limiting alternative, the container is cylindrically shaped such that it will spin axially and medially while the repellent is expelled. Spinning rapidly may lure sharks while spraying repellent in a wide area. In another alternative, the delivery device may have more than one nozzle such that repellent may be released in more than one direction at once.
[0184] In a non-limiting configuration, the device is heavier on an end of the container not comprising a nozzle. In a non-limiting preferred configuration, the device is heavier on an end of the container comprising a nozzle. When the container with pressurized repellent is placed in water, the weightier end initially sinks into the water and directs the nozzle into the water. When discharged into the water, the force of expulsion drives the nozzle into the air. When discharged into the air, the repellent travels considerably farther before settling to the surface of the water than it would after direct discharge into the water. In another non-limiting preferred configuration having a weightier nozzle end, when initial discharge occurs into the water, the pressure from the discharge drives the nozzle into the air. When the nozzle reaches the surface of the water, the combination of weightedness and pressure from discharge drive the nozzle to the water. A series of these opposing forces results in discharge of the semiochemical over a wide range, covering a large arc both in the air and in the water. Such an asymmetrical embodiment of a container would move more erratically in the water as the volume of pressurized repellent is released than would a symmetrical embodiment. Erratic movement is created by, among other things, the pressure of the released repellent acting against the weight of the nozzle-end of the container and the buoyancy of the container floating in the waves of the body of water. (See FIG. 25.) The erratic motion also acts as an attractant to sharks and serves as a mechanism to distract sharks away from swimmers or other endangered things and than to repel the shark from the surrounding area by directly exposing the shark to a concentration of repellent near the container.
[0185] B. Mortar-Launched Aerosol Bomb
[0186] A non-limiting delivery device of the invention comprises delivery of a pressurized container or a pouch containing repellent of the invention into the ocean from a mortar tube activated with compressed gas. (FIG. 26.) In one aspect of the delivery device, the container is an aerosol container (110) and is placed in a mortar tube (115) with a compressed-gas-mortar-charging-device (116) beneath the container (110). Activation of compressed gas (170) launches the canister in an arc toward a desired elasmobranch environment (163). The actuator (121) is triggered by dissolution of the actuator plug (123) when the canister encounters the water. In a preferred embodiment, dissolution of the actuator plug (123) actuates discharge of the repellent within several seconds. The mortar tube allows access to elasmobranch environments that are not immediately otherwise accessible. Discharge of the mortar tube propels the container over a distance toward an area where a shark may be expected or detected.
[0187] C. Raft/Buoy Delivery Device
[0188] Another non-limiting delivery device of the invention comprises a raft (201) or other floating or fixed device comprising a floating buoy (280), a solid platform (281), and a container (211) of repellent (250) connected to a pump (270) with a power source (not shown) that is capable of delivering repellent into a shark environment either by automatic timing, remote triggering or other actuating mechanism (271). The container (211) comprises a check valve (215) that allows the pump (270) to build pressure in the container (211) to a desired pressure. When a desired pressure is achieved, a release valve (223) or pressure-release cap releases the pressurized repellent (250) into a delivery tube (217). The repellent is expelled across the water, spreading a wide cloud of repellent. (FIG. 27.)
[0189] The pump may be automatically activated by a timer or may be activated remotely. The pump preferably delivers sufficient repellent into the water to repel sharks. Preferably, the discharge tube is long enough and not submerged such that when delivery begins, the repellent is sprayed a substantial distance onto the surface of the water and, under pressure, the discharge tube (217) moves erratically across a large radial area in relation to the raft (201). In a preferred embodiment the discharge tube is made of flexible material. Preferably the discharge tube will spray over an entire 360 degree arc.
[0190] A specific non-limiting preferred device in accordance with the invention is a raft/buoy that holds 2 liters of repellent. (See FIG. 27.) The raft is anchored, e.g., at a sandbar or a region where a shark might enter a shallow swimming area. If a shark is spotted by a lifeguard, the lifeguard would hit a remote control button. At the buoy or raft, a radio receiver switches on the air pump. Air is pumped quickly into the 2 liter plastic tank, which has a check valve to allow fast buildup of head pressure. Once enough pressure builds up, a cap on the delivery tube pops off of the tube, spraying repellent multi-directionally at about 20-30 psi.
[0191] D. Hand-Held Pressurized Discharge Delivery Device
[0192] A non-limiting delivery device in accordance with the invention is a delivery device and method of delivery of semiochemical repellent using a pressurized directional device. (See FIGS. 28 and 31.) The pressurized directional device comprises a sufficiently sized container for repellent to provide sufficient repellent to the environment of a shark to evoke a flight reaction. The pressurized directional device further comprises a pressurizing mechanism such as a pump or a compressed gas cylinder through which a pressure may be placed on the container of semiochemical repellent to expel the repellent. The pressurized directional device further comprises a discharge nozzle that preferably focuses a stream of semiochemical repellent in a particular direction under pressure when the repellent is expelled from the pressurized chamber. The pressure in the container of repellent is maintained, for example, with a check valve. The pressurized directional delivery device further comprises a mechanism for releasing the pressurized repellent through the delivery nozzle, such as a valve or cap that releases at a prescribed pressure or upon trigger by the user. In a non-limiting alternative, the gun is fitted with backpack straps (595). (See FIG. 31.)
[0193] A specific non-limiting semiochemical delivery device in accordance with the invention may also comprise a semiochemical repellent gun (510). (FIG. 28.) The gun may have one or more chambers (520) for repellent (550), each chamber connected to at least one source of compressed air (540) through a check valve (515). The end of the chamber may have a capped directional outlet (560). When the compressed air is introduced through the check valve (515) and the cap (523) is sprung from the end of the gun, the repellent (550) in the chamber is expelled in the direction of a shark or the environment of a shark. The discharge nozzle may be connected to a tube of any length to discharge the repellent over any length necessary to deliver the repellent into a desired environment.
[0194] E. Repellent Dispersing Pouch
[0195] A non-limiting semiochemical delivery device in accordance with the invention (see FIG. 28) also comprises a pouch (610) containing repellent (650). Repellent may be in the form of a solution or solid, preferably partly or wholly soluble. The repellent may be introduced to the environment of the shark by diffusion or by rupturing (655), tearing or otherwise penetrating the pouch. A pouch may also diffuse (656) repellent through its fabric. A diffusing pouch may be attached to a fishing net or longline (690) with a baited hook (691) on a snood (693) to allow repellent to slowly diffuse (656) into the water surrounding bate (692) or fishing net. The pouch will provide sufficient repellent around the baited hook to repel sharks while not repelling the desired teliost fish. A pouch to be placed on a longline may preferably be constructed of muslin or burlap.
[0196] F. Longline Fishing Delivery Device
[0197] Sharks represent a significant problem in the long line fishing industry. Commercial longline fishing operations routinely target swordfish and tuna, however, the hook is not selective, and sharks are sometimes caught in greater numbers than the intended catch. A non-limiting method of delivery of semiochemicals in accordance with the invention is a mass or part or piece of decayed elasmobranch treated with a polar solvent.
[0198] Semiochemicals on longlines in accordance with the invention are preferably soluble in seawater, and, at a sufficient concentration to produce flight responses in elasmobranches. Teleost fish are not affected by the semiochemicals. It is theorized that this phenomenon is determined by receptor specificity. Yellowfin tuna (T. albacares) and six species of reef fish were observed to feed directly in a cloud of the semiochemical.
[0199] Since shark-repelling semiochemicals can be derived from decayed shark carcasses, sections of an actual shark carcass are utilized in accordance with a non-limiting aspect of the invention to control by-catch. Small pieces of the carcasses, which have been aerobically decayed and exposed to polar solvents, are suitable as a source of semiochemicals and also remain on a hook for considerable periods of time. The piece of decayed polar solvent treated carcass is applied to the hook along with standard bait or attractant, such as mackerel or squid, in approximately equal mass. Therefore, each hook contains two pieces of material: an attractant for fish and sharks, and a shark repellent. Since the target fish do not detect the shark-repelling semiochemicals, they are likely to navigate to the attractant/bait and strike the hook. However, a shark navigating the odor plume towards the hook will continue to experience an increasing concentration of the semiochemical and will find the bait less attractive. The bait will therefore be avoided by sharks but attracted by commercially valuable fish.
[0200] When producing semiochemicals by extraction, it is desirable to utilize blue shark (Prionace glauda) carcasses, since this species presents the largest by-catch in commercial longline fisheries. Two adult blue carcasses are sufficient to produce at least 200 hooks worth of repelling mass. As a result, the carcass of two blue sharks has the potential to spare the lives of 198 other sharks.
[0201] The decayed polar solvent treated shark carcass must not be employed before the proper semiochemicals have been produced. A freshly-killed shark carcass, for example, serves as an attractant for other sharks. Even carcasses which have been decaying for days may not possess the proper flora of semiochemicals. Decay conditions must be carefully controlled. For example, anaerobically-decayed carcasses are not suitable. Also, most non-polar solvents kill or inhibit sufficient bacterial and enzymatic reaction necessary to produce semiochemicals. Therefore, the manufacturer must possess the proper analytical tools in order to detect the presence of semiochemicals.
[0202] Once semiochemicals in sufficient abundance are detected, the decay process may be halted either by lowering temperature, immersion in solvents for preservation until use, or by filtering the extraction. If catabolism continues unchecked, all tissue will be putrefied and the semiochemical compounds will be catabolized into other products. Usually, the detection of large quantities of uric acid signals that catabolism has progressed too far.
[0203] The mass of decayed shark carcass ranges from 40 g to 200 g, practically, but may be expanded to 10 g to 500 g in order to match the mass of the attractant bait used. Larger quantities of the decayed matter are typically used when whole mackerel are deployed as the bait.
[0204] It is desirable to encase the individual masses of decayed shark carcass in a disposable container or slow-dissolving polymer matrix which activates in water, such as a high molecular-weight DOW CHEMICAL POLYOX. Properly-decayed shark carcasses may also undergo a secondary chemical treatment which introduces other repellent compounds into the tissue. For example, COMPOSITION 3M4, produced by SHARKDEFENSE LLC, is a gustatory repellent in sharks. The decayed matter may be treated with a solution of dimethylsulfoxide and COMPOSITION 3M4, thereby impregnating the decayed matter with a second potent repellent.
[0205] Another non-limiting alternative comprises a tube extending the length of the longline comprising discharge tubes at each snood. (See FIG. 30.) A pump may meet sufficient repellent to each discharge tube to repel sharks. Another embodiment comprises the structure of FIG. 30 over a relatively small distance, such as 20 feet. This embodiment is especially useful for research related to shark repellents. This embodiment may also be applied, for example, to buoys surrounding, e.g., a swimming area.
[0206] Repellent may also be applied along the entire longline by brushing or soaking prior to placing the longline into the water. Likewise, the longline may comprise porous material that will allow adsorption of repellent and discharge of said repellent over time. In another non-limiting delivery device for longline fishing in accordance with the invention, semiochemicals or a mass of carcass comprising semiochemicals may be affixed to a net or other kind of fishing tackle.
[0207] A non-limiting semiochemical delivery device in accordance with the invention may also comprise an apparatus for administering repellent along longline fishing tackle. (FIG. 30.) The apparatus (710) comprises a pressurized chamber (720) connected to a source of compressed gas (740), contains repellent (750) and is connected to a primary delivery tube (717). The primary delivery tube is positioned adjacent to or otherwise in concert with the longline (718). Additional secondary delivery tubes (719) are joined to the primary delivery tube (717) in proximity to each snood (793) of the longline. The secondary delivery tubes terminate near the baited hook (791) of the snood. When pressurized repellent is released from the chamber (720), the repellent is delivered along the primary delivery tube (717) and into the secondary delivery tubes (719) thereby discharging repellent (750) near the baited hook (791) and repelling sharks from the bait.
[0208] G. Backpack Pressurized Delivery Device
[0209] A non-limiting semiochemical delivery device in accordance with the invention may also comprise a backpack (595) repellent delivery device (500) comprising two chambers (520) of pressurized repellent (550) and a nozzled spray gun (510). (FIG. 31.) The backpack may be worn by scuba divers or snorkelers or other and may provide two or more charges of elasmobranch repellent while diving or snorkeling without resort to a repellent source on shore.
[0210] H. Spear Gun with Pressurized Delivery Device
[0211] A non-limiting semiochemical delivery device in accordance with the invention may also comprise a spear gun (845) further fitted with a repellent gun (810), as described in section D above. (FIG. 32.)
[0212] I. Surfboard Fitted with Delivery Device
[0213] A non-limiting semiochemical delivery device in accordance with the invention may comprise a surfboard comprising a hollow chamber for containing semiochemical repellent. FIG. 33A illustrates a specific non-limiting embodiment of surfboard with a pressurized chamber that is discharged by the surfer in an emergency. A surf board (910) comprising a pressurized chamber (920) for repellent (950) comprising a source of compressed gas (925) for expelling repellent (950) through a release valve (984) and into a discharge tube (919) in, for example, an elasmobranch emergency situation. Discharge of repellent may be triggered by a surfer via a remote control triggering device (971) or with an ankle-band triggering device (972) or wrist-band triggering device. In another specific and non-limiting embodiment, the discharge tube (919) allows repellent to be periodically introduced into the environment of the surf board via a drip valve (983). In such an alternative, the chamber (920) need not be held under pressure and no source of compressed gas is necessary. Instead, the repellent may be allowed to leak through the drip valve (983) by supplying, for example, a source of air or vent (927) in a cap or other sealant (928) of a reservoir-filling end (926) of the chamber (920). FIG. 33B illustrates such a surfboard with a chamber for containing repellent (920) a drip valve (984) a vent (927) and a discharge tube (919) for continuous discharge of repellent (950) during surfing. A chamber alternatively may be strapped to the side of the surfboard. A further alternative comprises a plastic container drilled into the surface of the surfboard. One or more than one discharge tube is contemplated.
[0214] J. Wristwatch Delivery Device
[0215] FIG. 34 illustrates a non-limiting delivery device in accordance with the invention comprising a wristwatch (1010) and further comprising a repellent chamber or container (1020). In a non-limiting aspect in accordance with the invention the chamber is pressurized. Repellent is released from the chamber by activating a trigger (1021). In a specific non-limiting embodiment another non-limiting aspect in accordance with the invention a cap is removed. In another specific alternative embodiment the chamber is ruptured with a knife or by applying pressure.
[0216] K. Belt or Bracelet Delivery Device
[0217] FIG. 35 illustrates a non-limiting delivery device attached to a belt (FIG. 35A) or bracelet (FIG. 35B) and further comprising pressurized repellent in accordance with the present invention. A specific non-limiting embodiment alternative comprises a wristband (1101) or belt (1102) with a repellent gun (1103) with a chamber (1110) containing pressurized repellent (1150), a source of compressed gas (1170) a check valve (1115) a trigger (1197) and a nozzle with a pressure release valve (1122) to discharge the repellent into the environment of the wearer of the wristband and preferably in a desired direction.
[0218] The invention is further described with the following non-limiting examples, which are provided to further illuminate aspects of the invention.
IV. EXAMPLES
Example 1
Preparation and Testing of Semiochemical GWH
Example 1A
Preparation of Semiochemical GWH from Order Lamniformes
[0219] GWH was aerobically prepared from the head of a great white shark (C. carcharias, Order Lamniformes) in a polypropylene extraction vessel. The carcass head was allowed to decay aerobically for 10 days in a covered polypropylene container. The carcass head was then fully immersed in solvent in a polypropylene extraction vessel. The extraction solvent was 50:50 water:solvent, by weight. The solvent was 80% methanol, 17% ethanol, and 3% methyl isobutylketone. Extraction time was 6 months at 25[deg.] C. with slow agitation (container was shaken or stirred during sampling intervals). The extraction was periodically sampled by HPLC in accordance with the above described method. After several months signature peaks were noted at about 5, about 6 and about 7 minutes. The extraction process was terminated by filtering to remove tissue. The resulting filtrate was containerized in a polypropylene container. Extraction time was 6 months at room temperature. The carcass processed for semiochemical GWH was obtained from the U.S. Government (National Oceanic and Atmospheric Administration Fisheries Service-Galveston, Tex. (USA)), which had frozen the great white shark carcass after it had been caught as bycatch.
Example 1B
Testing of Semiochemical GWH for Repellent Activity
[0220] GWH was tested for repellent activity against blacknose sharks (C. acronotus Order Carcharhiniformes) and Caribbean reef sharks (C. perezii Order Carcharhiniformes) present in a population of 9 sharks. The target sharks were stimulated with bait. A 500 mL dose of GWH was introduced to the shark population as a cloud. The sharks were visibly repelled from the feeding zone. (See Table 1).
Example 1C
UV-Vis Spectrum of Semiochemical GWH
[0221] GWH was spectrophotometrically analyzed in the uv-visible range. A dual-beam Perkin Elmer Lambda 12 model scanning spectrophotometer was used. Neat semiochemical solutions were micron-filtered and loaded into quartz cuvettes. Representative uncontaminated solvents were used in the extraction process, at the same ratios used to perform the extraction, were used as a reference sample. The resulting spectrum is contained in FIG. 2 and labeled GWH. A distinct and strong absorbance peak is observable between about 300 nm and about 340 nm.
Example 1D
HPLC Chromatogram of Semiochemical GWH
[0222] A chromatograph of GWH was created to determine the chromatographic signature of active components of GWH. (See FIGS. 3 and 4.) HPLC parameters were:
[0223] Solvents: (1) Methanol and 0.1% acetic acid; (2) Water and 0.1% acetic acid
[0224] Ternary HPLC Pump: Gradient control
[0225] 0-10 minutes: 100% methanol/acetic acid
[0226] 10-12 minutes: Linear gradient to 100% water/acetic acid
[0227] 12-20 minutes: 100% water/acetic acid
[0228] 20-22 minutes: Linear gradient to methanol/acetic acid
[0229] 22-40 minutes: 100% methanol/acetic acid
[0230] Column: Waters Novapak C18 RP 3.9*150 mm with guard column
[0231] Column heater: 25 C
[0232] Detection: 240 nm-340 nm
[0233] Injector: 50 uL loop.
[0000] The early eluting chromatograph contained signature peaks at around 5, around 6 and around 7 minutes, respectively. (See FIG. 3.) The late eluting chromatogram contained the signature peaks at around 32 minutes, around 34.5 minutes, around 36.5 minutes and around 42 minutes. (See FIG. 4.)
Example 1E
HPLC Chromatogram of Ninhydrin Derivatized GWH
[0234] Semiochemical GWH was derivatized with 0.1 g ninhydrin at 40[deg.] C. for 15 minutes. The derivatized GWH was then subjected to HPLC analysis with detection at 570 nm to detect primary amines. (See FIG. 5.) The resulting chromatogram had a strong peak at around 7 minutes and two weaker peaks at around 5 and around 6 minutes, respectively.
[0235] Derivatized GWH was also subjected to HPLC analysis with detection at 440 nm to detect secondary amines. (See FIG. 6.) The resulting chromatogram demonstrated a first strong and sharp peak around 34 minutes and a strong broad peak with two components eluting about 2 minutes later.
Example 1F
GC-MS of Semiochemical GWH
[0236] Tests of GWH were run on Direct Injection GC-MS. The GWH semiochemical was injected neat into a Hewlett Packard model 6890 GC with 5973 MSD in accordance with the parameters on the chromatogram. (See FIG. 7.) Analysis of the resulting mass spectrogram using NIST 98.1 provided the following non-limiting components of GWH: glycerin, N,N-dimethylurea, urea, 5-methyl-2,4-imidazolidinedione (5-methylhydantoin), creatinine, methyl hexadecanoate (methyl palmitate), hexahydro-3-(2-methylpropyl)-pyrrolo, [1,2-a]pyrazine-1,4-dione. See Table 4.
Example 2
Preparation and Testing of Semiochemical CP from Order Charcharhiniformes
Example 2A
Preparation and Repellent Testing of Semiochemical CP
[0237] CP was aerobically prepared from the head of a Caribbean reef shark (C. perezii Order Charcharhiniformes). The carcass head was processed in the manner described above for semiochemical GWH.
[0238] CP was tested for repellent activity against blacknose sharks and Caribbean reef sharks present in a population of 12 sharks. The sharks were stimulated with bait. An aerosol canister containing 6 fluid oz. of CP was then introduced to the 12 sharks. All sharks were visibly repelled from the feeding zone. (See Table 1). In three ensuing tests delivery of semiochemical CP from an aerosol canister again repelled competitively-feeding blacknose and Caribbean reef sharks.
Example 2B
UV-Vis Spectral Analysis of Ninhydrin-Derivatized CP
[0239] Semiochemical CP was derivatized with 0.1 g ninhydrin at 40[deg.] C. for 15 minutes. A uv-visible spectrogram was determined on a dual-beam Perkin Elmer Lambda 12 model. Neat CP was micron-filtered and loaded into quartz cuvettes. Representative uncontaminated solvents used in the extraction process, at the same ratios used to perform the extraction, were used as a reference sample.
[0240] The absorbance spectra of semiochemical CP derivatized with ninhydrin provided clear maxima observable at 440 nm (around 4 AU) and 570 nm (2.9 AU). (See FIG. 8.) With ninhydrin derivatized extracts, 440 nm absorbance indicates secondary amines and 570 nm absorbance indicates the presence of primary amines. When primary and secondary amines are not present, and the sample is derivatized with ninhydrin, absorbances at 440 nm and 570 nm are not observed. A uv-visible spectrum of 50% w/w ammonium acetate (a discredited shark repellent) in water, derivatized with 0.1 g ninhydrin at 40[deg.] C. for 15 minute showed no maxima at 440 nm or 570 nm. (See FIG. 9.)
Example 2C
GC-MS of Semiochemical CP
[0241] CP was tested with Direct Injection GC-MS. The CP semiochemical was injected neat into a Hewlett Packard model 6890 GC with 5973 MSD operating in accordance with the parameters on the chromatogram. (See FIG. 10.) Analysis of the mass spectrogram using NIST 98.1 resulted in the following non-limiting components of semiochemical CP: glycerin, N,N-dimethylurea, urea, 5-methyl-2,4-imidazolidinedione (5-methylhydantoin), creatinine, hexahydro-3-(2-methylpropyl)-pyrrolo[1,2-a]pyrazine-1,4-dione, 2,3-butanediol, N-N-dimethylformamide, 2-butoxyethanol, DL-methyltartronic acid, 1,4-dimethyl-piperazine, 2-(1,1-dimethylethoxy)-thiophene, hexahydro-pyrrolo[1,2-a]pyrazine-1,4-dione.
Example 3
Preparation and Testing of Semiochemical A1 from Order Carcharhiniformes
Example 3A
Preparation of Semiochemical A1
[0242] Semiochemical A1 was aerobically prepared from the carcass of a lemon shark (N. brevirostris) a nurse shark (G. cirratum) and a spiny dogfish (S. acanthias) (each species in Order Carcharhiniformes). The carcasses were allowed to decay aerobically for 10 days in a covered polypropylene container RT. The carcasses were then fully immersed in solvent in a polypropylene extraction vessel. The extraction solvent was 50:50 water:solvent, by weight. The solvent was 80% methanol, 17% ethanol, and 3% methyl isobutylketone. Extraction time was 6 months at 25[deg.] C. with slow agitation. The extraction was periodically sampled and terminated after components of the extraction eluted from HPLC at the signature peaks of about 5, about 6 and about 7 minutes. The extraction process was terminated by filtering to remove tissue. The resulting filtrate was containerized in a polypropylene container.
Example 3B
Testing of Semiochemical A1 for Repellent Activity
[0243] Semiochemical A1 was tested for repellent activity against blacknose sharks and Caribbean reef sharks present in a population of 15 sharks. The sharks were stimulated with bait. A 500 mL dose of A1 was introduced as a cloud to the 15 sharks. The sharks were visibly repelled from the feeding zone. (See Table 1).
Example 3C
HPLC Chromatograph of Semiochemical A1
[0244] A chromatograph of A1 was created to determine the chromatographic signature of active components of the A1 extract. (See FIGS. 3 and 4.) HPLC parameters were the same as above for GWH. The early eluting chromatogram contained signature peaks at around 5, around 6 and around 7 minutes, respectively. (FIG. 3.) The late eluting chromatogram contained the signature peaks at around 31, around 34, around 36 and around 42 minutes. (See FIG. 4.)
Example 3D
HPLC Chromatogram of Ninhydrin Derivatized A1
[0245] Semiochemical A1 was derivatized with 0.1 g ninhydrin at 40[deg.] C. for 15 minutes. The derivatized A1 was then subjected to HPLC analysis with detection at 570 nm to detect primary amines. (See FIG. 5.) The resulting chromatogram had a strong peak at around 7 minutes, a weaker peak at around 6 minutes and a very weak peak at around 5 minutes.
[0246] Derivatized A1 was also subjected to HPLC analysis with detection at 440 nm to detect secondary amines. (See FIG. 6.) The resulting chromatogram demonstrated a strong and sharp peak around 39 minutes and a strong broad peak with two components eluting about 2 minutes later.
Example 4
Preparation and Testing of Semiochemical A2 from Multiple Orders
Example 4A
Preparation and Repellent Testing of Semiochemical A2
[0247] Semiochemical A2 was aerobically prepared using the method described for GWH above from two lemon shark carcasses (N. brevirostris), one nurse shark carcass (G. cirratum), and one spiny dogfish carcass (S. acanthias) (orders Carcharhiniformes, Orectolobiformes, and Squaliformes, respectively).
[0248] A2 was tested for repellent activity against blacknose sharks and Caribbean reef sharks present together in a population of 12 sharks. The 12 sharks were stimulated with bait. A 500 mL dose of A2 was introduced as a cloud to the 12 sharks. The sharks were visibly repelled from the feeding zone. (See Table 1).
[0249] A2 was tested for repellent activity against lemon shark (N. brevironstris Order Carcharhiniformes). One lemon shark was successively placed in a state of tonic immobility and successively subjected to administration of A2 in a range from 7 mL to 30 mL via a syringe. Each administration resulted in the termination of tonic immobility. (See Table 1). A2 was also tested against lemon shark in a diluted form. 30 mL and 60 mL of 0.1 ppm semiochemical A2 (diluted with HPLC-grade water) was introduced to a tonically immobile lemon shark. Tonic immobility was terminated with the dilute repellent. These data support a conclusion that the semiochemical A2 will meet the goal of the Johnson-Baldridge effective repellent concentration of 0.1 ppm.
[0250] During tonic immobility studies, the semiochemical was delivered using a plastic syringe, which was not in contact with the specimen. The test solutions were released within 3 inches of the specimen's nose. Controls were established using separate syringes with seawater. Some controls were released with a high flow rate (30 mL/sec) in order to establish that sharks were not awakened by the jet of fluid over their noses.
[0251] A2 was also tested for repellent activity against bull shark (C. leucas Order Carcharhiniformes). (See Table 1). Bull shark is considered the most dangerous inshore species of shark. Two sharks were stimulated with bait and subjected to 500 mL of A2 in a cloud. The sharks were visibly repelled from the feeding zone.
[0252] A2 was tested using the Johnson-Baldridge Test with blacktip sharks (C. limbatus Order Carcharhiniformes). (See Table 1). A PVC tripod with a peristaltic metering pump set to meter out 1 mL/min of A2 repellent, a video camera and a transmitter was situated in the ocean. A 6 cubic meter observation area under the tripod was marked off and compensated for tidal changes. A fish head was secured under the tripod, within view of the camera. In a series of control-only experiments, solvent was pumped into the observation area at the prescribed flow once a blacktip shark was present. Thereafter, in a series of treatment experiments, a fish head was secured, the pump was started, and the behavior of one blacktip shark was observed. The fish head was protected for one hour in the presence of the stimulated shark until the battery of the pump was exhausted.
Example 4B
UV-Vis Spectrum of Semiochemical A2
[0253] A uv-visible spectrum of one-year-old A2 was compared with spectra from one-year old A13N and one-year-old SQ1. All three of these extracts demonstrated good flight responses in target sharks. The three spectra together demonstrate matching strong peaks in the 300 nm range. (See FIG. 11.)
Example 4C
Head Space-GC-MS and Proposed Components of A2
[0254] A 10-mL aliquot of shark fluid was placed in a 100-mL headspace vial and capped with a Teflon butyl rubber septa. The vial was allowed to equilibrate at 30[deg.] C. overnight prior to analysis. The sample headspace was injected into a Hewlett Packard model 6890 GC with 5973 MSD operating under the following system conditions.
[0000]
Column: DB-5 40 m * 0.18 mm * 0.40 [mu]m film
Carrier: helium @ 1 mL/min
Injection: 10 cc manual cryo, split 25:1 @ 250[deg.] C.
Oven: 40[deg.] C. to 280[deg.] C. @ 10[deg.] C./min
Trans. Line: 280[deg.] C.
MSD: Scan 20-500 m/z.
A total ion chromatogram from Head Space Gas Chromatographic-Mass Spectrometric analysis of the semiochemical repellent A2 is shown in FIG. 17. Peaks are labeled with proposed chemical components of A2.
[0255] Components identified by headspace in combination with direct injection GC-MS are shown in Table 2. The components were identified with the aid of the NIST 2002 mass spectral search database and are tentative. Structures are proposed and are not intended to be limiting on the structure or makeup of the obtained semiochemical solution A2.
Example 4D
Direct Injection GC-MS and Proposed Components of A2
[0256] Semiochemical A2 was injected neat into a Hewlett Packard model 6890 GC with 5973 MSD operating under the following system conditions.
[0000]
Column: DB-5 40 m * 0.18 mm * 0.40 [mu]m film
Carrier: helium @ 1 mL/min
Injection: 2 [mu]L, splitless @ 280[deg.] C.
Oven: 40[deg.] C. hold 5 min, to 300[deg.] C. @ 10[deg.] C./min, hold 5 min
Trans. Line: 300[deg.] C.
MSD: Scan 20-700 m/z.
A total ion chromatogram from Direct Injection Gas Chromatographic-Mass Spectrometric analysis of the semiochemical repellent A2 is shown in FIG. 18. Peaks are labeled with proposed chemical components of A2. Structures are proposed and are not intended to be limiting on the structure or makeup of the obtained semiochemical A2.
[0257] Components identified by headspace and direct injection GC-MS are shown in Table 2. The components were identified with the aid of the NIST 2002 mass spectral search database and are tentative.
[0000]
TABLE 2
Summary of GC-MS Results from Various Analyses
Headspace GC-MS Direct Injection, small Direct Injection, large
ethanol ethanol Detector filament off
acetic acid, methyl ester acetic acid, methyl ester ''
2-methylpentane ''
3-methylpentane ''
hexane ''
ethyl acetate ethyl acetate ethyl acetate
acetic acid
2,4-dimethylpentane
2-methyl-1-pentene
1-ethoxy-2-methylpropane
3,3-dimethylpentane
2-methylhexane
cyclohexane cyclohexane
3-methylhexane
1,1-dimethylcyclopentane
1,3-dimethylcyclopentane
1,2-dimethylcyclopentane
heptane heptane
2,2-dimethylhexane
methylcyclohexane
2,4-dimethylhexane
methyl isobutyl ketone methyl isobutyl ketone methyl isobutyl ketone
1,2,3-trimethylcyclopentane
2,3-dimethylhexane
2-methylheptane
toluene toluene toluene
1,3-dimethylcyclohexane n,n-dimethylurea
octane urea
1,2-dimethylcyclohexane myristic acid, methyl
ester
1,3-dimethylcyclohexane myristic acid, ethyl ester
2-methyloctane palmitoleic acid, methyl
ester
1,2,3-trimethylcyclohexane palmitic acid, methyl ester
ethylcyclohexane palmitic acid
1,1,3-trimethylcyclohexane ethyl-9-hexadecenoate
1,2,4-trimethylcyclohexane palmitic acid, ethyl ester
1,3-diethylcyclopentane 8-octadecenoic acid,
methyl ester
2,3-dimethylheptane stearic acid, methyl ester
1,2,4-trimethylcyclohexane ethyl oleate
3-methyloctane stearic acid, ethyl ester
octahydropentalene arachidonic acid
1,2,4-trimethylcyclohexane
1,2,4-trimethylcyclohexane
1-ethyl-3-methylcyclohexane
1-ethyl-4-methylcyclohexane
propylcyclohexane
Example 4E
LC-MS and Proposed Components of A2
[0258] Semiochemical A2 was diluted 1:1 (v/v) for analysis with HPLC grade water. The resulting solution was filtered through a 0.45-[mu]m Gelman Acrodisc Nylon syringe filter prior to analysis. The filtered solution was transferred to autosampler vials for analysis.
[0259] The sample was analyzed using atmospheric pressure chemical ionization (APCI) in the positive ionization mode. In addition to mass spectrometry (MS), MS'' was also employed with n equal to 3 to afford fragmentation of the parent ion. The LC-MS conditions are given below:
[0000]
Pump: Agilent 1100 Series Binary Pump with Degasser
Detectors: Agilent 1100 Multi Wavelength Detector and LC/MSD
Trap
Column: YMC ODS-AQ column, 4.6 * 250 mm with a 5-[mu]m
particle
Wavelength: 210 nm
Run Time: 20 minutes
Autosampler: 6 minute equilibration time between sample injections
Injection Volume: 10 microliter
Mobile Phase: A) 0.1% Formic acid in methanol
B) 0.1% Formic acid in water
Time Flow
(min.) (mL/min.) % A % B
Gradient Initial 1.00 5 95
5.00 1.00 5 95
15.00 1.00 00 0
20.00 1.00 100 0
[0260] A total ion chromatogram from Liquid Chromatographic-Mass Spectrometric analysis of the semiochemical repellent A2 is shown in FIG. 19. Peaks are labeled with the mass/charge (m/z) ratio of proposed chemical components of A2. Table 3, below, also contains this data.
[0000]
TABLE 3
Component Observed by LC-MS
Retention Time Observed Mass
(min) (M + 1) Comments
3.7 147.4 Strong
4.1 151.1 Strong
4.2 132.7 Strong
4.2 207.2 Strong
4.2 227.1 Strong
4.2 263.1 Strong
4.3 114.6 Weak
5.5 150.5 Weak
6.0 228.9 Weak
7.5 132.5 Weak
8.4 137.4 Weak
8.4 182.2 Weak
11.6 268.2 Weak
12.5 269.6 Strong
12.8 166.3 Strong
15.2 371.9 Weak
Note:
observed mass is report as the M + 1 ion; the molecular weights are generally one mass unit lower than the observed mass.
[0261] Based on the LC-MS data, the following structures are proposed. However, structures are proposed and are not intended to be limiting on the structure or makeup of the obtained semiochemical A2 as characterized by the LC-MS.
[0262] Compounds that were detected using LC-MS are described below. A typical total ion chromatogram generated in the study is shown in FIG. 19. Supporting data are also included in Table 3. The structures that are proposed below are tentative assignments.
[0263] Retention time 3.7 min, m/z 147.2
[0000] This unknown was tentatively assigned as
[0000]
[0264] Retention time 3.9 min, m/z 151.3
[0000] It appears that the molecular weight of this unknown is 150 and ions at M/z 189 and 227 are probably potassium adducts. However, m/z 227 may belong to a slightly later eluting component (see one of the compounds eluting at 4.2 minutes). Limited fragmentation pattern and the molecular weight would support the tentative proposed structure (II) of tartaric acid in FIG. 2:
[0000]
[0265] Retention time, 4.2 min
[0000] Last significant peak is at m/z 263 and intense fragment ions at m/z 227, 207, 189, 151, 132.7 would appear to support a tentative proposed structure (III) of dibutyl tartarate.
[0000]
[0266] The possibility also exist that the above ions are related to different compounds. There is a question also whether m/z 263 may be a protonated dimer of a component at m/z 131 (leucine, IV), and m/z 207 could be the monobutyl ester of tartaric acid. Again, peak at m/z 227 may be attributed to the next eluting component (see Structure VI below). Other structures were proposed for molecular weight 131 including creatine, 3-hydroxy-dl-proline, leucine (IV), or (V).
[0000]
[0267] Retention time 4.2 min, m/z 227
[0000] This has been tentatively assigned before as (VI).
[0000]
[0268] Retention time 7.5 min, m/z 132.7
[0000] The molecular weight appears to be 131. The components mentioned at retention time of 4.2 minutes are also possible for this component.
[0269] Retention time 11.6 min, m/z 268.2
[0000] Molecular weight of this unknown is 267. At the beginning, oleylamine or pyrroridine structure (VII) was considered for this unknown, but second derivative fragment at m/z 135.9 (M-132) could not be explained for these structures. An adenosine structure (VIII) is tentatively proposed for this unknown.
[0000]
[0270] Retention time 12.5 min, m/z 269.5
[0000] An oleyl alcohol (IX) is proposed for this unknown.
[0000]
[0271] Retention time 12.8 min, m/z 166.3
[0000] This is a nitrogen containing compound with molecular weight of 165. This compound could be phenylalanine (X). The second derivative data would support this assignment.
[0000]
[0272] Ret. time 15.2 min, m/z 372.
[0000] This compound was observed in the previous study (R03-0299). A component at m/z 330 in the first study (R03-0215) was tentatively identified. This component is 42 mass units higher than the earlier identified compound and could be due to a tentatively proposed structure (XI). Fragmentation provides loss of 18 (loss of hydroxyl as water) and another loss of 17 mass units.
[0000]
Example 4F
HPLC Chromatographs of Ninhydrin-Derivatized Semiochemical A2
[0273] Semiochemical A2 was derivatized with 0.1 g ninhydrin for two hours at 40[deg.] C. The derivatized A2 was then subjected to HPLC analysis with detection at 570 nm to detect primary amines. (See FIG. 13.) The following system configuration was used with injection volume and mobile phase as set forth on the chromatograph in FIG. 13:
[0000]
Column: C18, reversed phase
Flow rate: 1 ml/min
Column temperature: 35[deg.] C.
The method produced around 5 characteristic peaks between 1 and 2 minutes for primary amines at 570 nm. With detection at 440 nm for secondary amines and injection volume and mobile phase as set forth in FIGS. 14 and 15, the method likewise produced around 5 characteristic peaks between 1 and 2 minutes. (See FIGS. 14 and 15.) An entity at both detection wavelengths is observed at 4.8 minutes with a trace concentration.
Example 4G
FTIR Spectrum of Semiochemical A2
[0274] An FTIR analysis was performed on semiochemical A2 using a waterless sample from the water-insoluble phase described above is set on a KBr crystal. A scans from 1100 nm to 3500 nm in butanol and diethylether of a semiochemical indicated the following groups:
[0000]
2800-3000 nm Asymmetric and symmetric CH3 groups
1300-1400 nm Scissor, asymmetric, and symmetric CH3 groups
1126.00 nm C-O bond stretching
1434.56 nm C-O bond stretching
1637.28 nm C-C bond stretching
2846.60 nm C-H bond stretching
2916.50 nm C-H bond stretching
2951.46 nm C-H bond stretching
3321.94 nm OH bond stretching, indicating alcohols with the
above three stretches.
(See FIG. 16.)
Example 5
Preparation of Semiochemical CL from Order Carcharhiniformes
[0275] Semiochemical CL solution from a carcass of C. limbatus (Order Carcharhiniformes) was aerobically prepared using the method described for GWH above. During the aerobic preparation process, the extraction vessel was sampled at 0, 7, 21 and 40 days to determine development of the semiochemical uv-vis signature peak at 300 nm. (See FIG. 12.) Signature absorbance at around 300 nm increased as extraction proceeded. A 300 nm shoulder was barely perceptible at 0 days and increased throughout 7, 21 and 40 days to a distinct peak at about 40 days.
Example 6
Preparation and Testing of Semiochemicals CPP and GCC from Order Carcharhiniformes
Example 6A
Preparation of Semiochemicals CPP and GCC
[0276] CPP and GCC were aerobically prepared from the head of a sandbar shark (S. plumbeus) and the cross section behind the pectoral fins of a tiger shark (G. cuvieri), respectively. Each carcass is within Order Carcharhiniformes. The extraction process was as described for GWH above.
Example 6B
Testing of Extracts CPP and GCC for Repellent Activity
[0277] CPP was tested for repellent activity against blacknose sharks and Caribbean reef sharks present together in a population of 7 sharks. The sharks were stimulated with bait. A 500 mL dose of CPP was introduced as a cloud to the 7 sharks. The sharks were visibly repelled from the feeding zone. (See Table 1).
[0278] GCC was tested for repellent activity against the highly migratory (pelagic) non-inshore blue shark (P. glauca Order Carcharhiniformes) in a population of 2 sharks. The sharks were stimulated bait and acoustical stimulation. A 500 mL dose of GCC was introduced as a cloud to the 2 sharks. The sharks were visibly repelled from the feeding zone. (See Table 1).
Example 7
Preparation and Testing of Semiochemical A13N from Order Carcharhiniformes
Example 7A
Preparation of Semiochemical A13N and UV-Vis Spectrum
[0279] A13N was prepared by mixing, in equal parts, three previously prepared semiochemicals, A1, A3 and N. A13N contained semiochemicals from a lemon shark carcass (N. brevirostris), a nurse shark carcass (G. cirratum), and a spiny dogfish carcass (S. acanthias) (each species in Order Carcharhiniformes). A1 was prepared as described above. A3 was prepared in the same manner as A1. N was prepared from the carcass of a nurse shark using the method described for GWH above. A uv-visible spectrum of a one-year-old sample of the mixed semiochemical A13N was prepared as discussed in Example 4B above. The spectrum contained the signature strong peak in the 300 nm range. (See FIG. 11.)
Example 7B
Testing of A13N for Repellent Activity
[0280] A13N was tested for repellent activity against a blacknose shark and a Caribbean reef shark. A 500 mL dose of A13N was introduced as a cloud to the two sharks, which were presently stimulated with bait and acoustic stimulation. The sharks were visibly repelled from the feeding zone.
Example 8
Preparation and Testing of Semiochemical B from Order Lamniformes
[0281] Semiochemical B was aerobically prepared from the cross-section behind the first dorsal fin of a shortfin mako shark (I. oxyrhincus Order Lamniformes). The carcass portion was allowed to decay aerobically for 10 days in a covered polypropylene container RT. The carcass portion was then fully immersed in solvent in a glass extraction vessel. The extraction solvent was 50:50 water:acetone, by weight. Extraction time was 6 months at 25[deg.] C. with slow agitation. The extraction was periodically sampled and terminated after components of the extraction eluted from HPLC at the signature peaks of about 5, about 6 and about 7 minutes. The extraction process was terminated by filtering to remove tissue. The resulting filtrate was containerized in a polypropylene container.
[0282] Composition B2 was aerobically prepared from the cross-section behind the first dorsal fin of a shortfin mako shark in one polypropylene extraction vessel. The extraction solvent was 100% water. Extraction time was 6 months at 25[deg.] C. with slow agitation. The extraction process was terminated by filtering to remove tissue. The resulting filtrate was containerized in a polypropylene container.
[0283] B was tested for repellent activity against blacknose sharks and Caribbean reef sharks present together in a population of 12 sharks. A 200 mL dose of B was introduced as a cloud to the 12 sharks, which were presently stimulated with bait. The sharks were visibly repelled from the feeding zone. (See Table 1).
[0284] Composition B2 was likewise tested for repellent activity against blacknose sharks and Caribbean reef sharks present together in a population of 6 sharks. A 1 liter dose of B2 was introduced as a cloud to the 6 sharks, which were presently stimulated with bait. No behavioral change was noted and feeding continued. (See Table 1).
Example 9
Preparation and Testing of Semiochemicals ML1 and ML2 from Order Lamniformes
[0285] Extracts ML1 and ML2 were separately aerobically prepared from two livers of a shortfin mako shark (I. oxyrhincus Order Lamniformes) in two polypropylene extraction vessels. The livers were initially allowed to decay aerobically for 10 days in covered polypropylene containers at room temperature (RT). The livers were then fully immersed in solvent in polypropylene extraction vessels. The extraction solvent was 50:50 water:acetone, by weight. Extraction time was 6 months at 25[deg.] C. with slow agitation. The extraction was periodically sampled and terminated after components of the extraction eluted from HPLC at the signature peaks of about 5, about 6 and about 7 minutes. The extraction process was terminated by filtering to remove tissue. The resulting filtrate was containerized in a polypropylene container.
[0286] ML1 and ML2 were tested for repellent activity against blacknose sharks and Caribbean reef sharks present together in two populations of 8 sharks. The sharks were stimulated with bait. Respective 700 mL doses of ML1 and ML2 were introduced to respective shark populations as a cloud. The sharks were visibly repelled from the feeding zones. (See Table 1).
Example 10
Preparation and Testing of Semiochemical SQ1 from Order Squaliformes
[0287] SQ1 was aerobically prepared from the carcass of a deep water Cuban dogfish (S. cubensis, Order Squaliforme) in one polypropylene extraction vessel as described for GWH above. A uv-visible spectrum of semiochemical SQ1 one year after the extraction process was terminated. The spectrum was prepared as discussed in Example 4B above. The spectrum contained the signature strong peak in the 300 nm range. (See FIG. 11.)
[0288] SQ1 was tested for repellent activity against blacknose sharks and Caribbean reef sharks present together in a population of 12 sharks. A 250 mL dose of SQ1 was introduced as a cloud to the 12 sharks, which were stimulated with bait. The sharks were visibly repelled from the feeding zone. (See Table 1).
Example 11
Preparation and Testing of Semiochemicals N2 and BB1 from Order Orectolobiformes
[0289] N2 and BB1 were aerobically prepared from the carcasses of a nurse shark (C. cirratum, Order Orectolobiformes, Family Ginglymostomatidae) and a brownbanded bamboo shark (C. punctatum, Order Orectolobiformes, Family Hemiscyllidae) in separate polypropylene extraction vessels. The carcasses were initially allowed to decay aerobically for 10 days in a covered polypropylene container RT. The carcasses were then fully immersed in solvent in a polypropylene extraction vessel. The extraction solvent was 50:50 water:solvent, by weight. The solvent was 80% methanol, 17% ethanol, and 3% methyl isobutylketone. Extraction time was 6 months at 25[deg.] C. with slow agitation. The extraction was periodically sampled and terminated after components of semiochemicals eluted from HPLC at the signature peaks of about 5, about 6 and about 7 minutes. The extraction process was terminated by filtering to remove tissue. The resulting filtrate was containerized in a polypropylene container.
[0290] N2 was tested for repellent activity against a lemon shark (Order Carcharhiniformes, Family Carcarhinidae, N. brevirostris). A 10 mL dose of N2 was introduced from a syringe into the environment of the shark in a tank. Aversive swimming behavior was observed. (See Table 1).
[0291] BB1 was tested for repellent activity against a lemon shark (Order Carcharhiniformes, Family Carcarhinidae, N. brevirostris). A 10 mL dose of BB1 was introduced from a syringe into the environment of the shark in a tank. Aversive swimming behavior was observed. (See Table 1).
Example 12
Repelling of Mako Shark of Order Lamniformes
[0292] A juvenile Mako shark (Order Lamniformes, Family Lamidae, Genus Isurus) was repelled by semiochemical repellent GCC. A buoy line baited with squid, blue fish, and striped bass was in the water. Mako sharks are known to attack swordfish, and eat prey such as blue fish and striped bass when it is available for consumption. A 500 mL charge to the line under CO2 pressure was applied. Diptubes were [1/4]'' HPDE. (See FIG. 30.) The bait was not taken.
[0293] After coming in contact With the GCC, the Mako retreated and did not appear again. Subsequently, the shark could not be lured back to the site despite the application of three types of stimulants and several hours applying traditionally adequate scent and bait. After extensive attempts to re-lure the shark, only new blue sharks appeared well after the repellent would be expected to have completely dispersed. Generally, when repellent is not deployed, sharks remain in the area where scent and bait has been administered for an extended period of time (up to many hours). The failure of the shark to return after fleeing the exposure to GCC was interpreted as support for the strong action of the GCC semiochemical against the juvenile Mako shark.
[0294] The test was done under the following conditions:
Live bait: Bluefish (2)
Carcass line: Bluefish (2) and striped bass (2)
Chum: Bunker, striped bass, and bluefish
Acoustics: Mako Magnet (http://www.makomagnet.com/)
Orange buoy lines with diptubes (see FIG. 30.)
Example 13
Repellent Tests of Semiochemicals from Four Different Orders
[0300] Semiochemical solutions from four orders of shark were prepared as described for semiochemical GWH above, using carcasses from Negaprion brevirostris, Ginglymostoma cirratum, Squalus cubensis, and Isurs oxyrinchus. All solutions were found to generally exhibit the sample characteristic absorbance maxima in the UV region. (See FIG. 2.) The four semiochemical solutions were derived from four different families of shark, Family Carcarhinidae, Ginglymostomatidae, Squalidae and Lanmidae, respectively. Each semiochemical exhibited the same repellency effects on other species, the common absorbance maxima, therefore, became a focus of finding an active chemical repellent entity. (See, e.g., Table 1). For example, a 100 mL dose of semiochemical solution prepared from spinal extracts of Squalus cubensis effectively repelled a small feeding school of Carcharhinus perezi and Carcharhinus acronatus.
Example 14
Repellent Test Controls
[0301] The following compounds have been established as effective controls in stimulated free-swimming sharks, tonic immobility tests and non-stimulated free-swimming sharks under chemical repellent evaluation: seawater (dose ranges 100 ul to 1000 ml); HPLC grade micron-filtered water (dose ranges 1 ml to 10 ml); methanol/ethanol/methyl isobutyl ketone/water solution (dose at approx 500 ml); (solvent for A, A2, A13N, SQ1, CPP, GWH, GCC, CP, N2 and BB1); methanol/ethanol/methyl isobutyl ketone solution (dose ranges 1 ml to 6 ml) (50% of solvent for A, A2, A13N, SQ1, CPP, GWH, GCC, CP, N2 and BB1); diethylene glycol monoethyl ether (dose ranges 1 ml to 6 ml); acetone/water solution (dose at approx 500 ml) (solvent for B, B2, ML1, and ML2).
Example 15
GC-MS of Composite of CF-Composite from Two C. falciformis Heads
[0302] Semiochemical CF-Composite was prepared from two C. falciformis heads subject to extraction with polar solvent as described for A2 above. C. falciformis is a pelagic Carcarhiniform known as the silky shark (Order Carcharhiniforme).
[0303] Tests were run by Direct Injection on a quadrupole GC-MS system with a selective mass detector, as described for GWH above. The resulting gas chromatogram is reported in FIG. 20. Analysis of the resulting mass spectrogram using NIST 98.1 provided the following resulting non-limiting components of CF-Composite: urea, 1-(2-hydroxyethyl)-2-imidazolidinone, ethyl acetate.
Example 16
GC-MS of Semiochemical B-Composite from P. glauca Head, Body and Tail
[0304] B-Composite was prepared from a head, body and tail of P. glauca the pelagic blue shark (Order Carcharhiniforme) subject to extraction as described for GWH above.
[0305] Tests were run by Direct Injection on a quadrupole GC-MS system with a selective mass detector, as described for GWH above. The resulting gas chromatogram is reported in FIG. 21. Analysis of the resulting mass spectrogram using NIST 98.1 provided the following resulting non-limiting components of B-Composite: glycerin, N,N-dimethylurea, urea, 5-methyl-2,4-imidazolidinedione (5-methylhydantoin), creatinine, methyl hexadecanoate (methyl palmitate), propanoic acid, dimethyl-propanedioic acid (dimethylmalonic acid), butanoic acid (butyric acid), 3-methyl-butanoic acid, 2-methyl-butanoic acid (isovaleric acid), phenol, 4-morpholinepropionitrile, n-hexadecanoic acid (palmitic acid), 10-octadecenoic acid, methyl ester (methyl elaidate), (E)-9-octadecenoic acid (eliadic acid).
Example 17
Comparison of Components Detected in Four Semiochemicals by GC-MS
[0306] The components of semiochemicals GWH, CF-Composite, CP and B-Composite were compared to determine shared chemistry. The comparison is in Table 4.
[0000]
TABLE 4
Comparison of components of Four Semiochemicals in GC-Mass-
Spectrometry (values represent relative percentage matches
with NIST 98.1 library)
CF- B-
Component GWH Composite CP Composite
glycerin 64 72 64
N,N-dimethylurea 91 91 91
urea 78 56 64 72
5-methyl-2,4-imidazolidinedione (5- 86 86
methylhydantoin)
creatinine 52 64 93
methyl hexadecanoate (methyl 95 94
palmitate)
hexahydro-3-(2-methylpropyl)- 72 56
pyrrolo [1,2-a] pyrazine-1,4-dione
propanoic acid 94
dimethyl-propanedioic acid 80
(dimethylmalonic acid)
butanoic acid (butyric acid) 64
3-methyl-butanoic acid 78
2-methyl-butanoic acid (isovaleric 83
acid)
phenol 90
4-morpholinepropionitrile 53
n-hexadecanoic acid (palmitic acid) 95
10-octadecenoic acid, methyl ester 53
(methyl elaidate)
(E)-9-octadecenoic acid (eliadic acid) 74
1-(2-hydroxyethyl)-2-imidazolidinone 45
ethyl acetate 72
2,3-butanediol 78
N-N-dimethylformamide 43
2-butoxyethanol 72
DL-methyltartronic acid 50
1,4-dimethyl-piperazine 64
2-(1,1-dimethylethoxy)-thiophene 64
hexahydro-pyrrolo [1,2-a] 62
pyrazine-1,4-dione
Example 18
Comparison of UV-Vis Spectra of Semiochemicals of Different Species and Different Carcass Parts of Shark
[0307] Semiochemicals of different species and carcass parts of shark were subjected to uv-vis spectral analysis according to the above-discussed method. (See FIG. 2.) All extracts demonstrated a peak around 300 nm. The control (solvent-first line) demonstrates no absorbance shoulder around 300 nm. The semiochemical showing the strongest absorption in the signature 300 nm range is semiochemical GWH, which is an extraction of a great white shark head.
[0308] Semiochemical abstracts GWH, GCC, N2 were demonstrated to have repellent activity. (See Table 1).
Example 19
Shelf-Life of A2 and N2
[0309] During a day of field tests on semiochemical repellents, experiments with a more-than-one-year-old sample of A2 semiochemical repellent evoked only weak measurable flight response in a variety of sharks. Similar results were obtained with a more-than-one-year-old sample of N2. Because both A2 and N2 had evoked strong flight responses in many tests in prior months, it was hypothesized that the A2 and N2 test samples had been degraded and the semiochemical components had been lost or reduced in concentration.
HPLC Chromatograph Analysis
[0310] Early eluting chromatograms of degraded A2 and N2 (FIGS. 22 and 23) were compared to chromatograms of GWH and A1 (FIGS. 3 and 4) to determine the chromatographic signature of active components of the GWH and A1 extracts. HPLC parameters were as discussed above. The chromatogram of GWH showed a strong peak around 7 minutes, a weaker peak at around 6 minutes and a weak peak at around 5 minute (See FIG. 3.) The chromatograms of degraded A2 and N2 contained no peak at around 7 minutes, a peak comparable with the chromatogram of GWH at around 6 minutes and very weak peaks at 5.2 and 5.4 minutes, respectively. (See FIG. 22.) The peaks at 5.2 and 5.4 minutes had no clear correlation with the 5 minute peak of GWH.
[0311] Late eluting chromatograms of A2 and N2 were likewise compared to GWH and A1. (FIGS. 4 and 23.) HPLC parameters were as discussed above. The chromatograms of GWH and A1 showed distinctive and expected peaks at around 34 minutes having a notably weaker earlier peak within the 34 minute peak. The GWH and A1 chromatograms likewise showed the expected broad peak about 2 minutes following the 34 minute peak, with two maxima within the broad peak. The late eluting chromatograms of degraded A2 and N2 had unexpectedly sharp peaks at around 32 minutes and somewhat sharp peaks about 2 minutes later that were distinctly different from the expected broad peak of an active semiochemical such as GWH or A1.
Example 20
Administration of Semiochemical Repellent Using a Canister
[0312] An aerosol container with a continuous-release actuator was pressurized with 6 fluid oz. of semiochemical CP. The container was constructed to be asymmetrically weighted so that it would not lie on its axis on the surface of the water. In this test, the container was lighter at its base and heavier at its discharging end. The actuator was depressed, initiating release of the CP semiochemical and thrown into a group of 12 feeding sharks. During the pressurized discharge of the extract, the canister moved erratically on the surface of the water. At times the semiochemical was expelled into the air creating a mist that subsequently fell on the surface of the water. At times, the semiochemical was expelled directly into the water. The feeding sharks were drawn to the erratic activity of the canister. When the sharks encountered the cloud of discharged semiochemical near the surface of the water, they fled. In a control, similar pressurized containers with 100% methanol instead of semiochemical were similarly thrown into a population of feeding sharks. The sharks were drawn to the container and did not flee. An expended container was recovered from the water with shark bite marks on it.
Example 21
Administration of Semiochemical Using a Remote Controlled Raft
[0313] A two liter repellent chamber was filled with repellent on a remote controlled raft. The raft was anchored at a sandbar where a shark might be expected to enter a shallow swimming area. A radio receiver was connected to a pump on the raft. The pump with its own power source was connected to the two liter chamber with tubing. The two liter chamber was provided a check valve for rapid build up of pressure from the pump. Tubing was then fixed from the two liter chamber exit portal away from the raft as a discharge tube. The tubing was not sufficiently long enough to enter the water where the raft was floating. The tubing exiting from the chamber was capped with a pressure release cap.
[0314] A person remote from the raft signaled the radio receiver to trigger activation of the pump. The pump compressed air into the 2 liter plastic tank. Head pressure in the chamber increased quickly. Once enough pressure built up, the cap popped off of the tubing and repellent was rapidly sprayed over a 2 meter surrounding area at about 20-30 psi. The chamber was emptied within 20 seconds.
Example 22\
Administration of Semiochemical Using a Pressurized Directional Device
[0315] A pressurized gun as described in FIG. 28 was charged with degraded semiochemical A2. A population of about 10 blacknose sharks and Caribbean reef sharks were stimulated with feed. A swimmer entered the water with the pressurized gun. When sharks approached, the swimmer discharged the first chamber in the direction of the sharks by pointing the directional nozzle and triggering the compressed gas canister. The sharks were partially repelled. The swimmer then discharged the second pressurized chamber in the direction of the sharks. The sharks were again partially repelled.
Example 23
Administration of Semiochemical on a Longline
[0316] A mass of elasmobranch carcass treated with polar solvent according to the methods of the invention is pressed together and placed on a longline hook at the end of a gangion. The hook is then baited, e.g., with mackerel. The longline is placed into the water. Sharks are deterred from striking the hook because of the semiochemical diffusing from the polar-solvent-treated-elasmobranch-carcass mass near the hook. Fish are not deterred. As a result, a tuna is caught on the hook.
Example 24
Preparation of Semiochemical in Jelly Form that Dissolves Over Time in Water
[0317] Semiochemical was prepared in a jelly form that would dissolve over time when placed in water. 100 g diethylene glycol monoethyl ether was warmed to around 40[deg.] C. in a mixture with 2 g of hydroxypropylmethylcelluose under heavy agitation. The mixture was allowed to cool with slow mixing to about 30[deg.] C. At around 30 C, around 20 mL of semiochemical CP was added with an eye dropper over about 2 minutes with slow mixing. The mixture was then cooled to room temperature. A firm gel was formed over night. About 10 g of gel was placed in about 125 mL of water. In about 8 hours the gel was fully dissolved. Such administration of semiochemical could be particularly advantageous to divers and snorklers who would want to repel elasmobranchs but who would not want to repel fish.
ELASMOBRANCH-REPELLING COMPOUNDS AND METHODS OF USE
US2010016346
ZA200710971
Compounds for repelling elasmobranch having an aldehyde or derivative, a carboxylic acid a derivative, a ketone or a derivative thereof, a di-ketone or a derivative thereof, a pyridine or derivative thereof, or an antipyrine or a derivative thereof and methods of use thereof.
INTRODUCTION
[0001] This invention relates generally to gustatory elasmobranch repellents comprising aldehydes, carboxylic acids, ketones, di-ketones, pyridines and anti-pyrines, separately or in combination.
BACKGROUND OF THE INVENTION
[0002] The reality of shark (elasmobranch) attacks and a pervasive fear of shark attacks in the modern world combine to create a great need for effective shark repellents. Effective shark repellents are also needed in the commercial fishing industry.
[0003] Elasmobranchs represent a significant problem in the commercial fishing industry. Elasmobranchs are often inadvertently caught on fishing hooks and tackle directed at other more commercially valuable kinds of fish. This inadvertent catching of elasmobranchs (or other non-valued fish) is called "by-catch." As many as 100 million elasmobranchs are killed each year as by-catch. This loss of life has resulted in a real threat to several shark species. Currently, as many as 80 species of shark are considered threatened with extinction.
[0004] Further, when elasmobranchs are caught as by-catch, fishing operations receive no return on their investment since the shark is caught on a hook that might have otherwise brought in a marketable fish. Additionally, the fishing tackle on which a shark is caught often must be cut loose for the safety of those working on the fishing vessel causing a loss of both equipment and time.
[0005] Longlining is a commercial fishing method that suffers significant losses from shark by-catch. Longlining uses multiple baited individual fish hooks with leaders strung at intervals along an often very long (2-3 mile) main fishing line. Longline fishing operations routinely target swordfish and tuna. The longline hooks and bait, however, are not selective and elasmobranchs are sometimes caught in greater numbers than the intended target catch. The result is great loss of life in elasmobranchs and significant financial losses in the longline industry. Elasmobranchs cause additional losses in the longline fishing industry by scavenging marketable fish caught on longlines before the fish may be retrieved for processing. This problem also applies to the commercial trawling industry.
[0006] There has been a long-felt need for methods and devices to deter elasmobranchs from commercial fishing lines and nets. Attempts in the middle of the twentieth century were made to protect trawl nets with electric discharge devices. Nelson, "Shark Attack and Repellency Research: An Overview," Shark Repellents from the Sea ed. Bernhard Zahuranec (1983) at p. 20). Nevertheless, no commercially effective repellent has been made available for reducing shark by-catch in the commercial fishing industry or for reducing loss of valuable fish or fishing tackle to shark predation.
[0007] An effective shark repellent would not only be valuable to the fishing industry but also would be valuable for protecting humans from shark attacks. An effective repellent has yet to be marketed for limiting the risk of shark attacks faced by humans exposed to elasmobranchs. Over the last 50 years antishark measures employed to protect humans from sharks have included electrical repellent devices (Gilbert & Springer 1963, Gilbert & Gilbert 1973), acoustical playbacks (Myrberg et al. 1978, Klimley & Myrberg 1979), visual devices (Doak 1974) and chemical repellents (Tuve 1963, Clark 1974, Gruber & Zlotkin 1982). None of these procedures proved satisfactory in preventing shark attacks. (Sisneros (2001)). As such, the long felt need for an effective repellent has not been satisfied.
[0008] Researchers have historically used several bio-assays to determine if a repellent evokes a flight response in shark. One such bio-assay measures the effect of a repellent on a shark that is immobilized in "tonic immobility." Tonic immobility is a state of paralysis that typically occurs when a shark is subject to inversion of its body along the longitudinal axis. This state is called "tonic," and the shark can remain in this state for up to 15 minutes thereby allowing researchers to observe effects of repellents. After behavioral controls are established, an object or substance that has a repelling effect will awaken a shark from a tonic state. Researchers can quantify the strength of a repellent effect from these studies.
BRIEF SUMMARY OF THE INVENTION
[0009] Applicant has discovered effective chemical repellents for elasmobranchs, which appear to affect the elasmobranch's gustatory (taste) receptors. According to the present invention, an elasmobranch repellent is provided comprising an aldehyde or a derivative thereof, a carboxylic acid or a derivative thereof, a ketone or derivative thereof, a di-ketone or a derivative thereof, a pyridine or a derivative thereof, or an antipyrine or a derivative thereof, separately or in combination. When tested, these elasmobranch repellents are capable of terminating tonic immobility of a tonic-immobile elasmobranch when introduced to elasmobranch gustatory receptors.
[0010] According to a first non-limiting embodiment of the present invention, a composition for repelling an elasmobranch is provided comprising an aldehyde or a derivative thereof. In a preferred non-limiting embodiment, the composition comprises a methylbutanal. In a more preferred non-limiting embodiment, the aldehyde is selected from 3-methylbutanal or 2-methylbutanal. In another preferred non-limiting embodiment, the composition comprises methylbutenal. In a more preferred non-limiting embodiment, the aldehyde is selected from 2-methylbutenal or 3-methylbutenal.
[0011] In an alternative non-limiting preferred embodiment of the first embodiment, the aldehyde comprises a linear carbon chain of about 5 carbons. In a more preferred embodiment, the aldehyde is selected from valeraldehyde, pentanal or trans-pentenal.
[0012] In an alternative non-limiting preferred embodiment of the first embodiment, the aldehyde comprises a saturated carbon chain comprising 1 carbon to about 6 carbons. In a more preferred embodiment, the saturated aldehyde is selected from formalin (the acetal form of formaldehyde gas in water), acetaldehyde, propionaldehyde, butyraldehyde, isobutyraldehyde, valeraldehyde (pentanal), capronaldehyde (hexanal), trimethylacetaldehyde (pivic aldehyde) and isovaleraldehyde (3-methylbutanal).
[0013] In another preferred embodiment of the first embodiment, the composition for repelling an elasmobranch comprises a natural aldehyde. In a more preferred embodiment, the natural aldehyde is selected from cinnimaldehyde, cuminaldehyde and acetaldehyde. In a more preferred embodiment, the natural aldehyde is piperonal.
[0014] In another alternative preferred embodiment of the first embodiment, the composition for repelling an elasmobranch comprises an aromatic aldehyde, solubilized in a suitable polar solvent. In a more preferred embodiment, the aromatic aldehyde is selected from one or more methoxybenzaldehydes and a tolualdehyde.
[0015] In an alternative preferred non-limiting embodiment, the composition comprises a combination of two or more aldehydes or aldehyde derivatives.
[0016] According to a second non-limiting embodiment of the present invention, a composition for repelling an elasmobranch is provided comprising a carboxylic acid or a derivative thereof. In a preferred non-limiting embodiment, the composition comprises butyric acid. In another preferred non-limiting embodiment, the composition comprises citric acid. In other preferred non-limiting embodiments, the carboxylic acid is selected from trans-cinnamic acid, 2-butenoic acid, lactic acid, 2,2-dimethylbutyric acid, 2,3,3-trimethylproprionic acid, 2-ethylbutyric acid, 2-ketobutyric acid, 3-aminobutyric acid, 4-acetylbutyric acid, 3-butenoic acid, tricarballylic acid and hydroxysuccinic acid. In an alternative preferred non-limiting embodiment, the composition comprises a combination of two or more carboxylic acids or carboxylic acid derivatives. In a more preferred non-limiting embodiment, the combination of carboxylic acids comprises at least two carboxylic acids selected from crotonic acid, cinnamic acid, maleic acid, citric acid and fumaric acid. In another more preferred non-limiting embodiment, the combination comprises crotonic acid, cinnamic acid and maleic acid. In an alternative preferred non-limiting embodiment, the combination of carboxylic acids comprises crotonic acid, citric acid and fumaric acid.
[0017] In a third non-limiting embodiment of the present invention, a composition for repelling an elasmobranch is provided comprising a ketone or a derivative thereof. In a preferred non-limiting embodiment, the composition for repelling an elasmobranch comprises ionone. In another preferred non-limiting embodiment, the composition for repelling an elasmobranch comprises zingerone. In an alternative preferred non-limiting embodiment, the composition comprises a combination of two or more ketones or ketone derivatives.
[0018] In a fourth non-limiting embodiment of the present invention, a composition for repelling an elasmobranch is provided comprising a di-ketone or derivative thereof. In a preferred non-limiting embodiment, the composition for repelling an elasmobranch comprises 2,3-butanedione. In another preferred non-limiting embodiment, the composition for repelling an elasmobranch comprises glyoxal. In another preferred non-limiting embodiment, the composition for repelling an elasmobranch comprises methylglyoxal. In an alternative preferred non-limiting embodiment, the composition comprises a combination of two or more di-ketones or diketone derivatives.
[0019] In a fifth non-limiting embodiment of the present invention, a composition for repelling an elasmobranch is provided comprising a pyridine or a derivative thereof. In a preferred non-limiting embodiment, the composition for repelling an elasmobranch comprises pyridine. In another preferred embodiment, the composition for repelling an elasmobranch comprises 3-methylpyridine or 2-amino-3-picoline. In an alternative preferred non-limiting embodiment, the composition comprises a combination of two or more pyridines or pyridine derivatives.
[0020] In a sixth non-limiting embodiment of the present invention, a composition for repelling an elasmobranch is provided comprising an anti-pyrine or a derivative thereof. In a preferred non-limiting embodiment, the composition for repelling an elasmobranch comprises anti-pyrine. In another preferred embodiment, the elasmobranch repellent comprises 4-amino-antipyrine. In an alternative preferred non-limiting embodiment, the composition comprises a combination of two or more anti-pyrines or an anti-pyrine derivative.
[0021] In a non-limiting embodiment of the present invention, a composition for repelling an elasmobranch is provided comprising a combination of two or more of aldehydes or derivatives thereof, carboxylic acids or derivatives thereof, ketones or derivatives thereof, diketones or derivatives thereof pyridines or derivatives thereof or antipyrines or derivatives thereof. In a preferred non-limiting embodiment, the composition for repelling an elasmobranch comprises an aldehyde and a diketone. In a more preferred non-limiting embodiment, the composition for repelling an elasmobranch comprises butyraldehyde, isobutyraldehyde, veratraldehyde and 2,3-butanedione.
[0022] A method of repelling an elasmobranch is provided comprising administering a composition for repelling an elasmobranch comprising an aldehyde or a derivative thereof, a carboxylic acid or a derivative thereof, a ketone or a derivative thereof, a di-ketone or a derivative thereof, a pyridine or a derivative thereof, or an antipyrine or a derivative thereof, separately or in combination, in the expected proximity of said elasmobranch. In a preferred non-limiting embodiment, the composition for repelling an elasmobranch is administered from an aerosol canister. In another preferred non-limiting embodiment, the composition for repelling an elasmobranch is administered in proximity of a longline.
[0023] A method of manufacturing an elasmobranch repellent is provided comprising the steps of combining an aldehyde or a derivative thereof, a carboxylic acid or a derivative thereof, a ketone or derivative thereof, a di-ketone or a derivative thereof, a pyridine or a derivative thereof, or an antipyrine or a derivative thereof, each alone or in combination with one another or other ingredients, with an acceptable solvent, carrier, diluent or other vehicle for administration or storage prior to administration.
[0024] A kit is provided comprising a composition for repelling an elasmobranch comprising a composition for repelling an elasmobranch comprising an aldehyde or a derivative thereof, a carboxylic acid or a derivative thereof, a ketone or derivative thereof, a di-ketone or derivative thereof, a pyridine or a derivative thereof, or an antipyrine or a derivative thereof, separately or in combination, and a vehicle for administering said composition for repelling an elasmobranch. In a preferred embodiment, the kit comprises a vehicle selected from a pressurized or pressurizable delivery device, a pressurized or pressurizable repellent gun, a miniature pressurizable repellent gun to be worn on a wrist or an ankle of a subject, a spear fishing gun with an adjacent pressurizable container for said composition, a time release sponge, a surfboard, a pump delivery system affixed to a surfboard, a pressurized delivery device affixed to a surfboard, a wristwatch comprising said composition, a syringe, a pressurized syringe, an aerosol bomb, a mortar-launched aerosol bomb, a remote-controlled buoy with a repellent tank, a fixed buoy with a metering pump, a repellent pouch, a jelly comprising glycol ether and hydroxypropylcelluose, a skin lotion containing said repellent, a porous fabric impregnated with repellent, rechargeable porous fabric impregnated with said repellent, a submerged repellent mine, a repellent-impregnated cable insulation for an undersea cable, and a repellent-impregnated cable jacket for an undersea cable.
DETAILED DESCRIPTION OF THE INVENTION
[0025] "Elasmobranchii" represents the subclass of class Chondrichthyes (cartilaginous fish), which includes the sharks and rays. In this specification, "elasmobranchs" represent the super-orders and orders of elasmobranchs that are of interest for producing a repellent based on availability and conservation, and also those that present a potential threat to humans or represent a bycatch problem in commercial fisheries. As such, "elasmobranchs" in this specification means one or more elasmobranchii in the super-orders Galeomorphii and Squalomorphii and orders Squaliforms (dogfish), Carcharhiniformes (requiem sharks), Lamniformes (mackerel sharks), and Orectolobiformes (carpet sharks).
[0026] "Derivative" is a chemical compound that may be produced from a compound of a similar structure in one or more steps, as in replacement of hydrogen by an alkyl, acyl, amino group, etc, wherein the derivative has a repellent function in elasmobranchs.
[0027] "Feeding zone" is the area in which sharks have been stimulated and demonstrate aggressive feeding behavior.
[0028] "Gustatory Response" is a response in an elasmobranch to a stimulation of taste receptors.
[0029] "Solvent" is a first substance capable of dissolving another substance.
[0030] "Carrier" is a first substance capable of mixing with a second substance.
[0031] "Diluent" is a first substance capable of mixing with a second substance such that the second substance is decreased in concentration.
[0032] "Tonic immobility" is the state of paralysis that typically occurs when an elasmobranch is subject to inversion of its body along the longitudinal axis of the body, i.e., is belly up. The elasmobranch can remain in this state for up to 15 minutes.
I. COMPOSITIONS FOR REPELLING ELASMOBRANCH
[0033] The elasmobranch repellent activity of aldehydes, carboxylic acids, ketones, diketones, pyridines or antipyrines has been demonstrated in eight species of elasmobranch. Tests demonstrate the repelling compounds are correlated with a flight response in elasmobranchs wherein the flight response is correlated with stimulation of elasmobranch taste receptors with the repelling compounds. As such, these compounds were effective as elasmobranch repellents.
[0034] Flight responses upon exposure to the repelling compounds disclosed herein have been observed in bioassays of eight different species across two different orders of elasmobranch and three different families of elasmobranch including lemon sharks of various sizes and ages (N. brevirostris, Order Carcharhiniformes, Family Carcarhinidae), nurse sharks of various sizes and ages (C. cirratum, Order Orectolobiformes, Family Ginglymostomatidae), tiger sharks of various sizes and ages (G. cuvieri, Order Carcharhiniformes, Family Carcarhinidae) blacktip sharks of various sizes and ages (C. limbatus Order Carcharhiniformes), blacknose sharks of various sizes and ages (C. acronotus, Order Carcharhiniformes, Family Carcarhinidae), Caribbean reef sharks of various sizes and ages (C. perezii, Order Carcharhiniformes, Family Carcarhinidae), great hammerhead sharks (Sphyrna mokarran, Order Carcharhiniformes, Family Sphyrnidae), and blue sharks (Prionace glauca, Order Carcharhiniformes, Family Carcarhinidae).
[0035] Among the above-listed species, flight responses have repeatedly been observed upon exposure to a wide variety of different aldehydes or combinations of aldehydes from one to ten carbons in length; including methylbutanals, methylbutenals, linear five-carbon aldehydes, saturated one-to-six carbon aldehydes, unsaturated two-to-six carbon aldehydes, natural aldehydes, aromatic aldehydes, piperonal and combinations of aldehydes. Aldehydes and there derivatives are disclosed herein as effective elasmobranch repellents.
[0036] Flight responses have likewise repeatedly been observed in elasmobranchs upon exposure to an extensive variety of carboxylic acids or combinations of carboxylic acids, including butyric acid, citric acid, crotonic acid and mixtures of crotonic acid, cinnamic acid and maleic acid, and crotonic acid, citric acid and fumaric acid. Carboxylic acids and their derivatives are disclosed herein as effective elasmobranch repellents.
[0037] Flight responses have likewise repeatedly been observed in elasmobranchs upon exposure to ketones such as ionone and zingerone. Ketones and their derivatives are disclosed herein as effective elasmobranch repellents.
[0038] Flight responses have also been observed in elasmobranchs upon exposure to diketones, such as 2,3-butanedione (diacetyl). Diketones and their derivatives are disclosed herein as effective elasmobranch repellents.
[0039] Flight responses have likewise repeatedly been observed upon exposure to pyridine and pyridine derivatives such as 3-methylpyridine, 2-amino-3-picoline and upon exposure to anti-pyrines and derivatives thereof, such as 4-aminoantipyrine and antipyrine solutions. See Tables 25-26. Pyridines and their derivatives and anti-pyrines and their derivatives are disclosed herein as effective elasmobranch repellents.
[0040] Surprisingly, fish appear unresponsive to these shark-repelling aldehydes. Tests involving captive Cobia and Yellowfin Tuna show that feeding behavior is actually slightly increased in the presence of aldehydes, particularly 3-methylbutanal, a potent shark gustatory repellent. Similarly, teleost reef fish, such as Triggerfish and Snappers, have been observed feeding and swimming in a cloud of shark-repelling aldehydes. This behavior is presumed to result from the lack of aldehyde-receptors in the fishes' gustatory system. Interestingly, aldehyde dehydrogenases (ALDH, ALDH2) have been found in certain species of fish.
[0041] In open water tests, the Queen triggerfish (Ballistes vetula), Durgeon Triggerfish (Melichthys niger), Bermuda Chub (Kyphosus sectatrix), Yellowtail Snapper (Ocyurus chrysurus) and Remora (Remora remora) were observed to be unaffected by exposure to elasmobranch repellents in numerous tests.
[0042] Flight responses, or repellency activity, may be demonstrated in any method described herein or known to one of skill in the art. Flight responses have been observed and measured using several bioassays known in the art to correlate with flight response.
[0043] One bioassay used to observe and measure flight response is the tonic immobility test. Tonic immobility is a state of paralysis that typically occurs when a shark is subject to inversion of its body along the longitudinal axis. This state is called "tonic," and the shark can remain in this state for up to 15 minutes thereby allowing researchers to observe effects of chemical repellents. The "tonic" state of the shark is first established by releasing seawater in proximity to the "tonic" shark with the same delivery instrument and at the same distance as a "test" repellent compound will be released. Some controls are released with a high flow rate (30 mL/sec) in order to establish that sharks are not awakened by a jet of fluid over their noses. Once behavioral controls are established, a compound or composition that may have a repelling effect is delivered to the shark. If the compound or composition engenders a flight response, the shark will awaken from the tonic state and rapidly attempt to flee the delivered repellent. Using this tonic immobility bioassay researches can quantify the strength of a repellent effect.
[0044] In the tonic immobility studies disclosed herein, several different methods were employed for delivery of repelling compounds. A first method for delivery of chemical repellent in tonic immobility studies employed a "Syringe 3/5/10 Assay" method. The "Syringe 3/5/10 Assay" method is so named because a test repellent is delivered to a shark from a distance of about "3" inches with a bolus of about "5" mL with a response to the test repellent considered positive if the shark reacts with a change in behavior within less than about "10" seconds from the time of delivery.
[0045] The "3/5/10 Syringe Assay" as employed herein delivered a dose of 5-6 mL of a test chemical repellent from a syringe fixed with a needle having a gauge of about 22 from a distance at least 3 inches in front of a shark. Because the test chemical repellent was delivered at a distance from the shark's nares and mouth, a cloud of test chemical repellent was dispersed over the shark within the water column. The dispersed test repellent was subject to water current direction, dispersion and dilution. As a result, a flight response within 10 seconds was considered a positive repellency response. Time from delivery of the test substance until a response was observed, measured and recorded. Time from delivery to response is related to the size of the bolus delivered from the syringe, distance of the shark from the syringe and water current. As such, a longer time to response does not reflect reduced potency for a particular compound. To the contrary, a longer time to response as compared to some other compound or test simply demonstrates potency even after a cloud of repellent has traveled some distance against water current.
[0046] A second delivery method called the "Syringe Assay" method delivered a dose of 60 mL or more of a test chemical repellent from at least one foot, and up to as many as five feet, from a shark. The distance of delivery was determined based on the strength of the water current in the direction of the shark. Time from delivery of the test substance until response was observed, measured and recorded. The "Syringe" method allows a researcher to observe how a diffusing and diluting cloud of test chemical repellent affects the shark's behavior when the shark encounters the delivered cloud of test substance. The "Syringe" method requires relatively large doses because of the diffusion of the cloud over time and distance. Time from delivery to response is related to the size of the bolus delivered from the syringe, distance of the shark from the syringe and water current. As such, a longer time to response does not reflect reduced potency for a particular compound, as discussed above.
[0047] A third method of delivery was called the "Bite Assay." In this method of delivery, a dose of typically less than 5 mL was presented directly into a shark's mouth using a pipette.
[0048] A fourth method of delivery was called the "Micropipette Assay" method of delivery. In this method, a very small dose (fraction of a mL) of a test substance was delivered directly into a shark's mouth. The Micropipette Assay did not consistently terminate tonic immobility in most chemical tests. A response, such as a cough or other notable action of the shark, was usually noted when effective gustatory repellents were delivered directly into a shark's mouth while in tonic immobility.
[0049] The Micropipette Assay method has not proven to be a particularly effective method of assaying for a flight response in elasmobranchs. The Micropipette Assay method is, nevertheless, an excellent method for specifying that a gustatory response has occurred. It is effective for specifying a gustatory response because the micropipette delivery method allows direct delivery of an entire bolus of test substance into the mouth of the shark being tested. A combination of data from micropipette assays demonstrating a gustatory response and other assays demonstrating a flight response is an excellent combination of data demonstrating both the repellent activity of a compound and its effectiveness as a gustatory repellent.
[0050] Another bioassay used to observe a flight response in sharks is a free-swimming test using a small metal cage containing bait. This assay is referred to as a "Cage Assay." The cage with bait is suspended below a float in the water column. A [3/8] inch diptube is secured from the cage to the boat and carried chemical compounds to the proximity of the cage where the test substances were delivered. Sharks are drawn to the vicinity of the boat with chum. Sharks are observed to immediately bump and bite at the cage wherein bait was contained. The number of interactions between the sharks and the cage are recorded over time. Test chemical repelling substance is delivered to the vicinity of the cage through the diptube. The frequency of bumps and strikes by sharks against the cage is then monitored and recorded. If bumps and strikes by sharks cease for a period of time, that time period is also recorded. In the free-swim ring tests disclosed herein using a baited cage, the volume of test chemical repellent delivered into the vicinity of the cage was about 500 mL.
[0051] Another bioassay used to observe a flight response in sharks is a cloud dispersion assay on competitively feeding population of sharks. This assay is referred to as a "Cloud Dispersion Assay" or "Cloud Assay." A pressurized fluid delivery system was designed to deliver repellent into large feeding populations of sharks. The repellent is released as a subsurface cloud, which follows the current. A 1 L plastic container containing the test chemical repellent solution is pressurized to approximately 20 psig with a battery compressor or hand pump. A globe valve is used to hold back the fluid. The fluid is delivered to the end of a long PVC pole using a Teflon tubing. This allows the operator to place the tip of the pole well into a population of feeding sharks. By actuating the small globe valve, a cloud of the chemical solution is released quickly and reliably into the feeding population. Controls are established using FD&C Red 40 dye and seawater, uncolored seawater, and air. These controls establish that sharks are not afraid to approach the delivery pole, nor are sharks deterred from feeding by the jet of control fluid or air.
A. Composition for Repelling Elasmobranchs Via Gustatory Receptors
[0052] Compositions for repelling an elasmobranch via said elasmobranch's gustatory receptors are disclosed herein. Gustatory repellent compositions may comprise an aldehyde or a derivative thereof, a carboxylic acid or a derivative thereof, a ketone or derivative thereof, a di-ketone or derivative thereof, a pyridine or a derivative thereof or an antipyrine or a derivative thereof, or any compound that terminates tonic immobility or otherwise evokes a behavioral response when administered to the mouth of an elasmobranch in tonic immobility.
[0053] The biological activity of a gustatory chemical shark repellent differs from olfactory and respiratory repellents. This is readily observed using the tonic immobility bioassay. Unlike mammals, a shark's "nose" (olfactory system) is isolated from its mouth, but its mouth and gills are interrelated.
[0054] Using a microliter syringe or microliter pipette, a bolus of test chemical can be directed precisely into one of the shark's nares, or its mouth. Gustatory repellents will terminate tonic immobility or evoke a behavioral response from a "tonic" shark almost immediately when injected into the mouth of the shark. Olfactory repellents will terminate tonic immobility almost immediately when injected into a nare of the shark.
[0055] Repellents that act upon the respiratory system, such as surfactants, saponins, and soaps, are typically introduced in the mouth, but a delayed coughing response is observed. The delayed response generally occurs after two gill pumps following the introduction of the test compound into the shark's mouth.
[0056] Gustatory repellent compounds typically will cause the shark to lock its mouth wide open, followed by head shaking. Respiratory repellent compounds will invoke coughing and violent gill pumping responses as the chemical contacts the gill rakes through pumping action.
[0057] During chemical repellent tests, a divider may be used to control the flow of trace amount of test chemical. A thin strip of plastic may be placed between the shark's mouth and nares, to minimize any chances that an olfaction substance will enter the mouth, or that a gustation compound will enter the nares. While the mouth may be separated from the nose in shark investigations, there is no way to segregate the gills from the shark's "palate" within the mouth. It has been hypothesized that the insertion of some kind of internal dam into the mouth might separate the "palate" from the gills but it is expected that this would injure the animal and, as such, would be an unsatisfactory research method.
[0058] Gustatory responses have been demonstrated in seven species of shark (lemon, nurse, blacktip, tiger, blacknose, Caribbean and blue) in a wide range of aldehydes and aldehyde mixtures; including methylbutanals, methylbutenals, linear five-carbon aldehydes, saturated one-to-six carbon aldehydes, unsaturated two-to-six carbon aldehydes, natural aldehydes, aromatic aldehydes, aldehydes of up to ten carbons in length and in combinations of aldehydes. See Tables 14-23. In Tables 14-23, results of tests using aldehyde and combinations of aldehydes on different species of elasmobranch are provided. Of particular interest for the differentiation of a gustatory response from an olfactory response are the tonic immobility assays using a micropipette delivery method wherein chemical repellent is delivered directly into the mouth of the test shark. A gustatory response is positive when tonic immobility is terminated and a flight response is observed (denoted under column "T?" as "Y") or when a change in shark behavior short of termination of tonic immobility is observed, such as a cough (denoted under column "T?" as "R"). "N" under column "T?" denotes no response.
[0059] Gustatory responses to aldehydes and aldehyde mixtures are likewise confirmed in Tables 1-8 using the delivery methods (other than "micropipette") that resulted in termination of tonic immobility or change of behavior. A review of the data in Tables 14-23 reveals that delivery of aldehydes or combinations of aldehydes directly to the mouth of a shark using a micropipette terminated tonic immobility in numerous tests among several different species of shark. Delivery of aldehydes or combinations of aldehydes directly to the mouth of nurse sharks in bite tests likewise resulted in termination of tonic immobility.
[0060] From the data presented herein, it is believed that a gustatory response (response based on detection of the repellent in the mouth) is different from an olfactory response (a response based on detection of the repellent in nares). Of particular interest for the differentiation of a gustatory response from an olfactory response are tonic immobility assays using a micropipette delivery method wherein chemical repellent is delivered directly into the mouth of the test shark. A gustatory response is positive when tonic immobility is terminated and a flight response is observed. See Tables 14-27.
[0061] The data in Tables 14-23 evidences that aldehydes stimulate gustatory receptors in creating a flight response. Gustatory responses have likewise repeatedly been observed upon exposure to pyridine and pyridine derivatives such as 3-methylpyridine, 2-amino-3-picoline as well as upon exposure to 4-aminoantipyrine and antipyrine solutions. See Tables 25-26. In Tables 25 and 26, results of tests using pyridine and antipyrine derivatives on different species of elasmobranch are provided. While delivery of about 500 microliters of pyridine from a micropipette directly into the mouth of a nurse shark did not evoke a response, "Syringe 3/5/10" assays and "Hd syringe" assays, which provide a stream of test substance to the mouth and nares of a shark, predominantly terminated tonic immobility. Delivery of 400 microliters of antipyrine solution from a micropipette evoked a response in one assay and did not evoke a response in another. "Hd Syringe" assays, which direct a precise bolus of test substance to the mouth and nose of a shark, with 4-aminoantipyrine terminated tonic immobility in all tests.
[0062] The data in Tables 25-26 evidence that pyridines and pyridine derivatives and antipyrines and antipyrine derivatives stimulate gustatory receptors in creating a flight response. The data in Table 27 and Example 5 evidence that ketones and di-ketones simulate gustatory receptors in creating a flight response.
B. Composition for Repelling Elasmobranchs Comprising Aldehydes
[0063] A composition for repelling an elasmobranch may comprise an aldehyde or a derivative thereof. Tables 1-7 and examples 1-8 and 12 provide data evidencing repeated observation of flight responses among seven species of elasmobranch upon exposure to more than twenty different aldehydes or combinations of aldehydes from one to ten carbons in length; including methylbutanals, methylbutenals, linear five-carbon aldehydes, saturated one-to-six carbon aldehydes, unsaturated two-to-six carbon aldehydes, natural aldehydes, aromatic aldehydes and combinations of aldehydes.
[0064] Exemplary and non-limiting aldehydes disclosed herein as elasmobranch repellents include, and are not limited to, 3-methylbutanal, 2-methylbutanal, 3-methylbutenal, 2-methylbutenal, valeraldehyde, trans-pentenal, propionaldehyde, butyraldehyde, isobutyraldehyde, capronaldehyde (hexanal), trimethylacetaldehyde (pivaldehyde or pivic aldehyde), trans-cinimaldehyde, cuminaldehyde, piperonal, methoxybenzaldehydes, vanillin, 2-ethylbutyraldehyde (diethylacetaldehyde), iso-butyraldehyde (2-methylpropionaldehyde), heptanal (heptyl aldehyde), octanal (octyl aldehyde), nonanal (nonyl aldehyde), decanal (decyl aldehyde), dimethylbenzaldehydes, o-anisaldehyde, m-anisaldehyde and p-anisaldehyde.
[0065] In general, the aldehyde function appears to be a tremendously powerful gustatory compound. In humans, aldehydes such as cuminal invoke spicy flavors, e.g., benzaldhyde (cherries), piperonal (black cherries), cinnimal (hot cinnamon), etc. However, in a shark, these receptors, if they exist, may invoke entirely different sensations. It is reasonable to expect that a shark would never encounter a free-aldehyde in the ocean, particularly aldehydes of C2-C6, and therefore would find them distasteful.
[0066] Most aldehydes having carbon chains of more than four carbons are not water soluble. In these cases, denatured alcohol may be used to solubilize the aldehyde. A preferred solvent may be a mixture of methanol and ethanol. A more preferred solvent may be a 50% w/w mixture of methanol and ethanol, denatured ethanol, or diethylene glycol monoethyl ether.
[0067] The electrophilic carbonyl function of the aldehyde makes it fairly reactive. If the aldehyde is soluble enough in seawater, which is slightly basic, cyclic addition products may be reversibly formed. These products are called "cyclic acetals" or simply "acetals." Because acetyls of the aldehydes disclosed herein likely form when the aldehydes are exposed to water, acetals of the aldehydes disclosed herein are also expected to play a role in gustatory repelling of elasmobranchs.
[0068] 1. Methylbutanals
[0069] Methylbutanals may be administered to elasmobranchs as a repellent, including methylbutanal or any derivative thereof. Excellent gustatory repellent activity has been observed in 3-methylbutanal and 2-methylbutanal as demonstrated in Table 1.
[0000]
TABLE 1
Syringe Total (Not including
Hd-Syringe 3/5/10 Bite Micropipette assays)
Compound Y R N Y R N Y R N Y R N
2- 5/5 5/5
methylbutanal
3- 20/27 6/27 1/27 7/7 2/2 29/36 6/36 1/36
methylbutanal (81%) (17%) (3%)
Total 25/32 6/32 1/32 7/7 2/2 34/41 6/41 1/41
Percent of 78% 19% 3% 100% 100% 83% 15% 2%
Methylbutanals
Trials
"Y" denotes termination of tonic immobility.
"R" denotes a behavior response within tonic immobility.
"N" denotes no response.
[0070] Table 1, which summarizes the data in Tables 14 and 15, evidences the gustatory repellent characteristics of methylbutanals. In 35 of 36 assays (combining columns "Y" and "R" under the column labeled "Total (Not including Micropipette Assays)" to arrive at a 97% effectiveness rate), including assays on lemon, nurse, tiger and blacktip sharks, the gustatory repellent activity of 3-methylbutanal was established. In 81% of assays, tonic immobility was fully terminated. Termination of tonic immobility demonstrates a flight response and good repellent activity. In 17% of assays, a behavioral change was observed in the shark being tested even though the shark remained paralyzed in tonic immobility. In only a single assay did a shark not respond to treatment with 3-methylbutanal.
[0071] In five of five assays for repellent effect of 2-methylbutanal in lemon and nurse sharks, tonic immobility was terminated with as little as 200 microliters of repellent.
[0072] Together the data in Table 1 evidence the effectiveness of methylbutanals as elasmobranch repellents. The acetyls of the methylbutanals that are created when the methylbutanals are exposed to water are also expected to play a role in the repellent activity of the methylbutanals. Methylbutanals may be administered into the vicinity of an elasmobranch in a method known in the art or herein disclosed.
[0073] The methylbutanal compounds, 3-methylbutanal and 2-methylbutanal, are preferred elasmobranch repellents because they are not prohibited by federal regulations, are easy to handle, and provide a very strong repellent response. Other derivatives of the methylbutanals including addition products, and hydroxy- or amino-substituted methylbutanals are also expected to provide good repellent effect because of the hydrogen bonding and polarity provided by such groups.
[0074] 2. Methylbutenals
[0075] Methylbutenals likewise may be administered to elasmobranchs as an effective repellent, including methylbutenals or any derivative thereof. Excellent repellent activity has been observed in 2-methylbutenal and 3-methylbutenal.
[0000]
TABLE 2
Hd-Syringe
Compound Y R N
2-methylbutenal 4/4
3-methylbutenal 4/4
Total 8/8
Percent of Trials 100%
"Y" denotes termination of tonic immobility.
"R" denotes a behavior response within tonic immobility.
"N" denotes no response.
[0076] Table 2, which summarizes data from Tables 16 and 17, evidences the effective repellent characteristics of methylbutenals. In four of four trials for 2-methylbutenal (with volumes as low as 300 microliters) and in four of four trials for 3-methylbutenal (with volumes as low as 350 microliters), all tests on nurse and lemon sharks terminated tonic immobility. This data demonstrates the effective repellent activity of the methylbutenals. Additionally, as may be seen in Table 17, Micropipette assays demonstrate the gustatory repellent nature of the methylbutenals by showing a response to repellent directed solely to the mouth and not including the nares. Acetyl derivatives of methylbutenals are also expected to play a role in the repellent effect of the methylbutenals.
[0077] Methylbutenals may be administered into the vicinity of an elasmobranch in any method of delivery known in the art or herein disclosed. Methylbutenals are preferred elasmobranch repellents because they are not prohibited by federal regulations, are easy to handle and provide a very strong repellent response. Derivatives of methylbutenals including its addition products, and hydroxy- or amino-substituted methylbutenals are also expected to provide good repellent effect.
[0078] 3. Linear 5-Carbon Aldehydes
[0079] It is demonstrated herein that aldehydes having a linear five carbon chain may be administered to elasmobranchs as a particularly effective repellent. Linear five carbon chain aldehydes are generally soluble in water and evoke a flight response in a wide range of shark species. Five carbon chain aldehydes include, methylbutanals or any derivative thereof, methylbutenals or any derivative thereof, valeraldehyde or any derivative thereof and trans-pentenal or any derivative thereof, such as pentenal. Effective repellent activity has been observed in each of these compounds.
[0080] Table 3, in combination with Tables 1 and 2 above, provides data evidencing the gustatory repellent characteristics of linear 5 carbon aldehydes. As established above methylbutanals and methylbutenals are effective elasmobranch repellents. See Tables 1-2.
[0000]
TABLE 3
Total (Not
including
Micropipette
Hd-Syringe Syringe assays)
Compound Y R N Y R N Y R N
Valeraldehyde 5/5 1/1 6/6
Trans-pentenal 5/5 5/5
Total 10/10 1/1 11/11
Percent 100% 100% 100%
"Y" denotes termination of tonic immobility.
"R" denotes a behavior response within tonic immobility.
"N" denotes no response.
[0081] Table 3, which summarizes data in Table 18, provides data evidencing the repellent activity of valeraldehyde and trans-pentenal. In six of six assays on lemon and nurse sharks using valeraldehyde, tonic immobility was terminated. Further, the results of micropipette assays in Table 18 using valeraldehyde support the conclusion that valeraldehyde is a gustatory repellent because all Micropipette assays showed a response by the shark to direct delivery of valeraldehyde to the mouth. Additionally, in five of five assays, trans-pentenal terminated tonic immobility in lemon and nurse sharks. Again, the results of micropipette assays in Table 18 support the conclusion that trans-pentenal is an effective gustatory repellent.
[0082] Together, the data in Tables 1, 2 and 3 establish the effectiveness of linear 5 carbon aldehydes as elasmobranch repellents. Linear 5 carbon aldehydes may be administered into the vicinity of an elasmobranch in any method of delivery known in the art or herein disclosed. As such, a composition for repelling an elasmobranch comprising a linear 5 carbon aldehyde including valeraldehyde or pentenal or trans-pentanal has been provided herein.
[0083] 4. Saturated C1-C6 Aldehydes
[0084] It is demonstrated herein that aldehydes having a saturated carbon chain comprising 1 carbon to about 6 carbons may be administered to elasmobranchs as a particularly effective repellent. Such aldehydes are generally soluble in water and evoke a flight response in a wide range of shark species. Saturated 1 carbon to 6 carbon aldehydes include, formalin, acetaldehyde, proprionaldehyde, butyraldehyde, isobutyraldehyde, valeraldehyde, capronaldehyde, trimethylacetaldehyde, 3-methylbutanal or any derivative of any of the before-listed compounds. Good repellent activity has been observed in each of these compounds.
[0085] Table 4, which summarizes data from Tables 14 and 19, establishes the repellent activity of saturated C1-C6 aldehydes.
[0000]
TABLE 4
Total (Not including
Hd-Syringe Syringe Bite Micropipette assays)
Compound Y R N Y N Y Y R N
Propionaldehyde 4/4 4/4
Butyraldehyde 4/4 4/4
Isobutyraldehyde 4/4 4/4
Valeraldehyde 5/5 1/1 6/6
Capronaldehyde 1/1 1/1
Trimethylacetaldehyde 5/5 1/2 1/2 6/7 1/7
(pivaldehyde)
3-methylbutanal 20/27 6/27 1/27 7/7 2/2 29/36 6/36 1/36
(Isovaleraldehyde)
Total 30/37 6/37 1/37 22/23 1/23 2/2 53/61 53/61 1/61
Percent of Trials 81% 16% 3% 96% 4% 100% 87% 10% 16%
"Y" denotes termination of tonic immobility.
"R" denotes a behavior response within tonic immobility.
"N" denotes no response.
[0086] As may be seen above, in 100% of assays performed using proprionaldehyde, butyraldehyde, isobutyraldehyde, valeraldehyde, capronaldehyde, and trimethylaldehyde, tonic immobility was terminated. These tests were done on nurse and lemon sharks. As was discussed above and shown in Table 1,3-methylbutanal is an excellent gustatory repellent.
[0087] The data in Table 4 evidences the utility of aldehydes having a saturated carbon chain comprising 1 carbon to about 6 carbons including propionaldehyde, butyraldehyde, isobutyraldehyde, valeraldehyde, capronaldehyde, trimethylacetaldehyde and 3-methylbutanal or derivatives of any of these compounds. Such aldehydes may be administered into the vicinity of an elasmobranch in any method of delivery known in the art or herein disclosed. Similarly, formalin and acetaldehyde are very water soluble and would be expected to produce similar gustation responses as the other C1-C6 aldehydes. As such, a composition for repelling an elasmobranch comprising a saturated aldehyde with a one to six carbon chain has been provided herein.
[0088] 5. Unsaturated C2-C6 Aldehyde
[0089] It is demonstrated herein that aldehydes that are soluble in water and have an unsaturated carbon chain of two to six carbons may be administered to elasmobranchs as a particularly effective repellent. Unsaturated C2-C6 aldehydes include pentenal, 2-methylbutenal, 3-methyl-butenal, or any derivative of any of the before-listed compounds. Excellent repellent activity has been observed in each of these compounds.
[0090] Table 5 provides data establishing the repellent activity of unsaturated C2-C6 aldehydes. The data for pentenal, 2-methylbutenal and 3-methylbutenal is repeated from Tables 2 and 3 above.
[0000]
TABLE 5
Hd-Syringe
Compound Y R N
Pentenal 5/5
2-Methylbutenal 4/4
3-Methylbutenal 4/4
Total 13/13
Percent of Trials 100%
"Y" denotes termination of tonic immobility.
"R" denotes a behavior response within tonic immobility.
"N" denotes no response.
[0091] The unsaturated aldehydes crotonaldehyde and acrolein fall within the family of unsaturated C2-C6 aldehydes and are also expected to act as gustatory repellents. The oxidize form of crotonaldehyde (crotonic acid) was shown to act as a gustatory repellent. The hydrogenated form of acrolein (propionaldehyde) was also shown to act as a gustatory repellent. Nevertheless, crotonaldehyde and acrolein are very toxic and are considered marine pollutants. As a result, testing of these compounds was not considered feasible. Further, while these compounds would be considered to be gustatory elasmobranch repellents as evidenced by the data contained herein, crotonaldehyde and acrolein are not preferred repellents.
[0092] The excellent elasmobranch repelling characteristics of pentenal, 2-methylbutenal and 3-methylbutenal is illustrated above. See Table 2.
[0093] The data in Table 5 evidences the utility of unsaturated C2-C6 aldehydes including pentenal, 2-methylbutenal and 3-methylbutenal. Such aldehydes may be administered into the vicinity of an elasmobranch in any method of delivery known in the art or herein disclosed. As such, a composition for repelling an elasmobranch comprising an unsaturated C2-C6 aldehyde has been provided herein.
[0094] 6. Natural Aldehydes
[0095] It is demonstrated herein that naturally occurring aldehydes such as cinnimaldehyde, cuminaldehyde and piperonal or any derivatives of any of the before-listed compounds may be administered to elasmobranchs as a particularly effective repellent. Table 6 provides data establishing the repellent activity of naturally occurring aldehydes.
[0000]
TABLE 6
Total (Effective
Hd-Syringe Syringe Cage Delivery Methods)
Compound Y R N Y Y Y R N
Cinnimal- 2/2 2/2
dehyde
Cuminaldehyde 1/1 1/1
Natural /4 3/4 1/4 3/4
Aldehydes
Piperonal 1/1 1/1
Total 1/4 3/4 3/3 1/1 4/8 1/8 3/8
Percent of 25% 75% 100% 100 50% 13% 38%
Trials
"Y" denotes termination of tonic immobility.
"R" denotes a behavior response within tonic immobility.
"N" denotes no response.
[0096] The data in Table 6 evidences the repellent activity of naturally occurring aldehydes as elasmobranch repellents including cinnimaldehyde, cuminaldehyde and piperonal. In two of two Syringe assays for cinnimaldehyde on lemon and nurse sharks and one Syringe assay for cuminaldehyde on lemon shark, tonic immobility was terminated in each assay. In an individual cage assay for piperonal, repellent activity was demonstrated by a decrease in the number of strikes by feeding sharks against a baited cage. In the piperonal assay, the sharks did not return to the baited cage after 10 minutes. In one of four Hd Syringe assays for natural aldehydes on nurse sharks, a behavioral change was observed within tonic immobility but tonic immobility was not terminated. In three of four Hd Syringe assays, no change was observed. The repellent activity evidenced in Table 6 should likewise apply to acetaldehyde. In view of the data in Table 6, effective compositions for repelling an elasmobranch comprising a natural aldehyde has been provided herein.
[0097] 7. Aromatic Aldehydes
[0098] It is demonstrated herein that aromatic aldehydes such as a methoxy/vanillin combination, tolualdehyde, veratraldehyde, or anisaldehyde or any derivatives of any of the before-listed compounds may be administered to elasmobranchs as a particularly effective repellent. Table 7 provides data evidencing the repellent activity of aromatic aldehydes.
[0000]
TABLE 7
Syringe
Compound Y R N
Methoxy/Vanillin 6/9 2/9 1/9
Combination
Tolualdehyde 1/1
Anisaldehyde 1/1
veratraldehyde 6/7 1/7
Total 14/18 2/18 1/18
Percent of Trials 78% 11% 6%
"Y" denotes termination of tonic immobility.
"R" denotes a behavior response within tonic immobility.
"N" denotes no response.
[0099] Table 7 demonstrates the repellent effect of aromatic aldehyde on sharks. In six of nine Syringe Assays for methoxy/vanillin aldehyde mixture, tonic immobility was terminated among lemon, nurse and blacknose sharks. In two of nine, a behavioral change was observed in tonic immobility. In one of nine, no change was observed. In a single Syringe Assay for tolualdehyde and another for anisaldehyde, tonic immobility was terminated in lemons sharks. In six of seven Syringe Assays of veratraldehyde, tonic immobility in nurse and lemons sharks was observed. In one of seven Syringe Assays no response was noted. Such aldehydes may be administered into the vicinity of an elasmobranch in any method of delivery known in the art or herein disclosed. As such, effective compositions for repelling an elasmobranch comprising an aromatic aldehyde have been provided herein.
[0100] 8. Longer Aldehydes and Combinations of Aldehydes
[0101] Aldehyde combinations or aldehydes having a chain length of 6 carbons or longer are effective elasmobranch repellents. It is demonstrated herein that aldehyde mixtures and aldehydes having a carbon chain of six carbons or longer or any derivatives thereof may be administered to elasmobranchs to repel them. Table 8 provides data evidencing the repellent activity of aldehydes with carbon chains longer than 6 carbons and combinations of aldehydes. Additional data evidencing the repellent activity of combinations of aldehydes may be seen in Table 23.
[0000]
TABLE 8
Syringe
Syringe Bite 3/5/10
Compound Y R N Y R N Y R N
octanal 1/1
nonanal 1/1
decanal 1/1
heptanal 1/1
mesityl oxide 1/1
octanal 1/1
Aldehyde Mixture 7/7 6/6 2/2
BA1
Total 12/13 1/13 6/6 2/2
Percent of Trials 92% 8% 100% 100%
[0102] Eight-carbon octanal, nine-carbon nonanal, ten-carbon decanal and six-carbon mesityl oxide were tested on lemon sharks using a Syringe Assay. In each case, tonic immobility was terminated. In one Syringe Assay with heptanal, no response was observed. In seven of seven Syringe Assay tests for an aldehyde combination containing proprional, butanal, isobutanal, pentanal, hexanal, heptanal, octanal, nonanal, decanal, cuminal, cinnimal, anisal, mesityl oxide, p-tolualdehyde and veratraldehyde on lemon and blacktip sharks, tonic immobility was terminated. In two of two Syringe 3/5/10 Assay tests of the aldehyde combination on lemon and blacktip sharks, tonic immobility was terminated. In six of six Bite Assay tests of the aldehyde combination on nurse sharks, tonic immobility was terminated. In only a single Micropipette Assay test wherein 500 microliters of aldehyde mixture was delivered was no response observed.
[0103] Table 8 evidences the repellent effect of combinations of aldehydes and aldehydes having carbon chains 6 carbons or longer on a variety of sharks. Such aldehydes may be administered into the vicinity of an elasmobranch in any method of delivery known in the art or herein disclosed. As such, compositions for repelling an elasmobranch comprising aldehydes of lengths of six carbons and greater and combinations of aldehydes have been provided herein.
C. Composition for Repelling Elasmobranchs Comprising Carboxylic Acid
[0104] Carboxylic acids or derivatives thereof alone or in combinations are disclosed herein as effective elasmobranch repellents. Exemplary and non-limiting carboxylic acids include n-butyric acid, isobutyric acid, valeric acid, isovaleric acid, propanoic acid, citric acid, 2-butenoic acid (crotonic acid), 3-butenoic acid (cinylacetic acid), trans-cinnamic acid, 2-hydroxy-1,2,3-propanetricarboxylic acid, 1,2,3-propanetricarboxylic acid (tricarballylic acid), hydroxysuccinic acid (di-malic acid), 2,2-dimethylbutyric acid, 2,3,3-trimethylpropionic acid, 2,3-dimethylbutyric acid, 2-ethylbutyric acid, 2-ketobutyric acid, 2-methylisovaleric acid, 3-aminobutyric acid, and 4-acetylebutyric acid. Non-limiting exemplary carboxylic acids include dicarboxylic acids and tricarboxylic acids. Other naturally occurring acids that repel sharks include malic acid, lactic acid, succinic acid, fumaric acid and tricarballylic acid. These compounds may be used in powder (crystalline) form, or in aqueous or polar solvent solutions.
[0105] Carboxylic acids may be solubilized in any manner known to the art for administration into the expected environment of an elasmobranch. In a preferred composition, the carboxylic acid is prepared at a concentration of 0.1% w/w to 100% w/w in powder or liquid form wherein the powder is solubilized in water, ethanol or a suitable polar solvent. An exemplary mixture is 20% w/w 3-butenoic acid, 10% w/w citric acid, and 5% w/w/trancinnamic acid solubilized in 50:50 w/w water:ethanol.
[0106] Carboxylic acids disclosed herein include all carboxylic acids having the COOH function of a carboxylic acid. A preferred non-limiting class of carboxylic acids includes carboxylic acids comprising one to ten carbons. A more preferred non-limiting class of carboxylic acids comprises two to about five carbons. Another preferred non-limiting class of carboxylic acids comprises the dicarboxylic acids. Another preferred non-limiting class of carboxylic acids comprises the tricarboxylic acids.
[0107] The presence of butyric acid was detected in semiochemical extractions of decayed shark tissue using gas chromatography coupled with mass spectrometry and NIST structure libraries. Semiochemicals from decayed shark tissue have been shown to have properties that repel elasmobranchs. When n-butyric acid was presented to juvenile lemon or nurse sharks in tonic immobility, the immobility was terminated at mouth doses of 100 microliters.
[0108] Because n-butyric acid presents a very unpleasant odor during handling, its derivatives were studied. Screening of derivatives of butyric acid revealed the following compounds as repellents in lemon and nurse sharks. 2,2-Dimethylbutyric Acid, 2,3,3-Trimethylproprionic Acid, 2,3-Dimethylbutyric Acid, 2-Ethylbutyric Acid, 2-Ketobutyric Acid, 3-Aminobutyric Acid, 4-Acetylbutyric Acid. Also, compounds having the -enoic form of butyric acid were tested revealing the following shark repelling compounds: 2-butenoic acid and 3-butenoic acid. Additionally, naturally-occurring carboxylic acids were found to have shark repelling properties. Other carboxylic acids and carboxylic acid combinations were tested. Tables 9 and 10 provide a portion of the data of some carboxylic acids.
[0109] Table 9 demonstrates the repellent effect of butyric acid, butyric acid derivatives, enoic acid derivatives of butyric acid and naturally occurring carboxylic acids.
[0000]
TABLE 9
Hd-
Syringe Syringe 3/5/10 Micropipette
Compound/Mixture Y Y R N Y
n-Butyric Acid 2/2
2,2-Dimethylbutyric Acid 2/2
2,3,3-Trimethylproprionic 2/2
Acid
2,3-Dimethylbutyric Acid 2/2
2-Ethylbutyric Acid 2/2
2-Ketobutyric Acid 2/2
2-Methylisovaleric Acid 2/2
3-Aminobutyric Acid 2/2
4-Acetylbutyric Acid 2/2
3-Butenoic Acid 2/2
(vinylacetic acid)
crotonic acid solution (2- 4/7 1/7 2/7 2/2
butenoic acid)
4-acetylbutyric acid 2/2
trans-Cinnamic acid 2/2
Citric acid 50% w/w 4/4 2/2
Tricarballylic Acid 2/2
Hydroxysuccinic Acid 2/2
Total 4/4 4/7 1/7 2/7 30/30
Percent of Trials 100% 71% 14% 29% 100%
"Y" denotes termination of tonic immobility.
"R" denotes a behavior response within tonic immobility.
"N" denotes no response.
[0110] In a first Micropipette Assay on a juvenile lemon shark, an oral dose of no more than 100 microliters of butyric acid terminated tonic immobility. An oral dose of no more than 400 microliters was then delivered by micropipette into the mouth of a juvenile nurse shark. Tonic immobility was terminated. For each derivative of butyric acid and each naturally occurring acid listed in Table 9, a first micropipette assay was performed on a juvenile lemon shark with no more than 100 microliters of test acid in an oral dose. A second micropipette assay was then performed on a juvenile nurse shark with no more than 400 microliters of test acid in an oral dose. In each test, tonic immobility was terminated. This data evidences the gustatory repelling activity of carboxylic acids.
[0111] In four of four Hd Syringe assays, citric acid between 0.3 mL and 2.4 mL of citric acid 50% w/w was delivered about three inches from the mouth of a lemon shark. Each assay terminated tonic immobility. Seven Syringe 3/5/10 assays on nurse and lemons sharks were employed with crotonic acid solution. In four of the seven assays the crotonic acid was delivered directly to the mouth on a longline or within 10 inches of the shark's mouth. Tonic immobility was terminated. In one of the seven assays, the crotonic acid was delivered directly to the mouth of the shark on a longline and a behavioral response was observed in tonic immobility. In two of the seven assays, 6 mL of crotonic acid was delivered to lemon sharks at a distance of 36 inches from the shark's mouth. No response was observed. The lack of response is explained by the small volume delivered at a very large distance.
[0112] The data in Table 9 evidences the broad repellent activity of carboxylic acids and the exemplary and non-limiting repellent activity of butyric acid, butyric acid derivatives, enoic acids and naturally occurring carboxylic acids. Table 10 additionally evidences the repellent effect of lactic acid and carboxylic acid combinations.
[0000]
TABLE 10
Cloud
Syringe 3/5/10 Dispersion
Compound/Mixture Y R N Y
lactic acid 2/3 1/3
Crotonic/Cinnamic/ 3/4 1/4 1/1
Maleic Acid
Crotonic/Citric/ 6/7 1/7
Fumaric Acid
Crotonic/Citric/ 7/7
Cinnamic
Total 15/21 3/21 1/27 8/8
Percent of Trials 71% 14% 19% 100%
"Y" denotes termination of tonic immobility.
"R" denotes a behavior response within tonic immobility.
"N" denotes no response.
[0113] In two of three Syringe 3/5/10 assays, lactic acid was delivered to lemon sharks and terminated tonic immobility. In a single Syringe 3/5/10 assay, lactic acid was delivered to a lemon shark and no behavioral change was observed. In three of four Syringe 3/5/10 assays, a mixture of Crotonic, Cinnamic and Maleic acids in glycol were delivered to tiger and blacknose sharks and terminated tonic immobility. In one of four Syringe 3/5/10 assays, delivery of the repellent actually missed the mouth of the shark and only a behavior change was seen in tonic immobility.
[0114] In a cloud dispersal assay of a mixture of Crotonic, Cinnamic and Maleic acids, 400 ml was dispersed from a diptube near the mouth of a great hammerhead shark. The shark fled the area and did not return.
[0115] In a surrounding cloud dispersal assay, 500 mL of a mixture of 20% w/w Crotonic acid, 10% w/w Citric acid and 5% w/w Cinnamic acid solubilized in 50:50 w/w water:ethanol was delivered in a subsurface dose in the vicinity of a population of competitively feeding sharks (5 Caribbean reef sharks and 2 blacknose sharks). The sharks were dispersed and did not return.
[0116] The data in Table 10 further evidences the broad repellent activity of carboxylic acids and mixtures of carboxylic acids. Together, Tables 9 and 10 evidence that carboxylic acids may be delivered into the vicinity of an elasmobranch in any method of delivery known in the art or herein disclosed to repel elasmobranchs. As such, a composition for repelling an elasmobranch comprising a carboxylic acid and derivatives thereof has been provided herein.
[0117] The composition for repelling an elasmobranch may comprise any carboxylic acid. It may preferably comprise a butyric acid, citric acid, a trans-cinnamic acid, 2-butenoic acid, lactic acid, 2,2-dimethylbutyric acid, 2,3,3-trimethylproprionic acid, 2-ethylbutyric acid, 2-detobutyric acid, 3-aminobutyric acid, 4-acetylbutyric acid, 3-butenoic acid, tricarballylic acid, hydroxysuccinic acid or any carboxylic acid that is deliverable to the environment of an elasmobranch, for example soluble in water or dissolved in a vehicle for delivery prior to delivery.
D. Composition for Repelling Elasmobranchs Comprising a Ketone or Di-Ketone
[0118] A composition for repelling an elasmobranch is provided herein comprising a ketone or a derivative thereof including, for example, ionone or zingerone, or a di-ketone or a derivative thereof, including, for example, 2,3-butanedione. Repelling characteristics of ketones and their derivatives and di-ketones and their derivatives are provided herein. Table 11 evidences the repellent activity of ketones and their derivatives and di-ketones and their derivatives.
[0119] Exemplary, non-limiting ketones and derivatives thereof include ionone, zingerone and derivatives thereof. Exemplary, non-limiting di-ketones and derivatives thereof include 2,3-butanedione, glyoxal and methylglyoxal. Data in Table 11 demonstrates the gustatory repellent activity of ketones and di-ketones.
[0120] In two individual cage assays for ionone and zingerone, repellent activity was demonstrated by a decrease in the number of strikes by feeding sharks against a baited cage. In both the ionone and zingerone assays, a decrease in the number of strikes at the cage was recorded when 500 mL of the repellent was delivered to the competitively feeding sharks. After a lull in feeding for 10 minutes, the sharks returned to the baited cage. See Example 5.
[0121] Tonic immobility studies were carried out on 2,3-butanedione and 2,3 butanedione (diacetyl) in denatured ethanol solution. In seven of eight Syringe Assays, tonic immobility was terminated in juvenile lemon and nurse sharks. In one Syringe Assay, a behavioral response was noted during tonic immobility. In one cloud dispersion assay in free-swimming Caribbean reef and blacknose sharks, no response was noted since the volume was only 290 microliters. No response would be expected with such a low volume. In one bite assay with a juvenile nurse shark, tonic immobility was terminated.
[0000]
TABLE 11
Cloud/
Syringe Bite Cage
Compound Y R N Y R N Y R N
Ionone 1/1
Zingerone 1/1
2,3-butanedione 7/8 1/8 1/1 1/1
Total 7/8 1/8 1/1 2/3 1/3
Percent of Trials 88% 13% 100% 67% 33%
"Y" denotes termination of tonic immobility.
"R" denotes a behavior response within tonic immobility.
"N" denotes no response.
[0122] The data in Table 11 evidences the gustatory repellent activity of ketones, such as ionone and zingerone, and di-ketones, such as 2,3-butanedione. Other ketones having demonstrated gustatory repellent activity would include glyoxal and methylglyoxal.
E. Composition for Repelling Elasmobranchs Comprising a Pyridine
[0123] A composition for repelling an elasmobranch is provided herein comprising a pyridine or a derivative thereof including 2-methylpyridine (alpha-picoline, 3-methylpyridine (beta-picoline), 4-methylpyridine (gamma-picoline), lutidine (dimethylpyridine), and isomers of lutidine, collidine (trimethylpyridine) and isomers of collidine, 2-amino-3-picoline and derivatives of each or all of the pyridine derivatives. Repelling characteristics of pyridine and its derivatives are provided herein.
[0124] In initial investigations using tonic immobility assays in juvenile sharks, pyridine, alpha-picoline (2-methylpyridine), beta-picoline (3-methylpyridine), gamma-picoline (4-methylpyridine), lutidine and isomers thereof, collidine (trimethylpyridine) and isomers thereof and 3-amino-2-picoline all terminated tonic immobility when introduced to the mouth using a micropipette or syringe.
[0125] Table 12 provides some additional data demonstrating the repellent effect of pyridines and its derivatives in further investigations.
[0000]
TABLE 12
Hd-Syringe Syringe 3/5/10
Compound Y R N Y R N
Pyridine 3/4 1/4
3- 3/3
methylpyridine
Total 3/3 3/4 1/4
Percent Total 100% 0% 0% 75% 25% 0%
"Y" denotes termination of tonic immobility.
"R" denotes a behavior response within tonic immobility.
"N" denotes no response.
[0126] In three of four Syringe 3/5/10 assays, pyridine was delivered to nurse and lemon sharks and terminated tonic immobility. In one of four Syringe 3/5/10 assays, delivery of pyridine evoke a change in behavior within tonic immobility of a nurse shark. In three of three Hd Syringe assays, delivery of 3-methylpyridine resulted in termination of tonic immobility. In two of two Micropipette assays on 2-amino-3-picoline 95% behavioral responses were noted in lemons sharks. In one, tonic immobility was terminated. In another, a violent seizure in the shark in response to the assay rendered measurement impossible.
[0127] Together the data in Table 12 demonstrate that pyridine and its derivatives such as 3-methylpyridine are good elasmobranch repellents. Pyridine is a simple heterocyclic aromatic organic compound that is structurally related to benzene, with one CH group in the six-membered ring replaced by a nitrogen atom. Pyridine has an equatorial lone pair of electrons at the nitrogen atom that does not participate in the aromatic pi-system. This makes pyridine a basic compound as well as a nucleophile. Pyridine is completely miscible in water.
[0000]
[0128] The addition of one methyl group to the pyridine ring has no appreciable reduction on miscibility, particularly in seawater. Methylpyridines are commonly called "picolines." Methyl groups may occur at the [alpha], [beta], [gamma] positions relative to the nitrogen:
[0000]
[0129] It was not considered necessary to test all isomers because in a shark, isomers would not be expected to have steric effects that would alter the bioactivity of the compound since there are no electrophilic reactions occurring. As long as the compound is miscible or soluble in seawater, it is expected that it will find its way to a gustatory receptor site and activate it.
[0130] Two methyl functions on the pyridine ring have slight reduction on miscibility, particularly in seawater. Dimethylpyridines are commonly called "lutidines." Dimethylation may occur in the following positions:
[0000]
[0131] As discussed above, it was not considered necessary to test all isomers because in a shark, isomers would not be expected to have steric effects that would alter the bioactivity of the compound since there are no electrophilic reactions occurring.
[0132] Likewise, addition of three methyl functions to the pyridine ring has an appreciable reduction on miscibility, particularly in seawater. This compound is now only partially miscible, but is still bioactive. Trimethylpyridines are commonly called "collidines." Trimethyl functions may occur at the following positions:
[0000]
[0133] Toxicity is slightly reduced as methylation increases. Pyridine itself is not a preferred repellent because pyridine is a carcinogen and considered a marine pollutant. Likewise, picolines are less preferred repellents because they are irritants (making handling difficult) and are suspected carcinogens. While collidines may be the least irritative of all the methylpyridines, their toxicity decreases their preferability. Nevertheless, the repellent activity of these compounds is clearly disclosed herein.
[0134] It is reasonable to expect that alkyl, alkenyl, amino, hydroxyl, nitro, and halo-functions on the pyridine ring are useful gustatory repellents as well. For example, aminopicoline exhibited violent responses in juvenile sharks in the tonic immobility assay. Once again, however, the toxicity of this compound makes it less preferred from a handling, storage, and environmental standpoint.
F. Composition for Repelling Elasmobranchs Comprising an Anti-Pyrine
[0135] A composition for repelling an elasmobranch is provided herein comprising an anti-pyrine or a derivative thereof including anti-pyrine (phenazone) or 4-amino-antipyrine (metapirazone). Table 13 provides data evidencing the repellent activity of the anti-pyrines. In five of five Hd Syringe assays, 4-amino-antipyrine terminated tonic immobility in lemon and nurse sharks. Additionally, the data from two Micropipette assays demonstrate that antipyrine is a gustatory repellent. See Table 26.
[0000]
TABLE 13
Hd-Syringe
Compound Y R N
4-amino- 5/5
antipyrine
(metapirazone)
Total 5/5
Percent of Trials 100%
G. Composition for Repelling Elasmobranchs Comprising a Combination of Elasmobranch Repellents
[0136] A composition for repelling an elasmobranch is provided herein comprising a combination of two or more of aldehydes or derivatives thereof, carboxylic acids or derivatives thereof, ketones or derivatives thereof, diketones or derivatives thereof, pyridines or derivatives thereof or antipyrines or derivatives thereof. It is expected that a combination of respective gustatory repellents will act together as a repellent composition. A composition may comprise a combination of any elasmobranch repellent.
[0137] For example, an effective elasmobranch repellent composition may comprise a number of aldehydes. See, e.g., Example 8, aldehyde repellent composition "BA1." A repellent composition may also, for example, comprise aldehydes and a di-ketone. A non-limiting preferred combination of aldehydes and a di-ketone may comprise butyraldehyde, isobutyraldehyde, veratraldehyde and 2,3-butanedione. See Example 8, aldehyde repellent composition "BA3." Likewise, a repellent composition may comprise, for example, a combination of crotonic acid, citric acid and fumaric acid, or a combination of crotonic acid, cinnamic acid and maleic acid. See Table 24. Each of these combinations, along with a variety of other combinations disclosed herein, evidence repellent characteristics. See, e.g., Tables 23-24.
[0138] The data in Examples 8 and 9 and Tables 23 and 24, together with the disclosure provided herein, evidence the effectiveness of combinations of elasmobranch repellents as elasmobranch repellent compositions.
H. A Method of Manufacturing an Elasmobranch Repellent
[0139] The repellents and methods describe herein provide the artisan with chemicals that have been demonstrated to repel, at very low concentrations, families of shark known to migrate in shallow coastal waters and species known to attack humans. As such, one of skill in the art will recognize from the breadth of repellents disclosed herein that an elasmobranch repellent may be manufactured by combining an aldehyde or a derivative thereof, a carboxylic acid or a derivative thereof, a ketone or a derivative thereof, a di-ketone or a derivative thereof, a pyridine or a derivative thereof, or an antipyrine or a derivative thereof, separately or in combination, with an acceptable solvent, carrier, diluent or other vehicle for administration or storage. An exemplary solvent is ethanol or ethanol:water (50:50 w/w). Ethanol and water are excellent solvents for elasmobranch repellents because they are not prohibited by federal regulations from dispersion in sea water. Other exemplary solvents include acetonitrile, dimethyl sulfoxide (DMSO), denatured alcohol, C3-C4 glycols (2,3-propanediol, butanediol), glycol ethers (diethylene glycol monoethyl ether), and glycol ether esters.
[0140] An elasmobranch repellent may also be manufactured by crystallizing any of the above-discussed compounds and preparing them as a powder to be dispensed into water. Powdered substances may be combined with carriers to improve solubility or handling. One skilled in the art would recognize many different carriers or diluents that may be combined with a powder of any of the repellents discussed herein.
III. METHODS AND DEVICES OF DELIVERY OF REPELLENT
[0141] A. Method for Repelling Elasmobranch with Gustatory Compounds
[0142] Also provided herein is a method of repelling an elasmobranch comprising administering a composition for repelling an elasmobranch comprising an aldehyde or a derivative thereof, a carboxylic acid or a derivative thereof, a ketone or a derivative thereof, a di-ketone or a derivative thereof, a pyridine or a derivative thereof, or an antipyrine or a derivative thereof, separately or in combination, in the expected proximity of said elasmobranch.
[0143] Any of the repellents disclosed herein may be delivered to the environment of an elasmobranch through a variety of methods and devices of delivery. These compounds are most useful when they can be directed into a shark's mouth or into the environment where the repellent may enter the shark's mouth. As such, a squirt gun or long syringe is a good delivery vehicle. The repellents disclosed herein may likewise be incorporated into lotions, longline time-release gels, time-release sponges, jelly's or any other delivery device or substance contemplated by one of skill in the art.
[0144] An extensive disclosure of devices for delivery of chemical repellents into the vicinity of an elasmobranch is provided in PCT/US06/05035. Delivery devices disclosed therein include, e.g., pressurized delivery pole apparatuses, syringes, cattle-treatment "drench" guns, aerosol canisters, mortar-launched aerosol "bomb" canisters, automated repellent dispensers on a raft or fixed to some other object, repellent dischargers, pouches containing repellents, apparatuses for administering repellent along fishing longline, repellent backpack dischargers for use, for example, by scuba divers and those who snorkel, spear guns fitted with a repellent discharge device, delivery devices for surfboards, wristwatches, belts and bracelets. Each of the devices and suggestions for devices disclosed therein may be applied to delivery of the compounds disclosed herein. PCT/US06/05035, filed Feb. 13, 2006, is incorporated in its entirety herein by reference.
[0145] Most aldehydes will oxidize in air. Therefore, it is prudent to stabilize the aldehyde when it is stored, especially in the warm climates. In a non-limiting preferred handling method, the practitioner may use a pinch of hydroquinone or sodium iodide with 3-methylbutanal or other aldehyde. The mixture may be stored under nitrogen. This method keeps the aldehyde fresh and effective. If an aldehyde oxidizes (in the case of 3-methylbutanal to becomes isovaleric acid, which smells like feet or cheese) the resulting acid may be less potent than the aldehyde.
[0146] Many of the gustation compounds disclosed herein are regulated under federal environmental regulations. Some are considered marine pollutants, and others, like pyridine and aminopyridine are considered toxic. However, some, such as 3-methylbutanal, 2-methylbutanal, 3-methylbutenal, 2-methylbutenal, trans-pentenal, piperonal, etc., are very safe and meet federal regulations.
B. Delivery Devices for Gustatory Compounds
[0147] Alternative methods for delivering a chemical repellent into an elasmobranch environment include a miniature pressurized repellent gun to be worn on the wrist or ankle, a spear fishing gun with an adjacent repellent cylinder, a time release sponge-material attached to a surfboard or otherwise placed near a diver, swimmer or in some other place of interest, a hollow surfboard with a calibrated drip to emit repellent, a pump delivery system affixed to a surfboard, a pressurized delivery device affixed to a surfboard wherein discharge of repellent may be triggered by the surfer, a floatation device, a wristwatch filled with repellent (pressurized or unpressurized), a carbon dioxide activated pressurized syringe, an aerosol bomb, a mortar-launched aerosol bomb, a remote-controlled buoy with a repellent tank that may be fired by a lifeguard or other person or mechanized system, a buoy with a metering pump that runs during swim time (daylight), a repellent pouch attached to longlines (muslin/burlap bags) or to clothing or surfboard or other water device, jellied repellent (glycol ether/hydroxypropylcelluose gels which time-dissolve in water), sunscreen/sun care formulations containing repellent, lotions containing repellent, porous fabric impregnated with repellent, rechargeable porous fabric impregnated with repellent, a kite- or balloon-deployed repellent bomb (remote control), a submerged repellent mine (remote control) for deeper water, a cattle-treatment drench gun converted to shark repellent gun (http://www.dr-register.com/drenchgun.htm), repellent-impregnated cable insulation and cable jackets for undersea lines.
[0148] Chemical repellents disclosed herein may be discharged through a pressurized tube that runs alongside an extended or extendable poll. The pressurized delivery pole apparatus may be useful for administering repellent to feeding or otherwise stimulated sharks. The apparatus may comprise a delivery device housing (pole) with a repellent discharge tube housed along or within the pole. The repellent discharge tube may be connected to a pressurized chamber or chambers containing repellent. The delivery device may contain a check valve to facilitate the maintenance of pressure. A trigger may allow the pressurized repellent to discharge through the tube and away from the pole. An alternative delivery device may be a pressurized syringe. Such a syringe may be filled with repellent.
[0149] 1. Pressurized Container Delivery Device
[0150] An alternative delivery method may be a pressurized container of repellent such as an aerosol canister. The container may be constructed of degradable material. The aerosol canister may have sufficient pressure and repellent to be discharged in the water and repel sharks in the area. The container may be asymmetrically weighted to provide an erratic movement in the water as it discharges repellent. The aerosol container may further comprise an actuator that when engaged allows the compressed contents of the aerosol container to be expelled. The device further preferably comprises a continuous discharge apparatus to allow the contents of the can to be expelled with a single activation of the discharge apparatus. Preferably, when the actuator is engaged, the nozzle remains open to allow the can to be continuously and fully evacuated. The actuator may be made of a soluble material that allows discharge when exposed to water.
[0151] 2. Raft/Buoy Delivery Device
[0152] Another delivery device is a raft or other floating or fixed device comprising a floating buoy, a solid platform, and a container of repellent connected to a pump with a power source that is capable of delivering repellent into a shark environment either by automatic timing, remote triggering or other actuating mechanism. The container comprises a check valve that allows the pump to build pressure in the container to a desired pressure. When a desired pressure is achieved, a release valve or pressure-release cap releases the pressurized repellent into a delivery tube. The repellent is expelled across the water, spreading a wide cloud of repellent.
[0153] The pump may be automatically activated by a timer or may be activated remotely. The pump preferably delivers sufficient repellent into the water to repel sharks. Preferably, the discharge tube is long enough and not submerged such that when delivery begins, the repellent is sprayed a substantial distance onto the surface of the water and, under pressure, the discharge tube moves erratically across a large radial area in relation to the raft. In a preferred method the discharge tube is made of flexible material. Preferably the discharge tube will spray over an entire 360 degree arc.
[0154] 3. Pouch
[0155] Another delivery device is a pouch containing repellent or a sponge treated with repellent. Repellent may be in the form of a solution or solid, preferably partly or wholly soluble. The repellent may be introduced to the environment of the shark by diffusion or by rupturing, tearing or otherwise penetrating the pouch. A pouch may also diffuse repellent through its fabric. A diffusing pouch may be attached to a fishing net or longline with a baited hook on a snood to allow repellent to slowly diffuse into the water surrounding bate or fishing net. The pouch will provide sufficient repellent around the baited hook to repel sharks while not repelling the desired teliost fish. A pouch to be placed on a longline may preferably be constructed of muslin or burlap.
[0156] 4. Longline Apparatus
[0157] Sharks represent a significant problem in the long line fishing industry. Chemical repellents on longlines in accordance with the invention are preferably soluble in seawater, and, at a sufficient concentration to produce flight responses in elasmobranches. Teleost fish are not affected by the chemical repellents.
[0158] Another delivery device is an apparatus for administering repellent along longline fishing tackle. The apparatus comprises a pressurized chamber connected to a source of compressed gas, contains repellent and is connected to a primary delivery tube. The primary delivery tube is positioned adjacent to or otherwise in concert with the longline. Additional secondary delivery tubes are joined to the primary delivery tube in proximity to each snood of the longline. The secondary delivery tubes terminate near the baited hook of the snood. When pressurized repellent is released from the chamber, the repellent is delivered along the primary delivery tube and into the secondary delivery tubes thereby discharging repellent near the baited hook and repelling sharks from the bait.
C. Kit for Repelling Elasmobranch
[0159] The skilled artisan will recognize from the disclosure herein that a kit may be compiled comprising a composition for repelling an elasmobranch comprising an aldehyde or a derivative thereof, a carboxylic acid or a derivative thereof, a ketone or a derivative thereof, a di-ketone or derivative thereof, a pyridine or a derivative thereof, or an antipyrine or a derivative thereof and a vehicle of administering said repellent.
[0160] In a preferred combination for a kit, the vehicle is selected from the group consisting of a pressurized or pressurizable delivery device, a pressurized or pressurizable repellent gun, a miniature pressurizable repellent gun to be warn on a wrist or an ankle of a subject, a spear fishing gun with an adjacent pressurizable repellent container, a time release sponge, a surfboard, a pump delivery system affixed to a surfboard, a pressurized delivery device affixed to a surfboard, a wristwatch comprising said repellent, a syringe, a pressurized syringe, an aerosol bomb, a mortar-launched aerosol bomb, a remote-controlled buoy with a repellent tank, a fixed buoy with a metering pump, a repellent pouch, a jelly comprising glycol ether and hydroxypropylcelluose, a skin lotion containing said repellent, a porous fabric impregnated with repellent, rechargeable porous fabric impregnated with said repellent, a submerged repellent mine, a repellent-impregnated cable insulation for an undersea cable, and a repellent-impregnated cable jacket for an undersea cable.
[0161] The invention is further described with the following non-limiting examples, which are provided to further illuminate aspects of the invention.
IV. EXAMPLES
Example 1
Methylbutanal Elasmobranch Repellents
[0162] Tonic immobility studies were carried out on lemon, nurse, tiger and blacktip sharks with 3-methylbutanal and 2-methylbutanal using Hd Syringe, Syringe 3/5/10 and Bite assays. In 40 of 41 total assays for repellent effect, tonic immobility was terminated or the sharks demonstrated a change of behavior during tonic immobility upon delivery of 3-methylbutanal. In Micropipette assays, a gustatory response to the deliver of 3-methylbutanal was observed in 16 of 20 assays.
[0163] Lemon, nurse, blacktip and tiger sharks were placed in tonic immobility by inverting the shark's body along its longitudinal axis. Each shark was observed to enter a tonic state of paralysis. The "tonic" state of each shark was first established by releasing a control of seawater in proximity to the shark with the same delivery instrument and at the same distance as the chemical repellent would later be delivered. In certain controls, sea water was released with a high flow rate (30 mL/sec) in order to establish that the sharks would not be awakened by a jet of fluid over their noses.
[0164] Once behavioral controls were established, the chemical repellent was delivered to the shark using the Hd Syringe Assay method, the Syringe 3/5/10 Assay method, the Bite Assay method or the Micropipette Assay method. The shark was observed for any behavioral response. If tonic immobility was terminated, the positive response was denoted as "Y." If tonic immobility was not terminated but a behavior change within tonic immobility was noted, such as the opening of the mouth or a cough, the response was denoted as an "R" for reduced response. If no behavior change was observed, the negative response was denoted "N."
[0165] In Hd-Syringe Assays, the 3-methylbutanal was delivered to the shark's mouth and nares using a 3 mL hypodermic syringe fitted with a 22 gauge needle. The needle was held within 3 inches of the shark's mouth and the test repellent was slowly released from the syringe with a very fine plume in the water column. Any response was denoted. If a response occurred during a measurable time after delivery of the test chemical, the time between delivery and response was noted. If a response occurred immediately or the response occurred before a measurable time could be established, no time to response was noted.
[0166] Because the test chemical repellent is delivered at a distance from the shark's nares and mouth, a cloud of test chemical repellent is dispersed over the shark within the water column. The dispersed test repellent is subject to water current direction, dispersion and dilution. As a result, the time between delivery of the chemical repellent and a response was not correlatable with volume of delivered repellent or potency of repellent. Instead, the time between delivery and response was usually related to water current.
[0167] In Syringe 3/5/10 Assays, 5-6 mL of 3-methylbutanal was delivered at least 3 inches in front of the shark's mouth. A cloud of the repellent dispersed over the shark within the water column. The shark was observed for a behavioral response. A behavioral response within 10 seconds was considered a positive flight response. Time from delivery of the repellent until behavioral response was recorded, if measurable. As noted above, because the dispersion of the chemical repellent upon delivery is affected by volume of repellent, water current, and other factors, the time between delivery and response was not correlatable with the potency of the repellent.
[0168] In Bite Assays, a dose of typically less than 5 mL of 3-methylbutanal was presented directly into the shark's mouth using a pipette. The shark was observed for behavioral response as above. Because the delivery was directly into the shark's mouth and responses were generally observed immediately upon delivery, time to response was not recorded.
[0169] Twenty seven Hd Syringe Assays were performed. Eleven assays on juvenile lemon sharks, 15 assays on juvenile nurse sharks and one assay on a tiger shark. See Table 14. In 74% of assays tonic immobility was terminated indicated a flight response. In 22% of assays a behavioral change was observed indicating a response to the chemical repellent. In one assay no response was observed. See Table 14.
[0170] Seven Syringe 3/5/10 Assays were performed. One assay on a juvenile lemon shark, two assays on juvenile nurse sharks, three assays on blacktip sharks and one assay on a tiger shark. 100% of assays terminated tonic immobility indicating a flight response. Two Bite assays were performed on nurse sharks each resulting in termination of tonic immobility indicating a flight response. In total, 97% of assays resulted in a positive response to 3-methylbutanal and 81% resulted in direct termination of tonic immobility demonstrating a strong repelling effect for 3-methylbutanal. See Table 14.
[0171] Twenty Micropipette Assays were performed on juvenile lemon sharks and juvenile nurse sharks. In fifteen assays a gustatory response was observed (five terminated tonic immobility, ten behavioral responses observed). In five assays no response was observed. These data demonstrate the gustatory repellent activity of 3-methylbutanal because the repellent is delivered directly into the mouth of the shark and no repellent is available to the nose of the shark. See Table 14.
[0172] Because Micropipette assays deliver very small volumes into the mouth of the shark, the volume of repellent is at times not sufficient to evoke termination of tonic immobility and, more rarely, is not sufficient to evoke a response. Further because the Micropipette assays were often done serially on the same set of sharks, second and third doses of the repellent over time would be expected to evoke less of a reaction. Nevertheless, the small doses delivered to the shark in the Micropipette assay provide important data on whether a compound is a gustatory stimulant because the small dose may be delivered directly into the mouth of the shark. This eliminates any olfactory response that might be acting in concert with a gustatory response to terminate tonic immobility.
[0173] In each assay disclosed herein, a control of sea water was performed prior to the testing of each test repellent. In the control, sea water was delivered to the shark to be tested in the same delivery manner as the test repellent was delivered. If the shark made no response to the delivery of sea water, the control was considered successful. Subsequently, the test repellent was delivered to the shark. In Cloud Dispersion assays and Cage assays, sea water or dye control was delivered to the test shark population prior to delivery of repellent.
[0174] Controls were performed in each assay reported herein and yielded no response from the subject shark. Control data is not illustrated in the data tables provided in this example or throughout. Nevertheless, each data point was subject to a control prior to the testing of each compound.
[0000]
TABLE 14
Tonic Immobility Assays with 3-Methylbutanal
Re-
Component Species TTI Delivery Dose sponse
3-methylbutanal Lemon R Hd syringe 3.6 ml
3-methylbutanal Lemon R Hd syringe 1.4 ml
3-methylbutanal Lemon Y Hd syringe 400 ul
3-methylbutanal Lemon Y Hd syringe 500 ul
3-methylbutanal Lemon Y Hd syringe 0.6 ml 6.27 sec
3-methylbutanal Lemon Y Hd syringe 0.3 ml 2.73 sec
3-methylbutanal Lemon Y Hd syringe 0.3 ml 2.17 sec
3-methylbutanal Lemon Y Hd syringe 0.5 ml 4.53 sec
3-methylbutanal Lemon R Hd syringe 1 ml 1.7 sec
3-methylbutanal Lemon Y Hd syringe 1.6 ml 9.52 sec
3-methylbutanal Lemon N Hd syringe 1.4 ml
3-methylbutanal Nurse Y Hd syringe 0.6 ml
3-methylbutanal Nurse R Hd syringe 0.8 ml
3-methylbutanal Nurse Y Hd syringe 1.6 ml
3-methylbutanal Nurse Y Hd syringe 400 ul
3-methylbutanal Nurse Y Hd syringe 150 ul
3-methylbutanal Nurse Y Hd syringe 350 ul
3-methylbutanal Nurse R Hd syringe 0.4 ml 1.28 sec
3-methylbutanal Nurse R Hd syringe 0.8 ml 2.80 sec
3-methylbutanal Nurse Y Hd syringe 1.2 ml 4.39 sec
3-methylbutanal Nurse Y Hd syringe 0.2 ml 0.64 sec
3-methylbutanal Nurse Y Hd syringe 0.2 ml 0.89 sec
3-methylbutanal Nurse Y Hd syringe 0.2 ml 0.76 sec
3-methylbutanal Nurse Y Hd syringe 0.15 ml 1.08 sec
3-methylbutanal Nurse Y Hd syringe 0.1 ml 0.82 sec
3-methylbutanal Nurse Y Hd syringe 0.2 ml 1.00 sec
3-methylbutanal Tiger Y Hd syringe 1 ml 1 sec
3-methylbutanal Lemon Y syringe 3/5/10 6 ml 2 sec
3-methylbutanal Nurse Y syringe 3/5/10 3 ml 2 sec
3-methylbutanal Nurse Y syringe 3/5/10 3 ml 2 sec
3-methylbutanal Blacktip Y syringe 3/5/10 6 ml 5 sec
3-methylbutanal Blacktip Y syringe 3/5/10 6 ml 2.75 sec
3-methylbutanal Blacktip Y syringe 3/5/10 6 ml 1.47 sec
3-methylbutanal Tiger Y syringe 3/5/10 6 ml 3.46 sec
3-methylbutanal Nurse Y bite 3.6 ml
3-methylbutanal Nurse Y bite 2 ml
3-methylbutanal Lemon Y micropipette 150 ul
3-methylbutanal Lemon R micropipette 150 ul
3-methylbutanal Lemon R micropipette 150 ul
3-methylbutanal Lemon R micropipette 200 ul
3-methylbutanal Lemon R micropipette 250 ul
3-methylbutanal Lemon Y micropipette 400 ul
3-methylbutanal Lemon R micropipette 400 ul
3-methylbutanal Lemon R micropipette 400 ul
3-methylbutanal Lemon N micropipette 250 ul
3-methylbutanal Lemon N micropipette 250 ul
3-methylbutanal Lemon N micropipette 200 ul
3-methylbutanal Lemon Y micropipette 200 ul
3-methylbutanal Lemon N micropipette 100 ul
3-methylbutanal Lemon R micropipette 150 ul
3-methylbutanal Lemon R micropipette 200 ul
3-methylbutanal Nurse R micropipette 400 ul
3-methylbutanal Nurse Y micropipette 400 ul
3-methylbutanal Nurse Y micropipette 400 ul
3-methylbutanal Nurse R micropipette 270 ul
3-methylbutanal Nurse R micropipette 270 ul
[0175] Five Hd Syringe assays were performed on juvenile lemon and nurse sharks using 2-methylbutanal. In each assay tonic immobility was terminated demonstrating a flight response and the repellent activity of 2-methylbutanal. See Table 15.
[0000]
TABLE 15
Tonic Immobility Assays with 2-Methylbutanal
Component Species T? Delivery Dose
2-methylbutanal Lemon Y hd syringe 400 ul
2-methylbutanal Lemon Y hd syringe 200 ul
2-methylbutanal Nurse Y hd syringe 500 ul
2-methylbutanal Nurse Y hd syringe 200 ul
2-methylbutanal Nurse Y hd syringe 200 ul
Example 2
Methylbutenal Elasmobranch Repellents
[0176] Tonic immobility studies were carried out on lemon and nurse sharks with 3-methylbutenal and 2-methylbutenal using Hd Syringe and Micropipette assays as described above in Example 1. One Hd Syringe assay was performed on a juvenile lemon shark using 2-methylbutenal. Three Hd Syringe assays were likewise performed on juvenile nurse sharks. All assays resulted in termination of tonic immobility demonstrating the repellent activity of 2-methylbutenal. See Table 16.
[0000]
TABLE 16
Tonic Immobility Assays with 2-Methylbutenal
Component Species T? Delivery Dose
2-methylbutenal Lemon Y hd syringe 300 ul
2-methylbutenal Nurse Y hd syringe 450 ul
2-methylbutenal Nurse Y hd syringe 400 ul
2-methylbutenal Nurse Y hd syringe 1700 ul
[0177] Hd Syringe assays were likewise performed using 3-methylbutenal. One assay was performed on a juvenile lemon shark. Three assays were performed on juvenile nurse sharks. All assays resulted in termination of tonic immobility demonstrating the repellent activity of 3-methylbutenal. See Table 17.
[0000]
TABLE 17
Tonic Immobility Assays with 3-Methylbutenal
Component Species T? Delivery Dose
3-methylbutenal Lemon Y hd syringe 350 ul
3-methylbutenal Nurse Y hd syringe 700 ul
3-methylbutenal Nurse Y hd syringe 100 ul
3-methylbutenal Nurse Y hd syringe 1000 ul
3-methylbutenal Lemon R micropipette 400 ul
3-methylbutenal Lemon R micropipette 400 ul
3-methylbutenal Lemon Y micropipette 400 ul
3-methylbutenal Nurse R micropipette 400 ul
3-methylbutenal Nurse R micropipette 400 ul
3-methylbutenal Nurse R micropipette 400 ul
[0178] As evidenced in Tables 16 and 17, methylbutenal is an effective elasmobranch repellent because tonic immobility was terminated in all test species when an HD Syringe Assay was employed and a gustatory response was observed in all Micropipette Assays. See Tables 16 and 17.
Example 3-5
Carbon Aldehyde Elasmobranch Repellents
[0179] Tonic immobility studies were carried out on juvenile lemon and nurse sharks using the linear 5-carbon aldehydes, valeraldehyde and trans-pentenal with Hd Syringe and Micropipette assays as described above in Example 1. One Syringe assay was carried out on a juvenile nurse shark.
[0180] In the Syringe assay about 60 mL or more of valeraldehyde was delivered from one to as many as five feet from the shark depending on the water current. Time from delivery of the test substance until a response was observed, measured and recorded. Time from delivery to response was related to the size of the bolus delivered from the syringe, distance of the shark from the syringe and water current. As such, a longer time to response does not reflect reduced potency for a particular compound. To the contrary, a longer time to response as compared to some other compound or test simply demonstrates that even after a cloud of repellent has traveled some distance against water current, the potency of the repellent is demonstrated.
[0181] Two Hd Syringe assays were performed on juvenile lemon sharks with valeraldehyde. Likewise, three Hd Syringe assays were performed on juvenile nurse sharks and one Syringe assay was performed on a juvenile nurse shark. In all assays valeraldehyde terminated tonic immobility demonstrating the repellent activity of valeraldehyde. In six of six Micropipette assays (three on lemon sharks and three on nurse sharks) a change in behavior during tonic immobility was observed. This demonstrates the gustatory activity of valeraldehyde. See Table 18.
[0182] Two Hd Syringe assays were performed on lemon sharks with trans-pentenal and three Hd Syringe assays were likewise performed on nurse sharks. In all assays tonic immobility was terminated demonstrating the repellent activity of valeraldehyde. In six of six Micropipette assays (three on lemon sharks and three on nurse sharks) a change in behavior during tonic immobility was observed. In five of the Micropipette assays tonic immobility was terminated. This strongly evidences the gustatory activity of trans-pentenal. See Table 18.
[0000]
TABLE 18
Tonic Immobility Assays with Valeraldehyde and Trans-Pentenal
Component Species T? Delivery Dose Response
valeraldehyde Lemon Y hd syringe 350 ul
valeraldehyde Lemon Y hd syringe 250 ul
valeraldehyde Nurse Y hd syringe 400 ul
valeraldehyde Nurse Y hd syringe 100 ul
valeraldehyde Nurse Y hd syringe 300 ul
valeraldehyde Nurse Y syringe 52 ml 15.5 sec
valeraldehyde Lemon R micropipette 400 ul
valeraldehyde Lemon R micropipette 400 ul
valeraldehyde Lemon R micropipette 400 ul
valeraldehyde Nurse R micropipette 400 ul
valeraldehyde Nurse R micropipette 400 ul
valeraldehyde Nurse R micropipette 400 ul
trans-pentenal Lemon Y hd syringe 300 ul
trans-pentenal Lemon Y hd syringe 250 ul
trans-pentenal Nurse Y hd syringe 150 ul
trans-pentenal Nurse Y hd syringe 300 ul
trans-pentenal Nurse Y hd syringe 400 ul
trans-pentenal Lemon Y micropipette 400 ul
trans-pentenal Lemon Y Micropipette 400 ul
trans-pentenal Lemon R Micropipette 400 ul
trans-pentenal Nurse R Micropipette 400 ul
trans-pentenal Nurse Y Micropipette 400 ul
trans-pentenal Nurse Y Micropipette 400 ul
[0183] As evidenced in Table 18, linear 5-carbon aldehydes, valeraldehyde and trans-pentenal, were observed to be effective elasmobranch repellents in two different species using three different assays. In combination with the data provided in Tables 14-17, the data herein evidences that linear 5-carbon aldehydes are effective elasmobranch repellents.
Example 4
Saturated C1-C6 Aldehyde Elasmobranch Repellents
[0184] Tonic immobility studies were carried out on juvenile lemon and nurse sharks using aldehydes with saturated carbon chains comprising 3 carbons to about 6 carbons including propionaldehyde, butyraldehyde, isobutyraldehyde, valeraldehyde, capronaldehyde, trimethylacetaldehyde. In combination with the study of 3-methylbutanal and 2-methylbutanal in Example 1 and the study of valeraldehyde and trans-pentenal in Example 3, the data provided herein evidences the repellent activity of aldehydes with saturated carbon chains comprising 3 carbons to about 6 carbons. These data may also be applied to the one and two carbon-chain compounds formalin and acetaldehyde because they are highly water soluble and are expected to exhibit the same bio-activity on gustatory receptors as the longer aldehydes.
[0185] Syringe, Hd Syringe, Syringe 3/5/10 and Micropipette assays were carried out as described in Examples 1 and 2.
[0186] Using propionaldehyde, a three carbon aldehyde, four Syringe assays were performed. Two on nurse sharks and two on lemon sharks. All resulted in termination of tonic immobility thereby demonstrating the repellent activity of propionaldehyde. See Table 19.
[0187] Using butyraldehyde, a four carbon aldehyde, two Syringe assays were performed on lemon sharks resulting in termination of tonic immobility; three Syringe assays were performed on nurse sharks, two resulted in termination of tonic immobility and one had no result. Because 4 of 5 assays resulted in termination of tonic immobility, the repellent activity of butyraldehyde was demonstrated. See Table 19.
[0188] Using isobutyraldehyde, another four carbon aldehyde, three Syringe assays were performed on lemon sharks and one Syringe assay was performed on a nurse shark. All resulted in termination of tonic immobility demonstrating the repellent activity of isobutyraldehyde. See Table 19.
[0189] Using trimethylacetaldehyde, a five carbon aldehyde, three Hd Syringe assays on juvenile nurse sharks, two Hd Syringe assays on juvenile lemon sharks and two Syringe 3/5/10 assays on juvenile nurse sharks resulted in termination of tonic immobility. The data clearly demonstrate the repellent activity of trimethylacetaldehyde. See Table 19.
[0190] Micropipette assays using trimethylacetaldehyde resulted in a change of behavior during tonic immobility in three juvenile nurse sharks and three juvenile lemon sharks. These data demonstrate the gustatory effect of trimethylacetaldehyde. See Table 19.
[0000]
TABLE 19
Tonic Immobility Assays with Saturated C1-C6 Aldehydes
Component Species T? Delivery Dose Response
propionaldehyde nurse Y syringe 35 ml 7.79 sec
propionaldehyde nurse Y syringe 20 ml 4.99 sec
propionaldehyde lemon Y syringe 18 ml 2.87 sec
propionaldehyde lemon Y syringe 37 ml 8.53 sec
butyraldehyde lemon Y syringe 23 ml 4.75 sec
butyraldehyde lemon Y syringe 23 ml 5.66 sec
butyraldehyde nurse Y syringe 21 ml 3.23 sec
butyraldehyde nurse N syringe 33 ml
butyraldehyde nurse Y syringe 23 ml 4.75 sec
isobutyraldehyde lemon Y syringe 4 ml 1.30 sec
isobutyraldehyde lemon Y syringe 9 ml 2.61 sec
isobutyraldehyde lemon Y syringe 6 ml 1.28 sec
isobutyraldehyde nurse Y syringe 60 ml 11.92 sec
capronaldehyde lemon Y syringe 27 ml 3.21 sec
trimethyl- Nurse Y Hd syringe 200 ul
acetaldehyde
trimethyl- Nurse Y Hd syringe 100 ul
acetaldehyde
trimethyl- Nurse Y Hd syringe 300 ul
acetaldehyde
trimethyl- Lemon Y Hd syringe 600 ul
acetaldehyde
trimethyl- Lemon Y Hd syringe 200 ul
acetaldehyde
trimethyl- nurse N syringe 3/5/10 4 ml
acetaldehyde
trimethyl- nurse Y syringe 3/5/10 5 ml
acetaldehyde
trimethyl- nurse R micropipette 400 ul
acetaldehyde
trimethyl- nurse R micropipette 400 ul
acetaldehyde
trimethyl- nurse R micropipette 400 ul
acetaldehyde
trimethyl- lemon R micropipette 400 ul
acetaldehyde
trimethyl- lemon R micropipette 400 ul
acetaldehyde
trimethyl- lemon R micropipette 400 ul
acetaldehyde
[0191] In combination with the data from Examples 1 and 3, which demonstrate the repellent activity of valeraldehyde and 3-methylbutanal, both five carbon aldehydes, the data demonstrate the repellent activity of aldehydes with saturated carbon chains comprising 1 to about 6 carbons.
[0192] Tonic immobility studies were carried out using diethylacetaldehyde on juvenile lemon, juvenile nurse, blacktip and tiger sharks. In six of six Syringe 3/5/10 Assays, tonic immobility was terminated in juvenile nurse sharks. In two of four Syringe 3/5/10 Assays, tonic immobility was terminated in blacktip sharks. In one 3/5/10 Syringe Assay, a behavioral response was noted during tonic immobility. In one 3/5/10 Syringe Assay, no response was noted. In one 3/5/10 Syringe Assay on a tiger shark, tonic immobility was terminated.
[0193] In twelve Hd Syringe Assays on juvenile lemon and nurse sharks, a behavioral response during tonic immobility was observed in 9 assays. In three assays tonic immobility was fully terminated. In eight Micropipette Assays in juvenile lemon and nurse sharks a gustatory response was noted with two full terminations of tonic immobility. In two Micropipette Assays, no response was noted. See Table 20.
[0000]
TABLE 20
Tonic Immobility Assays with Saturated C1-C6 Aldehydes
Component Species T? Delivery Dose Response Comments
diethylacetaldehyde nurse Y syringe 3/5/10 5 ml 4 sec
diethylacetaldehyde nurse Y syringe 3/5/10 2.6 ml 4 sec
diethylacetaldehyde nurse Y syringe 3/5/10 0.4 ml 5 sec
diethylacetaldehyde nurse Y syringe 3/5/10 1 ml 2 sec
diethylacetaldehyde nurse Y syringe 3/5/10 3.6 ml 3 sec
diethylacetaldehyde nurse Y syringe 3/5/10 1.2 ml 1 sec
diethylacetaldehyde blacktip Y syringe 3/5/10 6.5 ml 5 sec
diethylacetaldehyde blacktip Y syringe 3/5/10 6 ml 5 sec
diethylacetaldehyde blacktip R syringe 3/5/10 6 ml 5 sec cough/tensed
diethylacetaldehyde blacktip N syringe 3/5/10 4.9 ml
diethylacetaldehyde Tiger Y syringe 3/5/10 5.6 ml 2.83 sec 120 cm female
tiger on Longline
diethylacetaldehyde lemon R hd syringe 1 ml 1.86 sec cough
diethylacetaldehyde Lemon R hd syringe 0.6 ml cough
diethylacetaldehyde Lemon R hd syringe 0.8 ml cough
diethylacetaldehyde Lemon R hd syringe 1.1 ml cough
diethylacetaldehyde Lemon R hd syringe 1.3 ml cough
diethylacetaldehyde Lemon R hd syringe 1.5 ml cough
diethylacetaldehyde Lemon Y hd syringe 1.5 ml 5.96 sec
diethylacetaldehyde Nurse R hd syringe 0.3 ml 1.33 sec cough
diethylacetaldehyde Nurse R hd syringe 0.4 ml 3.23 sec cough
diethylacetaldeyde Nurse Y hd syringe 1.2 ml 5.62 sec
diethylacetaldehyde Nurse R hd syringe 0.3 ml 1.39 sec cough
diethylacetaldehyde Nurse Y hd syringe 1.5 ml 0.78 sec
diethylacetaldehyde lemon R micropipette 300 ul blinked
diethylacetaldehyde lemon N micropipette 200 ul
diethylacetaldehyde lemon R micropipette 150 ul cough
diethylacetaldehyde lemon R micropipette 150 ul cough
diethylacetaldehyde lemon R micropipette 150 ul cough
diethylacetaldehyde lemon N micropipette 100 ul
diethylacetaldehyde lemon N micropipette 200 ul
diethylacetaldehyde lemon R micropipette 250 ul cough
diethylacetaldehyde nurse Y micropipette 270 ul 1 sec
diethylacetaldehyde nurse Y micropipette 270 ul 1 sec
diethylacetaldehyde nurse R micropipette 500 ul
[0194] The data in Table 20 evidences the repellent activity of diethylacetaldehyde, further supporting the repellent activity of aldehydes with saturated carbon chains comprising 1 to about 6 carbons.
Example 5
Piperonal (Aromatic Aldehyde), Ionone (Ketone) or Zingerone (Ketone) Repellents on Free-Swimming Elasmobranchs
[0195] A series of chemical repellent tests on free-swimming Caribbean reef sharks (C. perezii) and blacknose sharks (C. acronotus) was performed in tropical waters. A small metal cage containing bait was suspended below a float in the water column. A [3/8]'' ID HDPE diptube was secured from the cage to the boat, so that chemical compounds could be transported to the cage's proximity. Sharks were stimulated using bunker chum in bags.
[0196] Sharks were observed to immediately bump and bite at the cage. The number of interactions was recorded using an underwater pole-camera. When 500 mL of 50% w/w piperonal in diethylene glycol monoethyl ether was presented, the number of strikes was dramatically reduced, and interactions ceased. When 500 mL of 50% w/w alpha-ionone in diethylene glycol monoethyl ether was presented, the number of strikes was reduced, but interactions continued after a 10 minute period. When 500 mL of 50% w/w zingerone in diethylene glycol monoethyl ether was presented, the number of strikes was reduced, but interactions continued after a 10 minute period.
Example 6
Natural Aldehyde Elasmobranch Repellents
[0197] Tonic immobility studies were carried out on juvenile lemon and nurse sharks using natural aldehydes including trans-cinnimaldehyde, cuminaldehyde and a combination of natural aldehydes. Syringe, and Syringe 3/5/10 assays were carried out as described in Examples 1 and 2.
[0198] Two Syringe assays (one on a lemon shark the other on a nurse share) using trans-cinnimaldehyde resulted in termination of tonic immobility. One Syringe assay on a lemons shark using cuminaldehyde resulted in termination of tonic immobility.
[0199] A combination of natural aldehydes was created from 4.4 g cuminaldehyde (cumin) and 5.3 g mixed isomers of anisaldehyde (anise) solubilized in 19.8 g denatured ethanol. In one Syringe 3/5/10 Assay on a juvenile nurse shark, a behavioral response within tonic immobility was observed. In three other Syringe 3/5/10 Assays, no response was observed. See Table 21. In two of the negative response assays only 500 microliters of chemical was delivered to the shark. This may explain the lack of response. See Table 21.
[0000]
TABLE 21
Tonic Immobility Assays with Natural Aldehydes
Re-
Component Species T? Delivery Dose sponse
Trans-cinnimaldehyde lemon Y syringe 21 ml 4.31 sec
trans-cinnimaldehyde nurse Y syringe 33 ml 4.97 sec
cuminaldehyde lemon Y syringe 19 ml 3.94 sec
natural aldehydes nurse R syringe 3/5/10 6 ml
natural aldehydes nurse N syringe 3/5/10 6 ml
natural aldehydes nurse N syringe 3/5/10 500 ul
natural aldehydes nurse N syringe 3/5/10 500 ul
[0200] Together these data demonstrate the repellent activity of natural aldehydes including trans-cinnimaldehyde and cuminaldehyde.
Example 7
Aromatic Aldehyde Elasmobranch Repellents
[0201] Tonic immobility studies were carried out on juvenile lemon sharks, juvenile nurse sharks and a blacknose shark using aromatic aldehydes including a mixture of methoxy/vanillin (containing methoxybenzaldehydes and vanillin) and tolualdehyde. Syringe and Micropipette assays were carried out as described in Examples 1 and 2.
[0202] A methoxybenzaldehyde combination with vanillin was made from 2 g Ortho-vanillin, 1 g 2,4,5-trimethoxybenzaldehyde, 1 g 2,3,4-trihydroxybenzaldehyde, 1 g 3-hydroxy-4-methoxybenzaldehyde, 1 g 2,3,4-trimethoxybenzaldehyde, 1 g 2,5-dimethoxybenzaldehyde, 1 g veratraldehyde, 1 g 4-hydroxy-3-methoxybenzaldehyde, 1 g 3-ethoxy-4-hydroxy-benzaldehyde, and 50 g denatured alcohol.
[0203] Seven Syringe assays were carried out using the methoxy/vanillin repellent combination on lemon sharks, one Syringe assay was carried out on a blacknose shark and one Syringe assay was carried out on a nurse shark. Five of six assays on lemon shark resulted in termination of tonic immobility. One assay on a lemon shark resulted in a change in behavior during tonic immobility. One assay on a nurse shark had no response. One assay on a nurse shark was inconclusive because the shark became ill. The assay on the blacknose shark resulted in termination of tonic immobility. See Table 22. In six of ten Micropipette assays on lemon shark, a response was observed. This demonstrates that methoxy/vanillin is a gustatory repellent. See Table 22.
[0204] Using p-tolualdehyde, one Syringe Assay on a lemon shark resulted in termination of tonic immobility. See Table 22.
[0205] Using veratraldehyde, six of six Syringe Assays on lemon and nurse sharks resulted in termination of tonic immobility. See Table 22. In a single Syringe Assay on a nurse shark no response was observed. In eight of twelve Micropipette Assays a gustatory response was observed in lemon sharks. In four Micropipette assays, no response was observed.
[0000]
TABLE 22
Tonic Immobility Assays with Aromatic Aldehydes
Component Species T? Delivery Dose Response Comments
methoxy/vanillin mixture lemon R syringe 27 ml
methoxy/vanillin mixture nurse N syringe 54 ml
methoxy/vanillin mixture lemon Y syringe 5 ml 1.91 sec
methoxy/vanillin mixture lemon Y syringe 14 ml 4.72 sec
methoxy/vanillin mixture lemon Y syringe 11 ml 6.92 sec
methoxy/vanillin mixture nurse N/A syringe 47 ml shark nearly dead, overdose
of alcohol/aldehydes
methoxy/vanillin mixture lemon R syringe 6 ml 2.4 sec
methoxy/vanillin mixture lemon Y syringe 10 ml 3.82 sec
methoxy/vanillin mixture lemon Y syringe 16 ml 5.2 sec
methoxy/vanillin mixture blacknose Y syringe 50 ml <20 sec
methoxy/vanillin mixture lemon Y micropipette 100 ul 5 sec
methoxy/vanillin mixture lemon R micropipette 100 ul 2 sec
methoxy/vanillin mixture lemon N micropipette 100 ul 8 sec
methoxy/vanillin mixture lemon R micropipette 100 ul 2 sec
methoxy/vanillin mixture lemon R micropipette 23 ul 10 sec
methoxy/vanillin mixture lemon N micropipette 25 ul
methoxy/vanillin mixture lemon Y micropipette 300 ul
methoxy/vanillin mixture lemon Y micropipette 300 ul
methoxy/vanillin mixture lemon N micropipette 300 ul
methoxy/vanillin mixture lemon N micropipette 300 ul
p-tolualdehyde lemon Y syringe 22 ml 3.54 sec
veratraldehyde lemon Y syringe 16 ml 2.67 sec
veratraldehyde lemon Y syringe 20 ml 6.45 sec
veratraldehyde lemon Y syringe 10 ml 1.06 sec
veratraldehyde lemon Y syringe 11.5 ml 0.94 sec
veratraldehyde nurse Y syringe 4.5 ml 0.54 sec
veratraldehyde nurse Y syringe 29 ml 9.71 sec
veratraldehyde nurse N syringe 47 ml
veratraldehyde lemon R micropipette 100 ul
veratraldehyde lemon N micropipette 100 ul
veratraldehyde lemon R micropipette 100 ul 3 sec
veratraldehyde lemon N micropipette 100 ul
veratraldehyde lemon R micropipette 100 ul 3 sec
veratraldehyde lemon Y micropipette 100 ul 12 sec
veratraldehyde lemon N micropipette 250 ul
veratraldehyde lemon Y micropipette 100 ul 3 sec
veratraldehyde lemon N micropipette 250 ul
veratraldehyde lemon N micropipette 280 ul
veratraldehyde lemon Y micropipette 300 ul 3 sec
veratraldehyde lemon Y micropipette 280 ul 2 sec
veratraldehyde lemon Y micropipette 310 ul 2 sec
[0206] Together these data evidence the gustatory repellent activity of aromatic aldehydes such as methoxy/vanillin, p-tolualdehyde and veratraldehyde.
Example 8
Longer Aldehydes and Combinations of Aldehydes
[0207] A combination of aldehydes was prepared in about 873 g of methanol in the following amounts: butanal 144.22 g; isobutanal 144.22 g; pentanal 172.26 g; hexanal 200.32 g; decanal 46.884 g; cuminal 44.463 g; cinnimal 52.864 g; anisal 68.075 g; mesityl oxide 29.445 g; p-tolualdehyde 36.045 g; and veratraldehyde 16.618 g. The combination was labeled BA1.
[0208] Tonic immobility studies were carried out with the aldehyde combination labeled BA1 on juvenile lemon sharks, juvenile nurse sharks and blacktip sharks using the above-described aldehyde mixture. Syringe, Syringe 3/5/10, Bite and Micropipette assays were carried out as described in Examples 1 and 2. In three Syringe and six Bite assays on juvenile nurse sharks all assays terminated tonic immobility. In three Syringe and one Syringe 3/5/10 assay on juvenile lemon sharks all assays terminated tonic immobility. In one Syringe assay and one Syringe 3/5/10 assay on blacktip sharks both resulted in termination of tonic immobility. These data demonstrate the excellent repellent activity of the above-described mixture of aldehydes. See Table 23.
[0209] In one Micropipette assay in a juvenile lemon shark no response was observed. Nevertheless, because the aldehydes that have been combined to create the above-described aldehyde mixture have demonstrated gustatory stimulation in other Micropipette assays, it is concluded that the aldehyde mixture tested here is a gustatory repellent. See Table 23.
[0210] In a cloud dispersion assay, a population of competitively feed Caribbean reef and blacknose sharks was repelled from the feeding zone with a delivery of 500 mL of BA1 repellent composition.
[0211] A combination of aldehydes in the following amounts was prepared in about 1294 grams of methanol: proprional 174.24 g; butanal 216.33 g; isobutanal 216.33 g; pentanal 172.26 g; hexanal 100.16 g; heptanal 28.5475 g; octanal 64.11 g; nonanal 35.5625 g; decanal 78.14 g; cuminal 74.105 g; cinnimal 66.08 g; anisal 68.075 g; and mesityl oxide 29.445 g. The combination was labeled BA2.
[0212] Tonic immobility studies were carried out with the aldehyde combination labeled BA2 on juvenile lemon sharks using the above-described aldehyde combination. Syringe, and Micropipette Assays were carried out as described in Examples 1 and 2. Pipette Assays were carried out in the same manner as Micropipette Assays with delivery of the repellent directly to the mouth except the volumes were sometimes larger. In three of four Syringe Assays in lemon shark, tonic immobility was terminated. In one Syringe Assay, a behavioral change was observed within tonic immobility. In four of four Pipette Assays, tonic immobility in lemon sharks was terminated. In three Micropipette Assays a gustatory response was observed. In three others no response was observed.
[0213] A combination of aldehydes and a ketone in the following amounts was prepared in 160 grams of denatured alcohol: Butyraldehyde 10 g; Isobutyraldehyde 10 g; Veratraldehyde 10 g; and 2,3-butanedione (Diacetyl) 10 g. The combination was labeled BA3. A cloud dispersion of the repellent composition was delivered to a population of Caribbean reef and blacknose shark competitively feeding. The sharks were dispersed from the feeding zone. In one Syringe Assay with the BA3 repellent on a juvenile lemon shark, tonic immobility was terminated. In two other Syringe Assays (one on a lemon shark and one on a nurse shark) behavioral changes were observed within tonic immobility. In one additional Syringe Assay, a nurse shark experienced a violent seizure and the assay could not be finished.
[0214] A combination of aldehydes and ammonium acetate was prepared in the following amounts in 258 grams of denatured alcohol and 200 g of water: butyraldehyde 72.1 g; isobutyraldehyde 36.2 g; veratraldehyde 35.0 g; and ammonium acetate 50 g. The repellent composition was labeled BA4. In two cloud assays in free-swimming competitively feeding Caribbean reef and blacknose sharks, a cloud dispersion of 500 mL of the repellent composition BA4 repelled the sharks from the feeding zone.
[0000]
TABLE 23
Tonic Immobility Assays with Aldehyde Mixture
Component Species T? Delivery Dose Response
Aldehyde mixture BA1 nurse Y syringe 4 ml 1.38 sec
Aldehyde mixture BA1 nurse Y syringe 6 ml 3.41 sec
Aldehyde mixture BA1 lemon Y syringe 5 ml 2.03 sec
Aldehyde mixture BA1 lemon Y syringe 9 ml 4.09 sec
Aldehyde mixture BA1 lemon Y syringe 5 ml 2.72 sec
Aldehyde mixture BA1 nurse Y syringe 9 ml 4.60 sec
Aldehyde mixture BA1 blacktip Y syringe 20 ml
Aldehyde mixture BA1 lemon Y syringe 3/5/10 6 ml 5 sec
Aldehyde mixture BA1 blacktip Y syringe 3/5/10 6 ml 5 sec
Aldehyde mixture BA1 nurse Y bite 5 ml
Aldehyde mixture BA1 nurse Y bite 5 ml
Aldehyde mixture BA1 nurse Y bite 5 ml
Aldehyde mixture BA1 nurse Y bite 2 ml 1 sec
Aldehyde mixture BA1 nurse Y bite 2 ml 1 sec
Aldehyde mixture BA1 nurse Y bite 2 ml 1 sec
Aldehyde mixture BA1 lemon N micropipette 500 ul
aldehyde mixture BA1 carib Y cloud - co2 500 mL
reef/black
nose
aldehyde mixture BA2 lemon Y syringe 9 ml 3.48 sec
aldehyde mixture BA2 lemon R syringe 15 ml 7.49 sec
aldehyde mixture BA2 lemon Y syringe 13 ml 2.99 sec
aldehyde mixture BA2 lemon Y syringe 5 ml 5.30 sec
aldehyde mixture BA2 lemon Y pipette 1 ml
aldehyde mixture BA2 lemon Y pipette 0.5 ml 2.98 sec
aldehyde mixture BA2 lemon Y pipette 0.5 ml
aldehyde mixture BA2 lemon Y pipette >0.5 ml
aldehyde mixture BA2 lemon N micropipette 25 ul
aldehyde mixture BA2 lemon R micropipette 25 ul
aldehyde mixture BA2 lemon R micropipette 18 ul
aldehyde mixture BA2 lemon N micropipette 10 ul
aldehyde mixture BA2 lemon N micropipette 10 ul
aldehyde mixture BA2 lemon R micropipette 25 ul
aldehyde mixture BA3 carib reef/ Y cloud 1000 ul
blacknose
aldehyde mixture BA3 lemon Y syringe 25 ml 8 sec
aldehyde mixture BA3 nurse R syringe 60 ml 5 sec
aldehyde mixture BA3 lemon R syringe 27 l
aldehyde mixture BA3 nurse N/A syringe 100 mL Violent
Seizure
aldehyde mixture BA4 carib reef/ R cloud - co2 500 mL
blacknose
aldehyde mixture BA4 carib reef/ R cloud - co2 500 mL
blacknose
octanal lemon Y syringe 37 ml 6.23 sec
nonanal lemon Y syringe 30 ml 5.00 sec
decanal nurse Y syringe 60 ml 17.72 sec
heptanal lemon N syringe 56 ml
mesityl oxide lemon Y syringe 38 ml 6.21 se
[0215] Some of the longer carbon chain aldehydes that had been included in the above-described aldehyde mixture were also tested for repellent activity.
[0216] In Syringe assays on lemon sharks using octanal, nonanal and mesityl oxide, tonic immobility was terminated. In a Syringe assay on a nurse shark using decanal tonic immobility was terminated. In a Syringe assay on a lemon shark using heptanal, no response was observed. This lack of response may have resulted from an unfavorable water current.
Example 9
Carboxylic Acid Elasmobranch Repellents
[0217] A wide range of carboxylic acids was tested on lemon, nurse, blacktip, blacknose, tiger and great hammerhead sharks. The carboxylic acids listed in Table 9 were each tested. Further, a range of doses of the following components were tested: cinnamic acid; citric acid; crotonic acid; lactic acid; aqueous succinic acid; crotonic acid, cinnamic acid, and maleic acid in glycol; and crotonic acid, citric acid and fumaric acid in solution. See Table 24. All compositions evidenced repellent characteristics.
[0218] The results together demonstrate the effective repellent characteristics of carboxylic acid compositions. Tonic immobility studies were carried out on many different carboxylic acids as well as cloud dispersal studies in free-swimming individual sharks. The data demonstrate the repellent activity of carboxylic acids.
[0219] In a first set of studies on the effectiveness of carboxylic acids as elasmobranch repellents, each tested substance was subjected to the following protocol. In a first micropipette assay on a juvenile lemon shark, an oral dose of no more than 100 microliters of carboxylic acid was observed to terminate tonic immobility. An oral dose of no more than 400 microliters was then delivered by micropipette into the mouth of a juvenile nurse shark. Tonic immobility was terminated. For butyric acid, each derivative of butyric acid and each naturally occurring acid listed in Table 9, the protocol was successfully repeated and each treatment resulted in termination of tonic immobility for each listed substance. The data evidences the gustatory repelling activity of carboxylic acids.
[0220] Further studies on carboxylic acids and mixtures of carboxylic acids were pursued. The data is contained in Table 24.
[0221] In four of four Hd Syringe assays, citric acid between 0.3 mL and 2.4 mL of citric acid 50% w/w was delivered about three inches from the mouth of a lemon shark. Each assay terminated tonic immobility. Seven Syringe 3/5/10 assays on nurse and lemons sharks were employed with crotonic acid solution. In four of the seven assays the crotonic acid was delivered directly to the mouth on a longline or within 10 inches of the shark's mouth. Tonic immobility was terminated. In one of the seven assays, the crotonic acid was delivered directly to the mouth of the shark on a longline and a behavioral response was observed in tonic immobility. In two of the seven assays, 6 mL of crotonic acid was delivered to lemon sharks at a distance of 36 inches from the shark's mouth. No response was observed. The lack of response is explained by the small volume delivered at a very large distance. See Table 24.
[0222] In two of three Syringe 3/5/10 assays, lactic acid was delivered to lemon sharks and terminated tonic immobility. In a single Syringe 3/5/10 assay, lactic acid was delivered to a lemon shark and no behavioral change was observed. See Table 24.
[0223] Crotonic Acid (25.0 g), Cinnamic Acid (10.0 g) and Maleic Acid (25.0 g) were combined in 100.0 g Diethyl Glycol Monoethyl Ether to create a repellent composition. In three of four Syringe 3/5/10 assays, the repellent carboxylic acid composition (crotonic/cinnamic/maleic) was delivered to tiger and blacknose sharks and terminated tonic immobility. In one of four Syringe 3/5/10 assays, delivery of the repellent actually missed the mouth of the shark and only a behavior change was seen in tonic immobility. See Table 24.
[0224] In a cloud dispersal assay 400 ml of the carboxylic acid composition (crotonic/cinnamic/maleic) was dispersed from a diptube near the mouth of a great hammerhead shark. The shark fled the area and did not return. See Table 24.
[0000]
TABLE 24
Tonic Immobility Assays with Carboxylic Acids
Component Species T? Delivery Dose Response Comments
cinnamic acid solution lemon N syringe 3/5/10 1.5 ml mouth
citric acid 50% w/w lemon Y hd syringe 2.4 ml both
citric acid 50% w/w lemon Y hd syringe 2.5 ml both
citric acid 50% w/w lemon Y hd syringe 0.3 ml both
citric acid 50% w/w lemon Y hd syringe 1.5 ml mouth
citric acid 50% w/w lemon Y syringe 3/5/10 6 ml mouth longline/231 cm
citric acid 50% w/w lemon Y syringe 3/5/10 2 ml mouth
citric acid 50% w/w lemon N syringe 3/5/10 2.5 ml distanced 6 inches
citric acid 50% w/w lemon Y syringe 3/5/10 6 ml distanced 12 inches
citric acid 50% w/w lemon N syringe 3/5/10 6 ml distanced 12 inches
citric acid 50% w/w lemon N syringe 3/5/10 6 ml distanced 10 inche
citric acid 50% w/w lemon Y syringe 3/5/10 6 ml distanced longline
citric acid 50% w/w lemon N syringe 3/5/10 6 ml mouth longline
citric acid 50% w/w lemon R syringe 3/5/10 6 ml mouth longline
citric acid 50% w/w nurse R syringe 3/5/10 6 ml mouth longline/3 coughs
citric acid 50% w/w nurse R syringe 3/5/10 6 ml mouth longline
citric acid 50% w/w blacktip Y syringe 3/5/10 6 ml mouth longline
citric acid 50% w/w blacknose R syringe 3/5/10 6 ml both longlinge/cough
citric acid 50% w/w blacknose Y syringe 3/5/10 6 ml mouth
crotonic acid solution lemon Y syringe 3/5/10 6 ml distanced
crotonic acid solution nurse Y syringe 3/5/10 6 ml mouth
crotonic acid solution nurse R syringe 3/5/10 6 ml mouth longline/cough
crotonic acid solution lemon Y syringe 3/5/10 6 ml mouth longline/231 cm
crotonic acid solution lemon Y syringe 3/5/10 6 ml distanced 10 inches
crotonic acid solution lemon N syringe 3/5/10 6 ml distanced 36 inches
crotonic acid solution lemon N syringe 3/5/10 6 ml distanced 36 inches
lactic acid lemon N syringe 3/5/10 1.5 ml mouth
lactic acid lemon Y syringe 3/5/10 1.5 ml r nare
lactic acid lemon Y syringe 3/5/10 0.5 ml mouth
saturated succinic acid lemon R micropipette 400 ul mouth
solution aq
saturated succinic acid lemon R micropipette 400 ul left nare
solution aq
succinic acid lemon N swab
succinic acid lemon N swab
crotonic/cinnamic/maleic in tiger Y syringe 3/5/10 5 ml mouth violent
glycol
crotonic/cinnamic/maleic in blacknose Y syringe 3/5/10 5 ml mouth
glycol
crotonic/cinnamic/maleic in tiger R syringe 3/5/10 5 ml mouth missed mouth, plumed
glycol
crotonic/cinnamic/maleic in tiger Y syringe 3/5/10 5 ml mouth
glycol
crotonic/cinnamic/maleic in great Y diptube to bait 400 ml poss mouth 13' hammerhead
glycol hammerhead at mini-barge, did not return
crotonic/citric/fumaric nurse R syringe 3/5/10 6 ml mouth longline/cough
solution
crotonic/citric/fumaric lemon Y syringe 3/5/10 0.5 ml both
solution
crotonic/citric/fumaric lemon Y syringe 3/5/10 6 ml distanced 36 inches/shark
solution moved into cloud
crotonic/citric/fumaric nurse Y syringe 3/5/10 6 ml mouth
solution
crotonic/citric/fumaric lemon Y syringe 3/5/10 6 ml mouth very large specimen in
solution pen
crotonic/citric/fumaric lemon Y syringe 3/5/10 6 ml mouth longline
solution
crotonic/citric/fumaric nurse Y syringe 3/5/10 6 ml mouth longline
solution
crotonic/citric/fumaric nurse Y micropipette 200 ul mouth spit
solution
crotonic/citric/fumaric lemon Y micropipette 300 ul mouth
solution
crotonic/citric/fumaric lemon Y micropipette 215 ul mouth
solution
crotonic/citric/fumaric lemon Y micropipette 120 ul mouth
solution
crotonic/citric/fumaric lemon Y micropipette 100 ul mouth
solution
crotonic/citric/fumaric lemon Y micropipette 100 ul mouth
solution
crotonic/citric/fumaric lemon Y micropipette 100 ul mouth
solution
crotonic/citric/fumaric lemon Y micropipette 200 ul mouth
solution
crotonic/citric/fumaric lemon Y micropipette 200 ul mouth
solution
crotonic/citric/fumaric lemon Y micropipette 150 ul mouth
solution
crotonic/citric/fumaric lemon Y micropipette 125 ul mouth
solution
crotonic/citric/fumaric lemon Y micropipette 125 ul mouth
solution
crotonic/citric/fumaric lemon Y micropipette 100 ul mouth
solution
crotonic/citric/fumaric lemon Y micropipette 100 ul mouth
solution
crotonic/citric/fumaric lemon Y micropipette 50 ul mouth
solution
crotonic/citric/fumaric lemon Y micropipette 50 ul mouth
solution
crotonic/citric/fumaric nurse R micropipette 300 ul mouth
solution
crotonic/citric/fumaric nurse R micropipette 300 ul mouth spit
solution
crotonic/citric/fumaric nurse R micropipette 300 ul mouth
solution
crotonic/citric/fumaric nurse Y micropipette 200 ul mouth
solution
crotonic/citric/fumaric nurse Y micropipette 100 ul mouth
solution
crotonic/citric/fumaric nurse Y micropipette 100 ul mouth
solution
crotonic/citric/fumaric nurse Y micropipette 50 ul r nare
solution
crotonic/citric/fumaric nurse Y micropipette 50 ul r nare
solution
crotonic/citric/fumaric nurse R micropipette 50 ul l nare cough
solution
crotonic/citric/fumaric nurse R micropipette 25 ul r nare
solution
crotonic/citric/fumaric nurse R micropipette 25 ul r nare cough
solution
[0225] In a surrounding cloud dispersal assay, 500 mL of a mixture of 20% w/w Crotonic acid, 10% w/w Citric acid and 5% w/w Cinnamic acid solubilized in 50:50 w/w water:ethanol was delivered in a subsurface dose in the vicinity of a population of competitively feed sharks (5 Caribbean reef sharks and 2 blacknose sharks). The sharks were dispersed and did not return.
[0226] Together, these data evidence the effectiveness of carboxylic acids and combinations of carboxylic acids as elasmobranch repellents.
Example 10
Pyridine Elasmobranch Repellents
[0227] Tonic immobility studies were carried out on juvenile lemon sharks and juvenile nurse sharks using 100% pyridine, 100% 3-methylpyridine and 100% 2-amino-3-picoline at 95% or 2-amino-3-picoline 95% cut to 10% w/w in desalinated water. Syringe 3/5/10, Hd Syringe and Micropipette assays were carried out as described in Examples 1 and 2. In three Syringe and six Bite assays on juvenile nurse sharks all assays terminated tonic immobility. In three Syringe and one Syringe 3/5/10 assay on juvenile lemon sharks all assays terminated tonic immobility. In one Syringe assay and one Syringe 3/5/10 assay on blacktip sharks both resulted in termination of tonic immobility. In one cloud dispersion assay with 2-amino-3-picoline 95% cut to 10% w/w with desalinated water, Caribbean reef sharks demonstrated a population decrease upon the administration of 500 mL of repellent. See Table 25.
[0000]
TABLE 25
Tonic Immobility Assays with Pyridine
Component Species T? Delivery Dose Response Comments
Pyridine nurse Y syringe 3/5/10 5.7 ml 2 sec
Pyridine nurse R syringe 3/5/10 6 ml
Pyridine lemon Y syringe 3/5/10 1.2 ml 6 sec
Pyridine lemon Y syringe 3/5/10 3 ml 5 sec
Pyridine nurse N micropipette 500 ul
Pyridine nurse N micropipette 500 ul
3-methylpyridine lemon Y hd syringe 0.7 ml
3-methylpyridine lemon Y hd syringe 0.3 ml
3-methylpyridine nurse Y hd syringe 0.7 ml
2-amino-3-picoline 95% lemon Y micropipette 400 ul mouth
2-amino-3-picoline 95% lemon F micropipette 400 ul left nare - EXTREMELY VIOLENT/SEIZURE RESPONSE
2-amino-3-picoline 95% Caribbean Y cloud - co2 500 mL population decreasedcut to 10% w/w in reefdesalinated water,TV = 500 mL
[0228] Together, the data in Table 25 evidence the effective gustatory repellent activity of pyridines and pyridine derivatives.
Example 11
Anti-Pyrine Elasmobranch Repellents
[0229] Compositions for repelling an elasmobranch comprising an anti-pyrine or a derivative thereof including anti-pyrine or 4-amino-antipyrine were tested. Tonic immobility studies were carried out on juvenile lemon sharks and juvenile nurse sharks using 4-aminoantipyrine and antipyrine solution. See Table 26. The 4-aminoantipyrine solution was prepared from 5 g 4-aminoantipyrine and 30 g water.
[0230] Hd Syringe, Syringe 3/5/10 and Micropipette assays were carried out as described in Examples 1 and 2. In four Hd Syringe assays on juvenile lemon sharks using 4-aminoantipyrines, all assays terminated tonic immobility. In one Hd Syringe assay on a juvenile nurse shark, tonic immobility was terminated even with a volume of 300 microliters. In only one Syringe 3/5/10 Assay on a blacktip shark, no response was observed using 4-aminoantipyrine. In one Micropipette assay on a juvenile lemon shark using antipyrine solution, a response was observed within tonic immobility. Together, these data evidence that antipyrine is a gustatory stimulant. In another Micropipette assay on a juvenile lemon shark, no response was noted.
[0000]
TABLE 26
Tonic Immobility Assays with Antipyrine
Component Species T? Delivery Dose
4-aminoantipyrine lemon Y hd syringe 1.05 ml
4-aminoantipyrine lemon Y hd syringe 0.45 ml
4-aminoantipyrine nurse Y hd syringe 0.3 ml
4-aminoantipyrine lemon Y hd syringe 0.5 ml
4-aminoantipyrine lemon Y hd syringe 0.7 ml
4-aminoantipyrine blacktip N syringe 3/5/10 6 ml
antipyrine solution lemon R micropipette 400 ul
antipyrine solution lemon N micropipette 400 ul
Example 12
Repellent Activity on Blue Sharks
[0231] In two assays each on two different blue sharks, 3-methylbutanal in dosages of 20 mL or less produced a behavioral response (classic mouth-agape response) from a direct delivery of the repellent to the mouth using a syringe. The sharks had been captured on rod and reel and were held in tonic immobility along a boat. The first shark had a total length of 6.5 feet. The second shark had a total length of 8 feet.
Example 13
Di-Ketones (Diacetyl) Elasmobranch Repellents
[0232] Di-ketones were tested for repellent activity on elasmobranchs. 2,3-butanedione evidenced a flight response in lemon and nurse sharks. See Table 27. The results, in combination with the results for ionone and zingerone on free-swimming sharks in Example 5 above, evidence the repellent activity of ketones and di-ketones. Tonic immobility studies were carried out on 2,3-butanedione and diacetyl in denatured alcohol. In seven of eight Syringe Assays, tonic immobility was terminated in juvenile lemon and nurse sharks. In one Syringe Assay, a behavioral response was noted during tonic immobility. In one cloud dispersion assay in free-swimming Caribbean reef and blacknose sharks, no response was noted since the volume was only 290 microliters. No response would be expected with such a low volume. In one bite assay with a juvenile nurse shark tonic immobility was terminated.
[0000]
TABLE 27
Tonic Immobility Assays with Acetyl
Component Species T? Delivery Dose Response
2,3-Butanedione lemon Y syringe 10 ml 3.34 sec
(diacetyl)
2,3-Butanedione lemon Y syringe 6 ml 3.34 sec
(diacetyl)
2,3-Butanedione lemon Y syringe 2 ml 2.43 sec
(diacetyl)
2,3-Butanedione lemon Y syringe 5 ml 3.56 sec
(diacetyl)
2,3-Butanedione nurse Y bite 3 ml
(diacetyl)
diacetyl nurse Y syringe 57 ml 11.14 sec
diacetyl/SLX carib reef/ N cloud - co2 290 ul
solution blacknose
diacetyl/SLX nurse R syringe 45 ml
solution
diacetyl mixture lemon Y syringe 7 ml 1.52 sec
diacetyl mixture lemon Y syringe 6 ml 1.40 sec
[0233] Together with the data provided in Example 5 for ionone and zingerone, the data in Table 27 evidence the repellent activity of ketones and di-ketones.
Elasmobranch-repelling electropositive metals and methods of use
US2007256623
CA2601682
INTRODUCTION
[0001] This invention relates generally to electropositive metals for repelling elasmobranchs and methods of using electropositive metals to repel elasmobranchs.
BACKGROUND OF THE INVENTION
[0002] Elasmobranchs represent a significant problem in the commercial fishing industry. Elasmobranchs are often inadvertently caught on fishing tackle directed at other more commercially valuable kinds of fish. This inadvertent catching of elasmobranchs (or other non-valued fish) is called "by-catch." As many as 100 million elasmobranchs are killed each year as by-catch. This loss of life has resulted in a real threat to several shark species. Currently, as many as 80 species of shark are considered threatened with extinction.
[0003] Further, when elasmobranchs are caught as by-catch, fishing operations receive no return on their investment since the shark is caught on a hook that might have otherwise brought in a marketable fish. Additionally, the fishing tackle on which a shark is caught often must be cut loose for the safety of those working on the fishing vessel causing a loss of both equipment and time.
[0004] Longlining is a commercial fishing method that suffers significant losses from shark by-catch. Longlining uses multiple baited individual fish hooks with leaders strung at intervals along an often very long (2-3 miles) main fishing line. Longline fishing operations routinely target swordfish and tuna. The longline hooks, however, are not selective and elasmobranchs are sometimes caught in greater numbers than the intended catch. The result is great loss of life in elasmobranchs and significant financial losses in the longline industry. Elasmobranchs cause additional losses in the longline fishing industry by scavenging marketable fish caught on longlines before the fish may be retrieved for processing.
[0005] Elasmobranchs also represent a problem in the commercial trawling industry. Trawling is a commercial fishing method that catches fish in nets. Elasmobranchs cause significant losses for trawlers because they scavenging fish caught in trawl nets before they are retrieved for processing. As such, valuable fish are often lost to shark predation. Also, sharks often tear holes in the nets, resulting in partial or complete loss of catch and significant repair costs.
[0006] There has been a long-felt need for methods and devices to deter elasmobranchs from commercial fishing lines and nets. Attempts in the middle of the twentieth century were made to protect trawl nets with electric discharge devices (Nelson, "Shark Attack and Repellency Research: An Overview," Shark Repellents from the Sea ed. Bernhard Zahuranec (1983) at pg .20). Nevertheless, no commercially effective repellent has yet to be made available for reducing shark by-catch in the commercial fishing industry or for reducing loss of valuable fish or fishing tackle to shark predation. Further, Applicant is unaware of any consideration in the art of the use of electropositive metals to repel elasmobranchs to limit by-catch and other losses from elasmobranchs.
[0007] An effective shark repellent would not only be valuable to the fishing industry but also would be valuable for protecting humans from shark attacks. No effective repellent has yet to be marketed for limiting the risk of shark attacks faced by humans exposed to elasmobranchs. Over the last 50 years antishark measures employed to protect humans from shark have included electrical repellent devices (Gilbert & Springer 1963, Gilbert & Gilbert 1973), acoustical playbacks (Myrberg et al. 1978, Klimley & Myrberg 1979), visual devices (Doak 1974) and chemical repellents (Tuve 1963, Clark 1974, Gruber & Zlotkin 1982). None of these procedures proved satisfactory in preventing shark attacks. (Sisneros (2001)). As such, the long felt need for an effective repellent had not been satisfied.
[0008] Researchers have historically used several bio-assays to determine if a repellent evokes a flight response in shark. One such bio-assay measures the effect of a repellent on a shark that is immobilized in "tonic immobility." Tonic immobility is a state of paralysis that typically occurs when a shark is subject to inversion of its body along the longitudinal axis. This state is called "tonic," and the shark can remain in this state for up to 15 minutes thereby allowing researchers to observe effects of repellents. After behavioral controls are established, an object or substance that has a repelling effect will awaken a shark from a tonic state. Researchers can quantify the strength of a repellent effect from these studies.
BRIEF SUMMARY OF THE INVENTION
[0009] The applicant has discovered that an electropositive metal is an effective elasmobranch repellent useful in limiting by-catch as well as protecting humans. Electropositive metals, particularly the Lanthanide metals, known or hereinafter developed, that are of sufficient electropositivity to repel elasmobranchs are acceptable in aspects of the present invention.
[0010] According to a non-limiting embodiment of the present invention, an apparatus for repelling elasmobranchs is provided comprising an electropositive metal. Preferably, the electropositive metal is a Lanthanide metal. More preferably, the electropositive metal is a Mischmetal. Electropositive metals may have a shape of a cylinder, a cone, a circle, a cube, a disk, a bar, a sphere, a plate, a rod, a ring, a tube, a stick or a block.
[0011] Electropositive metals of the present invention preferably have a revised Pauling electronegativity of less then 1.32. In a non-limiting embodiment, an electropositive metal has a cathode half-cell standard electrode potential greater then 1.9 volts in aqueous solution. In a non-limiting embodiment, the electropositive metal is a Lanthanide metal, a Mischmetal, an Alkaline Earth metal, an Alkali metal, or a Group 3 metal on the periodic table.
[0012] According to a first non-limiting aspect of the present invention, an apparatus is provided comprising an electropositive metal and a buoy, a barge, a net, fishing tackle or any combination thereof. Fishing tackle may comprise a longline, a main line, a gangion, a branchline, a weight, a buoy, a net, or any combination thereof.
[0013] According to a second non-limiting aspect of the present invention, an apparatus is provided comprising an electropositive metal and a fish hook. Such fish hook may be individual or attached to longline or mainline and such fish hook may have a single or multiple hooks.
[0014] According to a third non-limiting aspect of the present invention, an apparatus is provided comprising a surfboard and an electropositive metal.
[0015] In fourth non-limiting aspect of the present invention, a method is provided for repelling elasmobranchs comprising attaching an electropositive metal to a human body or to clothing or accessories associated with a human body. In an aspect of the invention, an electropositive metal may be attached to a human ankle or wrist. In a further aspect an electropositive metal may be attached to a bracelet. In yet a further aspect an electropositive metal may be attached to a belt, a weight belt for diving or flippers. In yet a further aspect, an electropositive metal may be housed within a surfboard or attached to a surfboard. In yet another aspect, an electropositive metal may be trailed along with a human in water.
BRIEF DESCRIPTION OF THE DRAWINGS
[0016] The invention will now be described by way of example with reference to the accompanying drawings wherein:
[0017] FIG. 1 illustrates a traditional circle hook (40) attached to a line (30) and preferred zone (I) for locating an electropositive metal in accordance with the present invention.
[0018] FIGS. 2A-C illustrate non-limiting positions within the zone (I) for locating an electropositive metal in accordance with the present invention. FIG. 2A illustrates an electropositive metal attached to the line above the hook. FIG. 2B illustrates an electropositive metal attached to the hook. FIG. 2C illustrates an electropositive metal attached to the hook shank and clear of the hook eye.
[0019] FIG. 3A-C illustrate non-limiting positions within the zone (I) for locating an electropositive metal on a J-hook in accordance with the present invention. FIG. 3A illustrates an electropositive metal attached to the line above the hook. FIG. 3B illustrates an electropositive metal attached to the hook. FIG. 3C illustrates an electropositive metal attached to the hook shank and clear of the hook eye.
[0020] FIG. 4A-B illustrate non-limiting positions within the zone (I) for locating an electropositive metal on a treble hook in accordance with the present invention. FIG. 4A illustrates an electropositive metal attached to the line above the hook. FIG. 4B illustrates an electropositive metal attached to the hook.
[0021] FIG. 5 illustrates a demersal longline with an electropositive metal in accordance with the present invention.
[0022] FIGS. 6A-B illustrate non-limiting apparatuses and methods of repelling elasmobranchs in accordance with the present invention. FIG. 6A illustrates a buoy and electropositive metal and a net with a plurality of electropositive metals in accordance with the invention. FIG. 6B illustrates a barge and an electropositive metal.
[0023] FIGS. 7A-B illustrate non-limiting surfboards with an electropositive metal in accordance with the invention. FIG. 7A illustrates a surfboard with an electropositive metal that is capable of spinning in accordance with the invention. FIG. 7A illustrates a surfboard with an electropositive metal embedded in or attached to the surfboard in accordance with the invention
[0024] FIGS. 8A-C illustrate accessories for attaching an electropositive metal to a human or other subject or object. FIG. 8A illustrates a belt or weight belt with an electropositive metal in accordance with the invention.
FIG. 8B illustrates a bracelet or wristband with an electropositive metal in accordance with the invention. FIG. 8C illustrates flippers for snorkeling or diving with an electropositive metal in accordance with the present invention.
DETAILED DESCRIPTION OF THE INVENTION
[0025] "By-catch" is any kind of fish that is caught in a fishing operation wherein the catching of the fish is not the object of the fishing operation. For example, if the target fish of a longline fishing operation is tuna, an elasmobranch caught on a hook of the longline is by-catch.
[0026] "Elasmobranchs" in this specification means one or more elasmobranchii in the super-orders Galeomorphii, Squalomorphii, and Batoidea and orders Squaliformes (dogfish), Carcharhiniformes (requiem sharks), Lamniformes (mackerel sharks), Rajiformes (true rays), Pristiformes (sawfishes), Torpediniformes (electric rays) and certain Orectolobiformes (carpet sharks). Elasmobranchs in this specification includes nurse sharks, an Orectolobiform, but this specification does not include the other carpet sharks, such as wobbegongs.
[0027] An "Electropositive metal" is a metal which readily donates electrons to form positive ions. Electropositive metals are strong reducing agents and all react with water to some degree, typically liberating hydrogen gas or forming a hydroxide. The most electropositive metals tends to be found on the left-hand side of the Periodic Table of the elements, particularly in Groups I, II, III, and the Lanthanides. In general, electropositivity decreases and electronegativity increases as one moves to the right hand side of the Periodic Table of the elements. The most electropositive metal known is Francium, which is radioactive. The most stable electropositive metal is Cesium which is highly reactive in water and air. Electropositive metals typically do not exhibit any permanent magnetism (ferromagnetism) at room temperature.
[0028] "Revised Pauling Electronegativity" is is a chemical property which describes the power of an atom to attract electrons towards itself. First proposed by Linus Pauling in 1932 as a development of valence bond theory it has been shown to correlate with a number of other chemical properties. Electronegativity cannot be directly measured and must be calculated from other atomic or molecular properties The Pauling electronegativity for an element is calculated using the dissociation energies of at least two types of covalent bonds formed by that element. Linus Pauling's original values were updated in 1961 to take account of the greater availability of thermodynamic data, and it is these "Revised Pauling" values of the electronegativity which are most usually used.
[0029] "Standard Electrode Potential" is the measure of the individual potential of any electrode at standard ambient conditions, which is at a temperature of 298K, solutes at a concentration of 1 M, and gases at a pressure of 1 bar. The basis for an electrochemical cell such as the galvanic cell is always a reduction-oxidiation reaction which can be broken down into two half-reactions: oxidation at anode (loss of electron) and reduction at cathode (gain of electron). Electricity is generated due to electric potential difference between two electrodes. This potential difference is created as a result of the difference between individual potentials of the two metal electrodes with respect to the electrolyte (In practice, seawater serves as the conductive electrolyte). In an electrochemical cell, an electropositive metal acts as the cathode, and the standard electrode potential represents the voltage of the reduction half-cell reaction.
[0030] A "Lanthanide metal" belongs to the series comprising the 15 elements with atomic numbers 57 through 71, from Lanthanum to Lutetium. All lanthanides are f-block elements, corresponding to the filling of the 4f electron shell, except for lutetium which is a d-block Lanthanide. The Lanthanide series is named after Lanthanum. The Lanthanide series is also commonly referred to as the "rare earths" or "rare earth elements".
[0031] "Mischmetal" is an alloy of Lanthanide elements in various naturally-occurring proportions. The term "Mischmetal" is derived from the German "Mischmetal" meaning mixed metals. Mischmetals are also called Cerium mischmetal, rare earth mischmetal or misch metal. A typical composition includes approximately 50% Cerium and 45% Lanthanum, with small amounts of Neodymium and Praseodymium. Other Mischmetal alloy mixtures include Lanthanum-rich Mischmetal, Ferrocerium, and Neodymium-Praseodymium Mischmetal.
[0032] An "Alkaline Earth" metal belongs to the series of elements comprising Group 2 of the Periodic Table of elements: Beryllium, Magnesium, Calcium, Strontium, Barium, and Radium. The alkaline earth metals are silvery colored, soft, low-density metals, which react readily with halogens to form ionic salts, and with water to form strongly alkaline hydroxides.
[0033] An "Alkali Earth" metal belongs to the series of elements comprising Group I of the Periodic Table of elements: Lithium, Sodium, Potassium, Rubidium, Cesium, and Francium. The alkali metals are all highly reactive and are rarely found in elemental form in nature. As a result, in the laboratory they are stored under mineral oil. They also tarnish easily and have low melting points and densities.
[0034] A "Group 3 metal" belongs to the third vertical column of the Periodic Table of elements. While Lanthanides are usually considered part of Group 3, the metallic elements Yttrium and Scandium all always considered Group 3 metals. The physical properties of Yttrium and Scandium resemble Lanthanides and these two metals are commonly considered part of the "rare earths".
[0035] "Longline" refers to a fishing line that may extend up to many miles wherein a mainline extends the full length of the longline and individual shorter gangion lines attached to the mainline are spaced at set intervals (perhaps several feet or meters or perhaps 1000 feet or greater apart). Hooks are attached to the individual gangion lines. Hooks may be baited and used to catch target fish. The addition of an electropositive metal repels elasmobranchs from the baited hooks as well as from the region of the longline generally.
[0036] "Target fish" is any kind of fish, the catching of which is the object of a fishing operation. For example, the target fish of a longline fishing operation may be tuna. A fish that is caught on the longline that is not tuna would not be a target fish.
[0037] "Tonic immobility" is the state of paralysis that typically occurs when an elasmobranch is subject to inversion of its body along the longitudinal axis of the body, i.e., is belly up. An elasmobranch can remain in this state for up to 15 minutes. While in tonic immobility, the shark is comatase and unresponsive to many external stimuli. Biologists often perform surgery on sharks using tonic immobility, precluding anesthesia. An effective shark repellent terminates tonic immobility, often violently, thus, tonic immobility is useful as a bioassay for testing the effectiveness of electropositive metals.
I. Electropositive Metals as Repellents of Elasmobranchs
[0038] The applicant first observed the unusual repellent effects of electropositive Lanthanide metals on sharks when tonically-immobilized juvenile lemon sharks (N. brevirostris) exhibited violent rousing behavior in the presence of a 153 gram 99.95% Samarium metal ingot. As the Samarium metal was moved towards the immobilized shark's head, the shark terminated tonic immobility, in the direction away from the approaching metal. For experimental controls, pure Chromium, an antiferromagnetic metal, and pyrolytic graphite, a highly diamagnetic substance, failed to produce any behavioral responses in juvenile lemon sharks.
[0039] A polystyrene white plastic blinder was used to remove any visual and motion cues from an approaching electropositive metal. This blinder was placed close to the shark's eye, sufficiently shielding its nares, eyes, gills, and head up to its pectoral fin. Again, Samarium metal terminated tonic immobility in all test subjects at a range of 2 to 50 cm from the blinder. Chromium metal and pyrolytic graphite did not produce any notable behavioral shifts. In order to confirm that pressure waves were not affecting the test subjects, the tester's hand was moved underwater towards the shark's head both with and without blinders at varying speeds. This motion also did not disrupt the immobilized state. The same series of experiments were repeated with juvenile nurse sharks (G. cirratum) and yielded the same behavioral results.
[0040] The same experimental protocol was repeated with a 73 gram ingot of 99.5% Gadolinium metal, an electropositive Lanthanide metal, and yielded the same behavioral results in both juvenile lemon sharks and nurse sharks. It is noted that the rousing behavior was most violent when Samarium metal was used. Additionally, the Gadolinium metal corroded quickly after seawater exposure, and therefore would be appropriate for a one-time use application.
[0041] In order to eliminate the possibility of galvanic cell effects, juvenile sharks were removed from their pens and brought at least 15 meters away from any submerged metal objects. All testers and witnesses removed watches, rings, and jewelry so that only the lanthanide metal was exposed to seawater. The same experimental method was repeated in lemon sharks and we report that tonic immobility was terminated with electropositive Samarium metal in all tests.
[0042] The application has discovered that waving Samarium or Gadolinium in air above immobilized or resting sharks does not effect behavior, even when the metal is very close to the water's surface. The electropositive metal must be in contact with seawater in order to produce the repellent effect. This is notably different from the effects of a rare-earth magnet, which will often terminate tonic immobility at close range in air.
[0043] The effects of an electropositive Lanthanide metal on free-swimming sharks were also evaluated. Two juvenile nurse sharks (less than 150 cm total length) were allowed to rest in an open-water captive pen. The tester approached the nurse sharks and moved his hand near the pen wall. His hand contained no metal. Both nurse sharks remained at rest. Next, the tester presented the 153 gram ingot of electropositive Samarium metal underwater to the pen wall and we note that both nurse sharks awakened and rapidly swam away from the tester's locale. Next, a highly-stimulated competitively-feeding population of six blacknose sharks (C. acronotus) (total length up to 120 cm) and six Caribbean reef sharks (C. perezii) (total length up to 210 cm) was established using chum and fish meat. A diver entered the water near the population of sharks with the 153 gram of Samarium metal secured to one end of a 1.5 meter-long polyvinyl chloride pole. As free-swimming sharks swam close to the diver, the control end of the pole (without metal) was presented in a left-right waving motion. Approaching sharks would swim past, bump, or briefly bite the pole. The diver then turned the Samarium metal-end of the pole towards the approaching sharks. All blacknose sharks exhibited a "twitching" or "jerking" behavior as they came near the metal ingot and quickly swam away. Caribbean reef sharks generally avoided the metal, but did not exhibit the twitching behavior.
[0044] Following the aforementioned initial experiments, many electropositive metals were procured and presented to tonic-immobilized juvenile sharks. The violence of the shark's response to each metal was scored on a scale of 0 to 4, with 0 equating to no response and 4 equating to a violent rousing reaction. All testing was performed in the Bahamas using open-water captive pens. Arc-melted 100 gram Lanthanide metal ingots, Calcium, and Strontium were obtained from Metallium Inc., USA. Lanthanum, Cerium, Neodymium, Yttrium, Praseodymium and Mischmetal samples were obtained from HEFA Rare Earth Metals, Canada. Magnesium, Beryllium, transition metals and nonmetals were procured as surplus items online from EBay.
[0045] In juvenile N. brevirostris and G. cirratum, the applicant has found that the following Lanthanide metals all terminated the tonic state at distances less than 0.1 meters: 100 grams of 99% purity Lanthanum metal, 90 grams of 99% purity Cerium metal, 100 grams of 99% purity Praseodymium metal, 100 grams of 99% purity Neodymium metal, 73 grams of 99.95% purity Samarium met al, 145 g of arc-melted 99% purity Terbium metal, 89 g of arc-melted 99% purity Erbium metal, 100 grams of arc-melted 99% purity Holmium metal, 100 grams of arc-melted 99% Gadolinium metal, 100 grams of arc-melted 99% Dysprosium metal, and 100 grams of arc-melted 99% purity Ytterbium metal.
[0046] In the same experiment, 75 grams of 99% purity Yttrium metal, a Group 3 metal, also terminated tonic immobility in juvenile N. brevirostris.
[0047] In the same experiment, a 30 gram 99% purity ingot of Strontium and separately, a 40 gram 99% purity ingot of Calcium terminated tonic immobility in juvenile G. cirratum. These metals were highly
reactive in seawater and dissolved before a second series of tests could be performed.
[0048] In the same experiment, the following Mischmetals terminated tonic immobility in N. brevirostris: An 80 gram slice of Cerium Mischmetal, and a 100 gram slice of Neodymium-Praseodymium Mischmetal.
[0049] In the same experimental, the following Alkaline Earth metals terminated tonic immobility in N. brevirostris: A 70 gram block of 99% Magnesium, and a 10 gram pellet of 99% purity Barium. The Barium pellet reacted violently with seawater and a subsequent test could not be performed.
[0050] Transition metals and nonmetals, which are much less electropositive than the Lanthanides, Alkali, Alkaline Earth, and Group 3 metals, were also screened using the tonic immobility bioassay. The following transition metals and metalloids failed to illicit a rousing response in immobilized juvenile N. brevirostris: A 20 gram disc of 99.95% purity Tellurium, a 20 gram cylinder of 99.5% purity Tungsten, a 20 gram cylinder of 99.5% purity Cobalt, a 20 gram cylinder of 99.5% purity Iron, a 20 gram cylinder of 99.5% purity Niobium, a 20 gram cylinder of 99.5% purity Zirconium, a 20 gram square of 99.95% Rhenium, a 100 gram pillow of Aluminum, and a 15 gram square of pyrolytic graphite (Carbon).
[0051] Based on the aforementioned experimental results, a close correlation was found between the revised Pauling electronegativity values for the electropositive metals, and behavioral response. As the revised Pauling electronegativity decreased, the violence of the shark's response seemed to increase. A significant repellency threshold was found at a revised Pauling electronegativity of 1.32 or less-Metals with electronegativities greater than 1.32 did not produce the response. Highly reactive metals, such as Strontium and Calcium (electronegativities of 0.89 and 1.00 respectively) produced a violent rousing reaction as expected.
[0052] An electropositive metal for repelling elasmobranchs may comprise the shape of a cylinder, a cone, a circle, a cube, a disk, a bar, a sphere, a plate, a rod, a ring, a tube, a stick, a block, a tapered cone, or any other shape.
[0053] The mode of action of electropositive metals on elasmobranchs is not fully understood. While not wishing to be bound by any particular theory, one plausible theoretical explanation for this surprising finding of repellent activity of electropositive metals is the possibility that relatively high voltages, ranging from 0.8 VDC to 2.7 VDC with currents up to 0.1 milliamperes, are created between the metal and the shark's skin. This electromotive force may over-stimulate the ampullae of Lorenzini (known to be used by elasmobranchs for navigation and orientation), which saturate below 100 nanovolts, causing a highly unnatural stimulus to the shark.
[0054] Electropositive metals exhibit no measurable permanent magnetism (ferromagnetism). The applicant hypothesized that a magnetic or electrical field was being induced by the metal's movement through seawater. The applicant attempted to measure minute magnetic fields being produced by the movement of Samarium metal through seawater in a closed system. A submersible calibrated milliGauss meter probe was secured in a plastic tank containing seawater with the same salinity, pH, and temperature of the water used in previous shark testing. After zeroing out the Earth's magnetic field, the applicant did not detect any magnetic fields being produced by the movement of Samarium metal through the tank, within tenths of a milliGauss
[0055] Electromotive forces generated by electropositive metals are effective repellents for elasmobranchs, excluding certain carpet sharks in the family Orectolobidae. It is believed that electropositive metals are not effective repellents against carpet sharks because carpet sharks, particularly spotted wobbegongs (Orectolobus maculatus), are ambush predators and rely more on visual, olfaction, and lateral line clues than this electromagnetic sense. This species of shark is found chiefly in Australia and Indonesia, and does not represent significant by-catch species or species that are known to be aggressive against humans. Electropositive metals, however, are effective against nurse sharks, another Orectolobiform.
[0056] Electropositive metals have been demonstrated to act as acceptable repellents of elasmobranchs. The repellent activity of electropositive has been shown to be better than existing shark-repellent technology with the exception of certain chemical repellents and magnetic repellents being developed by SHARK DEFENSE LLC that have a greater range of action.
[0057] A. Electromotive Forces
[0058] The repellency of an electropositive metal may be measured in a variety of ways. The applicant has found that the standard electrode potential of the cathode half-cell reaction of an electropositive metal in aqueous medium can be measured in a closed system using an electropositive metal at the anode (the site of oxidation), a piece of shark skin at the cathode (the site of reduction), and seawater as an electrolyte. Electromotive forces were measured using a calibrated direct current voltmeter. Electromotive forces greater than 0.8 volts were recorded for all electropositive metals, with Lithium metal, an Alkali earth metal, producing the highest measurable voltage at 2.71 volts. This demonstrated that cations and anions were exchanged through the electrolyte. These measured electromotive forces closely correlated to published standard electrode potentials for electropositive metals. A closed system using an electropositive metal at the external cathode (-) and a piece of shark skin at the external anode (+) with seawater electrolyte represents a simple and effective means of measuring electromotive forces and predicting repellency.
[0059] The strength of an electropositive metal's electromotive force field is inversely related to the distance an object is from the metal. As such, metals with a low standard electrode potential may repel elasmobranchs if the elasmobranch moves close enough to sense the electromotive force field of the metal. A highly electropositive metal having sufficient strength to repel an elasmobranch at sufficient distance such that the elasmobranch is deterred from striking a baited hook or coming near a person or other subject is preferred. It is more preferred that an electropositive metal have a standard electrode potential of at least 2.00 volts in seawater to provide sufficient electromotive force to repel an elasmobranch away from a baited hook or a person before the elasmobranch may bight the hook or harm the person. Because an elasmobranch may act to strike a hook or person at a distance from the target, the higher the standard electrode potential or the lower the revised Pauling electronegativity of the metal, the more effective it will be. II. Methods and Devices for Electropositive Metals
[0060] A. Electropositive Metals
[0061] Exemplary and non-limiting electropositive metals in accordance with the invention may be constructed of any metal that is capable of generating an electromotive force in seawater relative to the shark's skin.
[0062] Electromotive forces may be generated in any manner known to the skilled artisan who is practicing aspects of the invention or electrochemistry.
[0063] There are many varieties of electropositive metals including the Lanthanide metals, the Alkaline Earth metals, the Alkali metals, Mischmetals, and the Group 3 metals on the periodic table of elements. Any electropositive metal having sufficient standard electrode potential or a low revised Pauling electronegativity may be used as a repellent of elasmobranchs.
[0064] Exemplary electropositive metals include Lanthanum, Cerium, Neodymium, Praseodymium, Samarium, Europium, Gadolinium, Terbium, Dysprosium, Holmium, Erbium, Thulium, Ytterbium, Lutetium, Yttrium, Scandium, Lithium, Magnesium, Calcium, Strontium, Barium, Cerium Mischmetal, Neodymium-Praseodymium Mischmetal, and Lanthanum-rich Mischmetal. Electropositive metals may be flexible or inflexible.
[0065] A preferred electropositive metal contemplated within an aspect of the invention is Neodymium-Praseodymium Mischmetal. Neodymium-Praseodymium Mischmetal is a more preferred material than pure forms of Lanthanide or Alkaline earth metals due to cost and low corrosion reactivity in seawater. Pure Lanthanide metals, particularly the "late Lanthanides" comprising elements 63 through 71, are prohibitively expensive in pure form. Pure Alkali metals are extremely reactive in seawater and present fire hazards in storage. Certain Alkaline earth metals are also highly reactive in seawater, such as Barium and are too short-lived for commercial fishing applications. Highly electropositive metallic elements such as Promethium, Radium, and Francium are highly radioactive and are not feasible for any elasmobranch repelling application.
[0066] In selecting an electropositive metal, a revised Pauling electronegativity of less than 1.32 is preferred. A revised Pauling electronegativity of about 1.14 or less is more preferred since the impact of the electromotive force field will be felt at a slightly greater distance from the metal.
[0067] Early Lanthanide metals, particularly elements 57 through 62, commonly called the "early Lanthanides", possess revised Pauling Electronegativities less than 1.2, which is preferred. Similarly, Mischmetals containing combinations of Lanthanum, Cerium, Neodymium, and Praseodymium exhibit calculated revised Pauling electronegativities of less than 1.2, which is preferred.
[0068] In order to maximize electromotive forces, the surface area of an electropositive metal may be maximized. For example, a 6'' diameter by 2'' thick cylindrical Cerium Mischmetal block (revised Pauling electronegativity of 1. 15) may be effective in repelling elasmobranchs at a range of 8 inches.
[0069] A plurality of electropositive metals may be employed to repel elasmobranchs. For example, 1'' cube metals may be arranged in a 12'' long bar and used to repel elasmobranchs. The cube metals may be of any electropositive metal material capable of producing sufficient electromotive force at any distance of interest from the metal to repel elasmobranchs. Alternatively, a plurality of 1'' cube electropositive metals may be arranged linearly with a distance between each piece of metal.
[0070] B. Electropositive Metals in Combination with Hooks
[0071] A non-limiting aspect of the present invention is the use of electropositive metals to repel elasmobranchs from baited hooks. Exemplary and non-limiting combinations of an electropositive metal and a hook are illustrated in FIGS. 14. For example, in FIG. 1, an exemplary and non-limiting circle hook (140) is illustrated attached to a line (150) along with exemplary and non-limiting zone (I) in the circle hook and line where an electropositive metals may be placed or affixed. The preferred region (zone I) for metal placement is any region wherein the affixed or placed magnet does not obstruct the hook gap distance (zone II). Not more than 20% of the hook gap distance (zone II) is preferably obstructed by the metal such that the hook is not prevented from setting in the corner of the mouth of a target fish. Nevertheless, any arrangement wherein the hook is not prevented from catching target fish is acceptable. Tapered conical designs (not illustrated) are contemplated such that the diameter of the electropositive metal at the hook end is smaller than the diameter of the electropositive metal at the line end of zone I.
[0072] Exemplary and non-limiting combinations of an electropositive metal on a hook and line are illustrated in FIG. 2. An electropositive metal (210) may be placed in proximity to a circle or offset circle hook (240) such that it rests on the hook eye (241) providing an exemplary embodiment such as the hook-metal combination embodied at 260. An electropositive metal (210) may be placed in proximity to a circle or off-set circle hook (240) such that it rests on the shank (242) of the hook providing an exemplary embodiment such as the hook-metal combination embodied at 270. A metal (210) may be placed on a circle or offset circle hook (240) such that it is secured to the outside of the shank (242) and the hook eye (241) providing an exemplary embodiment such as the hook-metal combination embodied at 280. Vinyl electric tape (not illustrated) may be used to secure the metal. Black vinyl tape is preferred to reduce reflections of light.
[0073] Electropositive metals may be provided in any shape. It is preferred that a metal's shape not significantly obstruct the hook gap distance (zone II). The metal may comprise a hole through which a lead, or gangion, or mainline (250) or other filamentous object may pass. Exemplary non-limiting shapes may include a cube or block of any size or other object having at least one plane comprising four right angles and a hole passing through the object such that fishing line or other filament may be passed through to affix the magnet in place on fishing tackle or other object. Alternative, non-limiting shapes may also include cylindrical or other circular, oval or oblong three-dimensional shapes having a hole passing through some portion of the shape (210). Alternative, non-limiting shapes may also include a hollow pyramid or a hollow trapezoid.
[0074] Alternative, non-limiting shapes may also include a solid cube or similar shape, a solid rectangle or similar shape, a solid bar or similar shape, a solid pyramid or similar shape, a solid trapezoid or similar shape or any other shape. Metals may be shaped as a ring, a trapezoid, a series of trapezoids, a series of trapezoids arranged in a larger ring pattern, a cone, a tapered cone, a narrow or wide cylinder or in the shape of a Billy club. Preferably, the shape when combined with a hook provides a hook in proximity to an electropositive metal comprising sufficient electromotive force field strength to repel elasmobranchs.
[0075] Exemplary and non-limiting combinations of electropositive metal and hook are also illustrated in FIG. 3. An electropositive metal (310) may be placed in proximity to a j-hook (340) such that it rests on the hook eye (341) providing an exemplary embodiment such as the hook-metal combination embodied at 360. An electropositive metal (310) may be placed in proximity to a j-hook (340) such that it rests on the shank (342) of the hook providing an exemplary embodiment such as the hook-metal combination embodied at 370. An electropositive metal (310) may be placed on a j-hook (340) such that it is secured to the outside of the shank (342) and the hook eye (341) providing an exemplary embodiment such as the hook-metal combination embodied at 380. As described above in the illustration of FIG. 2, electropositive metal may be provided in any shape.
[0076] Exemplary and non-limiting combinations of an electropositive metal and hook are also illustrated in FIG. 4. An electropositive metal (410) may be placed in proximity to a treble hook (440) such that it rests on the hook eye (441) providing an exemplary embodiment such as the hook-metal combination embodied at 460. An electropositive metal (410) may be placed in proximity to a treble hook (440) such that it contacts the shank (442) of the hook providing an exemplary embodiment such as the hook-metal combination embodied at 470.
[0077] A hook in accordance with the invention may be any hook that is capable of catching target fish. The hook may comprise stainless steel, steel, galvanized metals, ferromagnetic metals or any other material, metallic or plastic or any other composite.
[0078] C. Electropositive Metals on Longlines
[0079] An exemplary and non-limiting method of repelling elasmobranchs involving repelling elasmobranchs from longlines in accordance with the invention is illustrated in FIG. 5. A longline (500) may be deployed from a boat (561) to fish for a target fish of interest. The main line (550) of the longline may be attached to a buoy (520) and at a set distance from the buoy may be attached to an anchor (562). A set of gangions (530) with hooks (540) may be attached to the mainline beginning at the anchor (562) and may be spaced sufficiently to limit interaction between individual gangion lines (530). Each hook may have an electropositive metal mounted resting on the hook eye (541). Alternatively, the electropositive metal may be mounted on a hook shank (542) or may be secured to the outside of the hook (540). The hooks may be baited. The longline may be a demersal longline such that the main line is proximal to the ocean or otherwise water's floor. The longline may be a pelagic long line, such that the main line is nearer to the surface of the water, suspending in the water column, typically at 100-500 feet below the surface. In the aspect of the invention where the longline is a pelagic longline, anchors (562) may have less weight or may be absent from the longline apparatus. The longline may also be a semipelagic longline wherein the mainline is further down the water column from the surface as compared to a pelagic line but is not proximal to the water's floor or is not proximal to the water's floor on at least one end of the longline. Use of electropositive metals with longlines reduces by-catch of elasmobranchs.
[0080] Longlines comprising electropositive metals may be handled in the commercial environment in a manner similar to those practices known in the art of longline commercial fishing. Because hooks must be carefully managed to control tangling and hooking of objects on a longlining boat, including other portions of the tackle of the longline, commercial fishing operations and those of skill in the art will recognize how to handle longlines with hooks. Electropositive metals on longlines likewise may be handled in the same manners as one would consider appropriate in the art to avoid entanglements.
[0081] As described above, electropositive metals of any size may be used in combination with a longline hook so long as the target fish may be caught on the hook. An exemplary electropositive metal on a longline hook may be 2''*0.25''*2''. Smaller electropositive metals are also acceptable. Electropositive metals of less than 0.5'' cubed may be appropriate for smaller hook settings.
[0082] D. Electropositive Metal Repellents on Buoys, Nets and Barges
[0083] An exemplary and non-limiting method of repelling elasmobranchs with an electropositive metal or a plurality of electropositive metals placed on a buoy or barge or net is illustrated in FIG. 6. Buoys with electropositive metals as their weighted bases are shown as element 660 and 661 in FIG. 6A. The floating portion of the buoy (620) allows the buoy to float while the electropositive metal portion of the buoy (610) remains in the water because of its weight. A series of buoys comprising electropositive metals may be placed in a region to repel elasmobranchs or may be placed around a swimming area or rescue area to repel elasmobranchs. A series of buoys with electropositive metals may be accompanied by a series of electropositive metals submerged (611) in an area of interest, such as a swimming area. As illustrated in FIG. 6B, very large electropositive metals may be placed on a large floating barge (670) comprising an electropositive metal (610).
[0084] An exemplary and non-limiting method of repelling elasmobranchs with a plurality of electropositive metals is illustrated in FIG. 6A as element 600, an elasmobranch repelling net apparatus. Buoys (660 and 661) may be employed to float a net (650) comprising a series of electropositive metals (640) held within the net and electropositive metal rings (630) holding the ropes of the net together. The net may be strung to the bottom of the water column using weighted electropositive metals (611). The net may be anchored to a specific location to provide a physical barrier. The net may provide a curtain of electromotive field forces to repel elasmobranchs from an area or to keep elasmobranchs from entering an area of interest, such as a swimming or working area. A net (650) comprising electropositive metals such as those illustrated as elements 610, 611, 630 and 640 may also be used to trawl for fish, shrimp or other aquatic species. In another non-limiting aspect of the invention, electropositive metals may be placed in aquaculture cages to repel sharks from predation or scavenging of cultured stock. Electropositive metals are useful to prevent damage by elasmobranchs to aquaculture cages, nets or other equipment.
[0085] E. Surfboard Fitted with Electropositive Metal
[0086] A non-limiting repelling device in accordance with the invention may comprise a surfboard comprising an electropositive metal device. FIG. 7B illustrates exemplary surfboards in accordance with an aspect of the invention. A surfboard (720) may comprise an electropositive metal device such as Mischmetal (710) imbedded, affixed, attached or otherwise associated in any manner contemplated by one of skill in the art with the surfboard An electropositive metal may be pressed into a space drilled into the surfboard (730). It may also be affixed with glue, waterproof tape, Velcro or any other mechanism known in the art now and hereafter.
[0087] In an alternative non-limiting example in Figure A, a surfboard (750) may comprise an electropositive metal or plurality of electropositive metals in association with one another wherein the electropositive metal or metals are capable of spinning when placed in water (740). Such a spinning electropositive metal (740) may comprise individual metal pieces attached to a hub (770) that is attached to an axle (760) to allow free spinning of the electropositive metal or metals attached to the surfboard (720) when water current is present.
[0088] An electropositive metal may be enclosed in the body of a surfboard or other watercraft or may be trailed behind a surfboard, other watercraft or swimmer.
[0089] F. Electropositive Metal Repellents on Swimming and Diving Clothing and Accessories
[0090] One exemplary non-limiting aspect of the present invention comprises an electropositive metal material for producing an electromotive force field near a swimmer or diver or other person or object in an elasmobranch environment.
[0091] Electropositive metals, such as for example, Mischmetal, or other electropositive metals may be worn as a bracelet or a band or otherwise placed in proximity of a person or object. An increase in the number of electropositive metals and an increase in the standard electrode potential of the metals that may be worn increases the electromotive force field around the wearer and increases the repelling activity of the bracelet, band or other metal article.
[0092] In a non-limiting example, an omnidirectional electromotive force field may be affixed or arranged near a subject or object exposed to an elasmobranch environment. The electromotive force field may be generated from, for example, an electropositive metal. An electropositive metal may be affixed, for example, to any portion of a swimmer's or diver's body such as the head, the leg, the arm, the torso, the ankle, the wrist, or any other portions of the body.
[0093] FIG. 8 illustrates a non-limiting example of electropositive metals (810) attached to a belt (801) (FIG. 8A) or bracelet (802) (FIG. 8B) or flippers (803) (FIG. 8C).
[0094] Electropositive metals may likewise be attached to clothing or water accessories such as swim trunks, wet suits, headbands, flippers, goggles or other piece of clothing or accessory. Electropositive metals may be sewn into such clothing or may be affixed with tape, glue, Velcro or any other mechanism for affixing to clothing or accessories for swimming, diving or otherwise working or playing in water.
[0095] Many human-shark interactions in shallow water, especially around the State of Florida in the United States, are hypothesized to be "mistaken identity" by the shark in water with poor visibility. The blacktip shark (C. limbatus) and nurse shark (G. cirratum) are often implicated in these encounters. The sharks do not have an olfactory clue in most of these "mistaken identity" cases. A series of electropositive metals, such as Mischmetal or other electropositive metal, may be used as means to repel the shark as it approaches within a few inches of the metal. With an electropositive metal, such as Cerium, or an increased number of electropositive metals, to increase electromotive force field strength, repellent activity increases and the chance that a shark will be repelled prior to an investigatory bump or bite is greatly increased.
[0096] The invention is further described with the following non-limiting examples, which are provided to further illuminate aspects of the invention.
III. EXAMPLES
Example 1
Tonic Immobility Responses to Electropositive Metals
[0097] In order to screen the repellency potential of various metals, 193 individual trials were conducted on juvenile sharks at South Bimini, Bahamas in open ocean pens. All sharks were placed into tonic immobility, and the behavioral response of the shark towards a test metal was scored using a scale of 0 to 4. A score of zero represented no response, with the shark remaining immobilized. A score of one represented a slight fin flinch or eye blink. A score of two represented a slight bend (less than 15 degrees) away from the metal, without rousing. A score of three represented a strong bend away from the metal (more than 15 degrees), without rousing. A score of four represents the termination of tonic immobility, with a rousing response, indicating adequate repellency. No more than three consecutive trials were performed on any one given shark. A minimum of 4 hours of rest was allotted before a shark was retested. Classifying the behavioral scores with a specific group on the Periodic Table of the element demonstrates that the electropositive metals found in Group 2 and Group 3 of the periodic table of elements produced a stronger repellent response than transition metals (Groups3 through 12), a poor metal (Group 13), a metalloid (Group 16), and a nonmetal (Group 14). See Table 1.
TABLE 1
Group Tests
(Periodic table) Performed Average Score
Group 1 1 4
Group 2 13 3.23
Group 2 Alloy 34 2.79
Group 3 84 2.28
Group 8 6 1.17
Group 13 4 0.75
Group 5 5 0.20
Group 14 21 0.10
Group 9 5 0
Group 7 6 0
Group 6 4 0
Group 4 5 0
Group 16 6 0
[0098] The aforementioned tests can also be analyzed in terms of the type of metal tested on the immobilized sharks. As expected, Alkali metals, Alkaline earths, Mischmetals, early Lanthanides, and late Lanthanides produced the highest repellency behavioral scores. These types of metals are electropositive and have revised Pauling electronegativities less then 1.32. See Table 2.
TABLE 2
Tests
Type of metal Performed Average Score
Alkali metal 1 4
Alkaline earth 13 3.23
Mischmetal 34 2.79
Early Lanthanide 49 2.66
Late Lanthanide 29 1.83
Rare Earth 6 1.333
Poor metal 4 0.75
Transition metal 31 0.26
Nonmetal 21 0.10
Metalloid 6 0
Example 2
Published Standard Electrode Potentials of Electropositive Metals
[0099] The published standard electrode potentials (SEP) for the cathode half-cell reaction of electropositive metals is a practical means of determining the repellency of the metal without performing a bioassay. As the cathode half-cell reaction voltage increases, the repellent effect is also expected to increase. The published voltage represents the electromotive force between the electropositive metal and the reference electrode. Published standard electrode potentials typically use a standard hydrogen electrode as the reference electrode. In practice, shark skin is the reference electrode and produces measurable voltages at about 88% of the published standard electrode potentials. The safe handling of highly electropositive metals must be considered, as well as the longevity of the metal in seawater. See Table 3.
TABLE 3
Cathode SEP Terminates Tonic metal (Volts) Immobility? Safety Comments
Lithium 3.05 YES Short-lived in water
Rubidium 2.98 PROBABLE Explosive in water
Potassium 2.93 PROBABLE Fire hazard in water
Cesium 2.92 PROBABLE Explosive in water
Barium 2.91 PROBABLE Short-lived in water
Strontium 2.89 YES Short-lived in water
Calcium 2.76 YES Short-lived in water
Sodium 2.71 PROBABLE Fire hazard in water
Lanthanum 2.52 YES Safe for repellent use
Cerium 2.48 YES Safe for repellent use
Praseodymium 2.47 YES Safe for repellent use
Neodymium 2.44 YES Safe for repellent use
Samarium 2.41 YES Safe for repellent use
Europium 2.41 PROBABLE Corrodes quickly in air
Gadolinium 2.40 YES Safe for repellent use
Terbium 2.39 YES Safe for repellent use
Magnesium 2.38 YES Safe for repellent use
Yttrium 2.37 YES Safe for repellent use
Dysprosium 2.35 YES Safe for repellent use
Holmium 2.32 YES Safe for repellent use
Erbium 2.31 YES Safe for repellent use
Thulium 2.31 PROBABLE Safe for repellent use
Lutetium 2.30 PROBABLE Safe for repellent use
Ytterbium 2.22 YES Safe for repellent use
Beryllium 1.847 NOT PROBABLE Weakly repellent,
toxic oxides
Aluminum 1.662 NO Not a repellent
Zirconium 1.45 NO Not a repellent
Niobium 1.099 NO Not a repellent
Chromium 0.744 NO Not a repellent
Rhenium 0.3 NO Not a repellent
Tungsten 0.1 NO Not a repellent
[0100] Beryllium and Magnesium metals are Alkaline earths in Group 2 of the periodic table of elements. These metals exhibit larger revised Pauling electro-negativities (1.56 and 1.31 respectively) than the Lanthanide metals. Magnesium, however, has a higher standard electrode potential (see Table 3) than beryllium and therefore is expected to be a better shark repellent than beryllium. Tonic immobility testing has confirmed that magnesium indeed produces aversive behavior in immobilized juvenile sharks. It is anticipated the beryllium would be weakly repellent based on the published standard electrode potentials. Additionally, the highly toxic nature of beryllium compounds preclude its use as a safe shark repellent.
Example 3
Target Fish not Repelled by Electropositive Metals
[0101] Preliminary research conducted on the effects of electropositive metals on adult cobia, Rachycentron canadum, suggests that electromotive forces produced by electropositive metals had little effect on captive cobia. Digital video of cobia striking at electropositive metals was recorded. Cobia were observed directly biting electropositive metals as well as transition metals. It is hypothesized that the shiny nature of the metals acted as a visual attractant to the fish. Since bony fish lack the ampullae of Lorenzini organ found in sharks, the fish were unable to detect the electromotive forces produced by the electropositive metals.
Shark Repellant Patents
Composition and device for discouraging the predatory intentions of carnivorous fish
US2458540
Shark repellant and preparation method thereof
CN103834189
Wetsuit System With Shark Deterrents
US2013091610
Electronic controls and options for powered riding machine
US8306673
Multi-factorial electronic shark repellant
US2012031343
Shark repellant surfboard wax and method of making same
US2009013902
POWERED RIDING APPARATUS WITH ELECTRONIC CONTROLS AND OPTIONS
US8290636
Shark repellant device
ZA9810482
Shark repellant devices
ZA9603377
Shark repellant patch
US5616333
Diver's knife
GB2176732
LIFE SAVING EQUIPMENT FOR VESSELS
GB1416048
A Sprayable Web-Forming Composition
GB1183297
LIFE SAVING ARRANGEMENT FOR A VESSEL
GB1426995