
rexresearch.com
David SINCLAIR, et al
Nicotinamide ( NAM ) vs Ageing
Nicotinamide ( NAM ) vs Ageing
http://www.news.com.au/lifestyle/health/university-of-nsw-research-finds-compound-that-can-reverse-ageing/story-fneuzlbd-1226786877989
University of NSW research finds
compound that can reverse ageing
AUSTRALIAN researchers have found what could become the elixir of life - a chemical compound that can reverse ageing.
The discovery of nicotinamide mononucleotide (NMN) by University of NSW researchers could lead to new treatments for ageing, cancer, type 2 diabetes and muscle wasting and inflammatory diseases within five to ten years.
Human trials of the compound that turns back ageing by improving communication between parts of a cell could start as early as next year.
The only hiccup is the cure is unaffordable for most people because it costs $1,000 per gram.
The research used the equivalent of 500mg of NMN for every kilogram of body weight per day.
This means the substance would cost the average 86 kilogram man $43,000 a day and the average 71 kilogram woman $35,000 a day.
Lead researcher University of NSW Professor David Sinclair says he soon hopes to find a way to produce it more cheaply.
The compound is fast acting and could also benefit healthy people by making them super charged.
Just a week after older mice were injected with the compound they had improvements in their muscles that made them indistinguishable from younger animals.
"It's something like a 60 year old being similar to a 20 year old on some measures," says University NSW pharmacologist and co-author Dr Nigel Turner.
Very old mice that were the equivalent of a human aged 85 also benefited from the substance with their body improving to be like that of a 40 year old.
"If those results stand, then ageing may be a reversible condition, if it is caught early", says Professor Sinclair who is based at Harvard Medical School.
Underpinning the breakthrough is the discovery that when there is a communication breakdown between the mitochondria, the battery pack of a cell, and the nucleus of the cell ageing accelerates.
A chemical called NAD is central to kick starting this cellular communication process but it begins to decline as we age.
The only way to combat the decline in NAD is excessive calorie restriction and intensive exercise.
In a research paper published in the journal Cell today the researchers report they have found that NMN injected into an animal body transforms into NAD to repair the broken communication channels.
This compound mimics the effect of diet and exercise.
"It was shocking how quickly it happened," says Dr Nigel Turner.
"If the compound is administered early enough in the ageing process, in just a week, the muscles of older mice were indistinguishable from the younger animals," he said.
The research is also examining another molecule called HIF-1 that also interferes with cellular communication and has a role in cancer.
This molecule is switched on in many cancers and researchers have now found it also switches on as we age.
"We become cancer-like in our ageing process," says Professor Sinclair.
"This may explain why the greatest risk of cancer is age," he says.
Professor Sinclair has previously been behind research that found resveratrol a substance found in red wine and certain nuts, made an anti-ageing gene SIRT1 run faster.
This new compound, NMN, activates all seven of the sirtuin genes implicated in longevity.
Further studies will test whether NMN leads to mice living longer lives, whether it helps them lose weight or has any side effects.
Professor Sinclair stresses that although NMN is a naturally occurring "I wouldn't advise anyone to take it until we know it is safe, we wouldn't want any surprises".
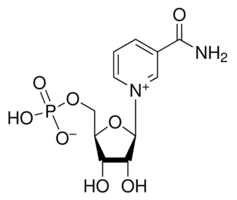
NMN
http://www.cell.com/retrieve/pii/S0092867413015213?cc=y
Cell, Volume 155, Issue 7, 1624-1638, 19 December 2013
Declining NAD+ Induces a
Pseudohypoxic State Disrupting Nuclear-Mitochondrial
Communication during Aging
Authors
Ana P. Gomes, Nathan L. Price, Alvin J.Y. Ling, Javid J. Moslehi, Magdalene K. Montgomery, Luis Rajman, James P. White, João S. Teodoro, Christiane D. Wrann, Basil P. Hubbard, Evi M. Mercken, Carlos M. Palmeira, Rafael de Cabo, Anabela P. Rolo, Nigel Turner, Eric L. Bell, David A. Sinclair
Summary
Ever since eukaryotes subsumed the bacterial ancestor of mitochondria, the nuclear and mitochondrial genomes have had to closely coordinate their activities, as each encode different subunits of the oxidative phosphorylation (OXPHOS) system. Mitochondrial dysfunction is a hallmark of aging, but its causes are debated. We show that, during aging, there is a specific loss of mitochondrial, but not nuclear, encoded OXPHOS subunits. We trace the cause to an alternate PGC-1a/ß-independent pathway of nuclear-mitochondrial communication that is induced by a decline in nuclear NAD+ and the accumulation of HIF-1a under normoxic conditions, with parallels to Warburg reprogramming. Deleting SIRT1 accelerates this process, whereas raising NAD+ levels in old mice restores mitochondrial function to that of a young mouse in a SIRT1-dependent manner. Thus, a pseudohypoxic state that disrupts PGC-1a/ß-independent nuclear-mitochondrial communication contributes to the decline in mitochondrial function with age, a process that is apparently reversible.
http://www.news.com.au/lifestyle/health/aussie-scientist-david-sinclair-claims-anti-aging-superbug-breakthrough/story-fneuzlbd-1226592865613
Aussie Scientist David Sinclair Claims
Anti-Aging Breakthrough
IT sounds too good to be true, but a respected Australian scientist believes he has invented a new class of superdrug that could prevent cancer and Alzheimer's disease.
What's more, Professor David Sinclair says his drugs have the potential to help some people enjoy a healthy life until the age of 150. However, this needs further research.
A paper in the March 8 issue of the journal, Science, explains how the drugs have the ability to switch on the body's defences against ageing.
Three of the drugs are in human trials for the treatment of specific illnesses such as type 2 diabetes and inflammatory bowel disease, says the University of New South Wales geneticist.
Prof Sinclair is most excited about the potential to prevent illness and hopes to prove the drugs will have a dual purpose of treating and preventing disease at the same time.
"My research has been criticised because it sounds too good to be true. This paper shows it is true," he says in a telephone interview from Harvard Medical School, where he is based.
Prof Sinclair's drugs target the enzyme SIRT1, which is switched on naturally by calorie restriction and exercise, but it can also be enhanced through activators such as resveratrol in red wine.
He and his colleagues have developed 4000 synthetic activators. Each one is 100 times more potent than a glass of red wine and the best three are the ones in human trials.
"Our drugs can mimic the benefits of a healthy diet and exercise, but there is no impact on weight," says Prof Sinclair, who suggests the first medicine to be marketed could be for diabetes in about five years.
Once a significant number of people are using the drugs, it will be possible to assess other benefits.
"We can look at 10,000 people and see if they are healthier and living longer than the general population."
In animal tests, overweight mice given synthetic resveratrol were able to run twice as far as slim mice and they lived 15 per cent longer.
"My prediction is that we will delay the onset of diseases and will not have so many people becoming chronically sick in their 50s and 60s," says Prof Sinclair.
The hope is that people will live healthily into their hundreds.
Patents
METHODS AND COMPOSITIONS FOR
EXTENDING THE LIFE SPAN AND INCREASING THE STRESS RESISTANCE
OF CELLS AND ORGANISMS
US7977049
[ PDF , 8 MB ]
US7977049
[ PDF , 8 MB ]
Also published as: US2012022013 // US2005267023 // US7977049 // WO2006086454 // WO2006086454 // JP2012176962 // AU2010219395
The invention provides methods and compositions for modulating the life span of eukaryotic and prokaryotic cells and for protecting cells against certain stresses, e.g., heatshock. One method comprises modulating the flux of the NAD+ salvage pathway in the cell, e.g., by modulating the level or activity of one or more proteins selected from the group consisting of NPT1, PNC1, NMA1 and NMA2. Another method comprises modulating the level of nicotinamide in the cell.
BACKGROUND OF THE INVENTION
Physiological studies and, more recently, DNA array analysis of gene expression patterns have confirmed that aging is a complex biological process. In contrast, genetic studies in model organisms have demonstrated that relatively minor changes to an organism's environment or genetic makeup can dramatically slow the aging process. For example, the life span of many diverse organisms can be greatly extended simply by limiting calorie intake, in a dietary regime known as caloric restriction (1-3).
How can simple changes have such profound effects on a complex process such as aging? A picture is emerging in which all eukaryotes possess a surprisingly conserved regulatory system that governs the pace of aging (4,5). Such a regulatory system may have arisen in evolution to allow organisms to survive in adverse conditions by redirecting resources from growth and reproduction to pathways that provide stress resistance (4,6).
One model that has proven particularly useful in the identification of regulatory factors of aging is the budding yeast, S. cerevisiae. Replicative life span in S. cerevisiae is typically defined as the number of buds or "daughter cells" produced by an individual "mother cell" (7). Mother cells undergo age-dependent changes including an increase in size, a slowing of the cell cycle, enlargement of the nucleolus, an increase in steady-state NAD<+> levels, increased gluconeogenesis and energy storage, and sterility resulting from the loss of silencing at telomeres and mating-type loci (8-13). An alternative measure of yeast life span, known as chronological aging, is the length of time a population of non-dividing cells remains viable when deprived of nutrients (14). Increased chronological life span correlates with increased resistance to heat shock and oxidative stress, suggesting that cumulative damage to cellular components is a major cause of this type of aging (14,15). The extent of overlap between replicative and chronological aging is currently unclear.
One cause of yeast replicative aging has been shown to stem from the instability of the repeated ribosomal DNA (rDNA) locus (16). This instability gives rise to circular forms of rDNA called ERCs that replicate but fail to segregate to daughter cells. Eventually, ERCs accumulate to over 1000 copies, which are thought to kill cells by titrating essential transcription and/or replication factors. (16-18). Regimens that reduce DNA recombination such as caloric restriction or a fob1 deletion extend replicative life span (17,19,20).
A key regulator of aging in yeast is the Sir2 silencing protein (17), a nicotinamide adenine dinucleotide (NAD<+>)-dependent deacetylase (21-24). Sir2 is a component of the heterotrimeric Sir2/3/4 complex that catalyzes the formation of silent heterochromatin at telomeres and the two silent mating-type loci (25). Sir2 is also a component of the RENT complex that is required for silencing at the rDNA locus and exit from telophase (26,27). This complex has also recently been shown to directly stimulate transcription of rRNA by Pol I and to be involved in regulation of nucleolar structure (28).
Biochemical studies have shown that Sir2 can readily deacetylate the amino-terminal tails of histones H3 and H4, resulting in the formation of 1-O-acetyl-ADP-ribose and nicotinamide (21-23,29). Strains with additional copies of SIR2 display increased rDNA silencing (30) and a 30% longer life span (17). It has recently been shown that additional copies of the C. elegans SIR2 homolog, sir-2.1, greatly extend life span in that organism (31). This implies that the SIR2-dependent regulatory pathway for aging arose early in evolution and has been well conserved (4). Yeast life span, like that of metazoans, is also extended by interventions that resemble caloric restriction (19,32). Mutations that reduce the activity of the glucose-responsive cAMP (adenosine 3'5'-monophosphate)-dependent (PKA) pathway extend life span in wild type cells but not in mutant sir2 strains, demonstrating that SIR2 is a key downstream component of the caloric restriction pathway (19).
In most organisms, there are two pathways of NAD+ biosynthesis (see FIG. 1). NAD+ may be synthesized de novo from tryptophan or recycled in four steps from nicotinamide via the NAD+ salvage pathway. The first step in the bacterial NAD<+> salvage pathway, the hydrolysis of nicotinamide to nicotinic acid and ammonia, is catalyzed by the pncA gene product (33). An S. cerevisiae gene with homology to pncA, YGL037, was recently assigned the name PNC1 (SGD) (34). A nicotinate phosphoribosyltransferase, encoded by the NPT1 gene in S. cerevisiae, converts the nicotinic acid from this reaction to nicotinic acid mononucleotide (NaMN) (35-38). At this point, the NAD<+> salvage pathway and the de novo NAD<+> pathway converge and NaMN is converted to desamido-NAD<+> (NaAD) by a nicotinate mononucleotide adenylyltransferase (NaMNAT). In S. cerevisiae, there are two putative ORFs with homology to bacterial NaMNAT genes, YLR328 (39) and an uncharacterized ORF, YGR010 (23,39). We refer to these two ORFs as NMA1 and NMA2, respectively. In Salmonella, the final step in the regeneration of NAD<+> is catalyzed by an NAD synthetase (40). An as yet uncharacterized ORF, QNS1, is predicted to encode a NAD synthetase (23).
In yeast, null mutations in NPT1 reduce steady-state NAD<+> levels by ~2-fold (23) and abolish the longevity provided by limiting calories (19). One current hypothesis explaining how caloric restriction extends replicative life span is that decreased metabolic activity causes an increase in NAD<+> levels, which then stimulate Sir2 activity (reviewed in Campisi, 2000 and Guarente, 2000).
Transcriptional silencing involves the heritable modification of chromatin at distinct sites in the genome. Silencing is referred to as long-range repression as it is promoter non-specific and often encompasses an entire genomic locus (1',2'). In yeast these silent regions of DNA, which are similar to the heterochromatin of higher eukaryotes, are subject to a wide variety of modifications (3'). Among the most well studied of these modifications is the reversible acetylation of histones (reviewed in 4',5').
There are two classes of enzymes that affect the acetylation state of histones: histone acetyltransferases (HATs) and the opposing histone deacetylases (HDACs). Compared with more transcriptionally active areas of the genome, histones within silent regions of chromatin are known to be hypoacetylated, specifically on the NH2-terminal tails of core histones H3 and H4 (6'). Three classes of histone deacetylases have been described and classified based on homology to yeast proteins. Proteins in class I (Rpd3-like) and class II (Hda1-like) are characterized by their sensitivity to the inhibitor trichostatin A (TSA) (7',8'). Studies using this inhibitor have provided a wealth of information regarding the cellular function of these proteins, including their involvement in the expression of regulators of cell cycle, differentiation, and apoptosis (reviewed in 9').
Yeast Sir2 is the founding member of Class III HDACs. Sir2-like deacetylases are not inhibited by TSA and have the unique characteristic of being NAD<+>-dependent (10'-13'). Proteins of this class are found in a wide array of organisms, ranging from bacteria to humans. At least two Sir2 homologues, yeast Hst2 and human SIRT2, are localized to the cytoplasm and human SIRT1 has recently been shown to target p53 for deacetylation (11',13'-15'). These results indicate that not all members of this family are specific for histones or other nuclear substrates.
The term, silent information regulator (SIR), was first coined to describe a set of non-essential genes required for repression of the mating type loci (HML and HMR) in S. cerevisiae (16'). Silencing in yeast is also observed at telomeres and the ribosomal DNA (rDNA) locus (2',17'). The formation of heterochromatin at mating type loci and the poly(TG1-3) tracts of yeast telomeres is mediated by a heterotrimeric complex of Sir2, Sir3 and Sir4 (18',19'). At the rDNA locus, Sir2 is part of the RENT (regulator of nuleolar silencing and telophase exit) complex, which includes Net1 and Cdc14 (20',21'). Of these proteins, Sir2 is the only factor that is indispensable for silencing at all three silent regions (22'-24').
The yeast rDNA locus (RLN1) consists of 100-200 tandemly-repeated 9 kb units encoding ribosomal RNAs. A major cause of yeast aging has been shown to stem from recombination between these repeats (25'-27') which can lead to the excision of an extrachromosomal rDNA circle (ERC). ERCs are replicated but they fail to segregate to daughter cells, resulting in their exponential amplification as cells divide. ERCs can accumulate to a DNA content greater than that of the entire yeast genome in old cells and are thought to kill cells by titrating essential transcription and/or replication factors (28'). Although Sir2 silences Pol II-transcribed genes integrated at the rDNA, there is evidence that its primary function at this locus is to suppress recombination. Deletion of SIR2 eliminates rDNA silencing and increases the frequency that a marker gene is recombined out of the rDNA 10-fold (29'). This results in increased ERC formation and a dramatic shortening of life span (29',30').
Sir2 is a limiting component of yeast longevity. A single extra copy of the SIR2 gene suppresses recombination and extends life span by 40% (26',31',32'). Recently, it has been shown that SIR2 is essential for the increased longevity provided by calorie restriction (31''), a regimen that extends the life span of every organism it has been tested on. Moreover, increased dosage of the Sir2 homologue sir2.1 has been shown to extend the life span of the nematode C. elegans (33') and the nearest human homologue SIRT1, has been shown to inhibit apoptosis through deacetylation of p53 (34',35'). These findings suggest that Sir2 and its homologues have a conserved role in the regulation of survival at the cellular and organismal level.
Recently, a great deal of insight has been gained into the biochemistry of Sir2-like deacetylases (reviewed by 36'). In vitro, Sir2 has specificity for lysine 16 of histone H4 and lysines 9 and 14 of histone H3 (10',12',13'). Although TSA sensitive HDACs catalyze deacetylation without the need of a cofactor, the Sir2 reaction requires NAD<+>. This allows for regulation of Sir2 activity through changes in availability of this co-substrate (10'-13'). Sir2 deacetylation is coupled to cleavage of the high-energy glycosidic bond that joins the ADP-ribose moiety of NAD<+> to nicotinamide. Upon cleavage, Sir2 catalyzes the transfer of an acetyl group to ADP-ribose (10',11',15',37'). The product of this transfer reaction is O-acetyl-ADP-ribose, a novel metabolite, which has recently been shown to cause a delay/block in the cell cycle and oocyte maturation of embryos (38').
The other product of deacetylation is nicotinamide, a precursor of nicotinic acid and a form of vitamin B3 (39'). High doses of nicotinamide and nicotinic acid are often used interchangeably to self-treat a range of conditions including anxiety, osteoarthritis, psychosis, and nicotinamide is currently in clinical trials as a therapy for cancer and type I diabetes (40'). The long-term safety of the high doses used in these treatments has been questioned (41') and the possible effects of these compounds at the molecular level are not clear.
SUMMARY OF THE INVENTION
In one embodidment, the invention provides methods for modulating the life span of a cell or its resistance to stress, comprising modulating the flux through the NAD+ salvage pathway in the cell. The method may comprise increasing or extending the life of a cell or increasing its resistance against stress, comprising increasing the flux through the NAD+ salvage pathway in the cell. Modulating the flux through the NAD+ salvage pathway may occur essentially without changing steady state levels of NAD+ and NADH and essentially by maintaining the NAD+/NADH ratio in the cell.
Increasing the flux through the NAD+ salvage pathway may comprise increasing the level or activity of a protein selected from the group consisting of NPT1, PNC1, NMA1 and NMA2. The method may comprise introducing into the cell at least one nucleic acid encoding a protein selected from the group consisting of NPT1, PNC1, NMA1 and NMA2, or a nucleic acid comprising at least 5 copies of a gene. Alternatively, the method may comprise introducing into the cell at least one protein selected from the group consisting of NPT1, PNC1, NMA1 and NMA2. The method may comprise contacting the cell with an agent that upregulates the expression of a gene selected from the group consisting of NPT1, PNC1, NMA1 and NMA2. The cell may live at least about 40% longer, or at least about 60% longer.
The invention also provides methods for increasing the resistance of the cell against stress, e.g., heat shock, osmotic stress, DNA damaging agents (e.g., U.V.), and inadequate nitrogen levels, comprising increasing the flux through the NAD+ salvage pathway in the cell.
In one embodiment, modulating the life span of a cell comprises modulating silencing in the cell. Silencing may include telomeric silencing and rDNA recombination.
The cell whose life span can be extended or who can be protected against stress can be a eukaryotic cell, such as a yeast cell or a prokaryotic cell, such as a bacterial cell. The cell can be in vitro or in vivo.
In another embodiment, modulating the life span of a cell or its resistance to stress comprises modulating the amount of nicotinamide and/or the ratio of NAD:nicotinamide in the cell. The ratio of NAD:nicotinamide may be modulated by a factor of at least about 50%, 2, 3, 5, 10 or more. For example, reducing the life span of a cell or rendering a cell more sensitive to stress may comprise increasing the level of nicotinamide in the cell. This may comprise contacting the cell with an amount of nicotinamide of about 1 to 20 mM, preferably of about 2 to 10 mM. The level of nicotinamide in a cell may also be increased by increasing the level or activity of enzymes involved in the biosynthesis of nicotinamide or by decreasing the level or activity of enzymes that degrade or inactivate nicotinamide. Enzymes which directly or indirectly inactivate nicotinamide include PNC1; nicotinamide N-methyl transferase (NNMT and NNT1); NPT1, and human homologs thereof; nicotinamide phosphoribosyltransferase (NAMPRT); and optionally nicotinamide mononucleotide adenylyltransferase (NMNAT-1 and 2); NMA1 and 2 and human homologs thereof.
On the contrary, extending the life span of a cell or rendering the cell more resistant (i.e., less sensitive) to stress may comprise decreasing the level of nicotinamide in the cell. This may be achieved by decreasing the level or activity of enzymes involved in the biosynthesis of nicotinamide or by increasing the level or activity of enzymes that degrade or inactivate nicotinamide. Accordingly, increasing lifespan or stress resistance in a cell can be achieved by increasing the activity or level of expression of a protein selected from the group consisting of NPT1, PNC1, NMA1, NMA2, NNMT, NAMPRT, NMNAT-1, and NMNAT-2. Increasing lifespan or stress resistance can also be achieved by contacting the cell with nicotinamide riboside, an NAD+ precursor, or a biologically active analog thereof or prodrug thereof, and optionally increasing the protein level or activity of nicotinamide riboside kinase, e.g., Nrk1 and Nrk2 (see, Bieganowski et al. (2004) Cell 117:495).
The invention further provides methods for identifying compounds that modulate the life span of a cell or its resistance to stress, comprising (i) contacting a protein selected from the group consisting of NPT1, PNC1, NMA1, NMA2, NNMT, NAMPRT, NMNAT-1, and NMNAT-2 with a test compound for an amount of time that would be sufficient to affect the activity of the protein; and (ii) determining the activity of the enzyme, wherein a difference in the activity of the enzyme in the presence of the test compound relative to the absence of the test compound indicates that the test compound is a compound that modulates the life span of the cell or its resistance to stress. The method may further comprise contacting a cell with the test compound and determining whether the life span of the cell has been modulated. The method may also further comprise contacting a cell with the test compound and determining whether the resistance of the cell to stress has been modulated.
In another embodiment, the invention provides a method for identifying a compound that modulates the life span of a cell or its resistance to certain types of stresses, comprising (i) contacting a cell or a lysate, comprising a transcriptional regulatory nucleic acid of a gene selected from the group consisting of NPT1, PNC1, NMA1, NMA2, NNMT, NAMPRT, NMNAT-1, and NMNAT-2 operably linked to a reporter gene, with a test compound for an amount of time that would be sufficient to affect the transcriptional regulatory nucleic acid; and (ii) determining the level or activity of the reporter gene, wherein a difference in the level or activity of the reporter gene in the presence of the test compound relative to the absence of the test compound indicates that the test compound is a compound that modulates the life span of the cell or its resistance to certain types of stresses. The method may further comprise contacting a cell with the test compound and determining whether the life span of the cell has been modulated. The method may also further comprise contacting a cell with the test compound and determining whether the resistance of the cell to stress has been modulated.
Also provided herein are methods for identifying an agent, e.g., a small molecule that modulates the nicotinamide level in a cell. The method may comprise (i) providing a cell or cell lysate comprising a reporter construct that is sensitive to the level of nicotinamide in a cell; (ii) contacting the cell with a test agent; and (iii) determining the level of nicotinamide in the cell contacted with the test agent, wherein a different level of nicotinamide in the cell treated with the test agent relative to a cell not treated with the test agent indicates that the test agent modulates the level of nicotinamide in the cell. The cell may further comprise a vector encoding a fusion protein that can bind to a DNA binding element operably linked to the reporter gene. The fusion protein may comprise at least an NAD+ binding pocket of a nicotinamide sensitive enzyme, e.g., a Sir2 family member, and a heterologous polypeptide. The heterologous polypeptide may be a transactivation domain of a transcription factor. The method may further comprise contacting a cell with the test compound and determining whether the life span of the cell or its resistance to stress has been modulated.
Also within the scope of the invention are computer-assisted methods for identifying an inhibitor of the activity of a Sir2 family member comprising: (i) supplying a computer modeling application with a set of structure coordinates of a molecule or molecular complex, the molecule or molecular complex including at least a portion of a Sir2 family member comprising a C pocket; (ii) supplying the computer modeling application with a set of structure coordinates of a chemical entity; and (iii) determining whether the chemical entity is an inhibitor expected to bind to or interfere with the molecule or molecular complex, wherein binding to or interfering with the molecule or molecular complex is indicative of potential inhibition of the activity of the Sir2 family member. The chemical entity may be an analog of nicotinamide. Another method for identifying an inhibitor of the activity of a Sir2 family member comprises: (i) contacting a protein of the Sir2 family comprising at least the C pocket with a test compound for a time sufficient for the test compound to potentially bind to the C pocket of the protein of the Sir2 family; and (ii) determining the activity of protein; wherein a lower activity of the protein in the presence of the test compound relative to the absence of the test compound indicates that the test compound is an inhibitor of the activity of a Sir2 family member.
In addition, the invention provides methods for treating or preventing diseases that are associated with aging or cell death (e.g., apoptosis) in a subject or diseases that may benefit from the effects of calorie restriction. A method may comprise administering to a subject in need thereof an agent that increases the flux through the NAD+ salvage pathway or reduces nicotinamide levels or the ratio of nicotinamide/NAD+ in the cells susceptible or subject to cell death. Diseases can be chronic or acute and include Alzheimer's disease, Parkinson's disease, stroke, myocardial infarction or a metabolic disease, such as insulin resistance. The methods of the invention for extending life span or increasing resistance to stress can also be used to reduce aging, e.g., for cosmetic purposes. The agent can be administered locally or systemically. Methods for extending life span or increasing resistance to stress can also be used on cells, tissues or organs outside of a subject, e.g., in an organ or tissue prior to transplantation.
The invention also provides methods for treating or preventing diseases in which reducing the life span of cells or rendering cells sensitive to stress is beneficial. Such diseases include those in which cells are undesirable, e.g., cancer and autoimmune diseases. Methods may also sensitize cells to killing by other agents, e.g., chemotherapeutic agents.
The methods of the invention can also be used to modulate the lifespan and stress resistance of organisms other than mammals. For example, the method can be used in microorganisms and plants. In particular, the methods of the invention permit to increase the resistance of plants to high salt, drought or disease, e.g., by treating these with a chemical that lowers nicotinamide levels or by genetically modifying genes that modulate the NAD+ salvage pathway or the level of nicotinamide in cells.
Also provided are diagnostic methods, e.g., a method for determining the general health of a subject or whether a subject has been exposed, e.g., unknowingly exposed, to a stress condition. A diagnostic method may also be used for diagnosing the presence or likelihood of developing cancer. A method may comprise (i) providing a sample of cells or bodily fluid, e.g., blood or serum, from a subject; and (ii) determining the level of expression of a gene or level of protein or activity thereof encoded thereby selected from the group consisting of NPT1, PNC1, NMA1, NMA2, NNMT, NAMPRT, NMNAT-1, and NMNAT-2, wherein a higher level of expression of a gene or the level of protein encoded thereby or activity thereof relative to a control sample indicates that the general health of the subject is not adequate, acceptable or optimal. A diagnostic method may also comprise determining the level of NAD+, NADH, nicotinamide or other intermediate compound of the NAD+ salvage pathway. In one embodiment, the method comprises determining the level of NAMPRT in serum of a subject.
METHODS AND KITS FOR MEASURING ENZYME
ACTIVITY
WO2011005289
WO2011005289
Provided herein are sirtuin-modulating compounds and methods of use thereof. The sirtuin-modulating compounds may be used for increasing the lifespan of a cell, and treating and/or preventing a wide variety of diseases and disorders including, for example, diseases or disorders related to aging or stress, diabetes, obesity, neurodegenerative diseases, cardiovascular disease, blood clotting disorders, inflammation, cancer, and/or flushing. Also provided are compositions comprising a sirtuin-modulating compound in combination with another therapeutic agent.
0001] NICOTINAMIDE RIBOSIDE AND ANALOGUES THEREOF
[0002] BACKGROUND
[0003] The Silent Information Regulator (SIR) family of genes represents a highly conserved group of genes present in the genomes of organisms ranging from archaebacteria to a variety of eukaryotes (Frye, 2000). The encoded SIR proteins are involved in diverse processes from regulation of gene silencing to DNA repair. The proteins encoded by members of the SIR gene family show high sequence conservation in a 250 amino acid core domain. A well-characterized gene in this family is S. cerevisiae SIR2, which is involved in silencing HM loci that contain information specifying yeast mating type, telomere position effects and cell aging (Guarente, 1999; Kaeberlein et al., 1999; Shore, 2000). The yeast Sir2 protein belongs to a family of histone deacetylases (reviewed in Guarente, 2000; Shore, 2000). The Sir2 homolog, CobB, in Salmonella typhimurium, functions as an NAD (nicotinamide adenine dinucleotide)-dependent ADP-ribosyl transferase (Tsang and Escalante- Semerena, 1998).
[0004] The Sir2 protein is a class III deacetylase which uses NAD as a cosubstrate (Imai et al., 2000; Moazed, 2001; Smith et al., 2000; Tanner et al., 2000; Tanny and Moazed, 2001). Unlike other deacetylases, many of which are involved in gene silencing, Sir2 is insensitive to class I and II histone deacetylase inhibitors like trichostatin A (TSA) (Imai et al., 2000; Landry et al., 2000a; Smith et al., 2000).
[0005] Deacetylation of acetyl-lysine by Sir2 is tightly coupled to NAD hydrolysis, producing nicotinamide and a novel acetyl-ADP ribose compound (Tanner et al., 2000; Landry et al., 2000b; Tanny and Moazed, 2001). The NAD-dependent deacetylase activity of Sir2 is essential for its functions which can connect its biological role with cellular metabolism in yeast (Guarente, 2000; Imai et al., 2000; Lin et al., 2000; Smith et al., 2000). Mammalian Sir2 homologs have NAD-dependent histone deacetylase activity (Imai et al., 2000; Smith et al., 2000). Most information about Sir2 mediated functions comes from the studies in yeast (Gartenberg, 2000; Gottschling, 2000).
[0006] Biochemical studies have shown that Sir2 can readily deacetylate the amino- terminal tails of histones H3 and H4, resulting in the formation of 1-Oacetyl-ADP- ribose and nicotinamide. Strains with additional copies of SIR2 display increased rDNA silencing and a 30% longer life span. It has recently been shown that additional copies of the C. elegans SIR2 homolog, sir-2.1, and the D. melanogaster dSir2 gene greatly extend life span in those organisms. This implies that the ,S'/i?2-dependent regulatory pathway for aging arose early in evolution and has been well conserved. Today, Sir2 genes are believed to have evolved to enhance an organism's health and stress resistance to increase its chance of surviving adversity.
[0007] Caloric restriction has been known for over 70 years to improve the health and extend the lifespan of mammals (Masoro, 2000). Yeast life span, like that of metazoans, is also extended by interventions that resemble caloric restriction, such as low glucose. The discovery that both yeast and flies lacking the SIR2 gene do not live longer when calorically restricted provides evidence that SIR2 genes mediate the beneficial health effects of this diet (Anderson et al., 2003; Helfand and Rogina, 2004). Moreover, mutations that reduce the activity of the yeast glucose-responsive cAMP (adenosine 3'5'-monophosphate)-dependent (PKA) pathway extend life span in wild type cells but not in mutant sir2 strains, demonstrating that SIR2 is likely to be a key downstream component of the caloric restriction pathway (Lin et al., 2001).
[0008] SUMMARY
[0009] The present invention is directed to nicotinamide riboside and analogs thereof, including their use in methods of treating diseases or conditions, such as diabetes/insulin resistance, hyperlipidemia and obesity. It is believed that nicotinamide riboside and its analogs directly or indirectly activate sirtuins, such as the human protein SIRTl. For convenience, the compounds disclosed herein are referred to as "sirtuin modulating compounds"; however, Applicants do not intend this designation to mean that the biological effects of these compounds are dependent upon sirtuin modulation (activation).
[0010] In certain embodiments of the invention, the invention is directed to analogs of nicotinamide riboside, particularly compounds that are metabolized, hydrolyzed or otherwise converted to nicotinamide riboside in vivo...
http://en.wikipedia.org/wiki/Nicotinamide-nucleotide_adenylyltransferase
Nicotinamide-nucleotide
adenylyltransferase
Identifiers
EC number 2.7.7.1
CAS number 9032-70-6
In enzymology, a nicotinamide-nucleotide adenylyltransferase (EC 2.7.7.1) is an enzyme that catalyzes the chemical reaction
ATP + nicotinamide ribonucleotide \rightleftharpoons diphosphate + NAD+
Thus, the two substrates of this enzyme are ATP and nicotinamide ribonucleotide, whereas its two products are diphosphate and NAD+.
This enzyme belongs to the family of transferases, specifically those transferring phosphorus-containing nucleotide groups (nucleotidyltransferases). The systematic name of this enzyme class is ATP:nicotinamide-nucleotide adenylyltransferase. Other names in common use include NAD+ pyrophosphorylase, adenosine triphosphate-nicotinamide mononucleotide transadenylase, ATP:NMN adenylyltransferase, diphosphopyridine nucleotide pyrophosphorylase, nicotinamide adenine dinucleotide pyrophosphorylase, nicotinamide mononucleotide adenylyltransferase, and NMN adenylyltransferase. This enzyme participates in nicotinate and nicotinamide metabolism. The human version of this protein is NMNAT1.
Structural studies
As of late 2007, 11 structures have been solved for this class of enzymes, with PDB accession codes 1EJ2, 1GZU, 1HYB, 1KKU, 1KQN, 1KQO, 1KR2, 1M8F, 1M8G, 1M8J, and 1M8K.
References
ATKINSON MR, JACKSON JF, MORTON RK (1961). "Nicotinamide mononucleotide adenylyltransferase of pig-liver nuclei. The effects of nicotinamide mononucleotide concentration and pH on dinucleotide synthesis". Biochem. J. 80 (2): 318–23. PMC 1244001. PMID 13684981.
Dahmen W, Webb B, Preiss J (1967). "The deamido-diphosphopyridine nucleotide and diphosphopyridine nucleotide pyrophosphorylases of Escherichia coli and yeast". Arch. Biochem. Biophys. 120 (2): 440–50. doi:10.1016/0003-9861(67)90262-7. PMID 4291828.
Kornberg A and Pricer WE (1951). "Enzymatic cleavage of diphosphopyridine nucleotide with radioactive pyrophosphate". J. Biol. Chem. 191 (2): 535–541. PMID 14861199.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3204926/
Cell Metab. 2011 October 5; 14(4): 528–536.
doi: 10.1016/j.cmet.2011.08.014
Nicotinamide mononucleotide, a key
NAD+ intermediate, treats the pathophysiology of diet- and
age-induced diabetes in mice
Jun Yoshino,* Kathryn F. Mills,* Myeong Jin Yoon, and Shin-ichiro Imai
Summary
Type 2 diabetes (T2D) has become an epidemic in our modern lifestyle, likely due to calorie-rich diets overwhelming our adaptive metabolic pathways. One such pathway is mediated by nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme in mammalian NAD+ biosynthesis, and the NAD+-dependent protein deacetylase SIRT1. Here we show that NAMPT-mediated NAD+ biosynthesis is severely compromised in metabolic organs by high-fat diet (HFD). Strikingly, nicotinamide mononucleotide (NMN), a product of the NAMPT reaction and a key NAD+ intermediate, ameliorates glucose intolerance by restoring NAD+ levels in HFD-induced T2D mice. NMN also enhances hepatic insulin sensitivity and restores gene expression related to oxidative stress, inflammatory response, and circadian rhythm, partly through SIRT1 activation. Furthermore, NAD+ and NAMPT levels show significant decreases in multiple organs during aging, and NMN improves glucose intolerance and lipid profiles in age-induced T2D mice. These findings provide critical insights into a potential nutriceutical intervention against diet- and age-induced T2D.
Introduction
Recent studies have raised an interesting possibility that various physiological mechanisms that mediate metabolic adaptation have evolved in response to nutritionally scarce conditions such as famine and drought (Lazar, 2005). In our modern, sedentary lifestyle with calorie-rich diets, such adaptive mechanisms could be seriously overwhelmed, causing an epidemic of obesity and T2D worldwide (Yach et al., 2006). In mammals, one such mechanism comprises NAMPT-mediated NAD+ biosynthesis and the NAD+-dependent protein deacetylase SIRT1 (Haigis and Sinclair, 2010; Imai, 2010; Imai and Guarente, 2010). NAMPT-mediated NAD+ biosynthesis and SIRT1 together play critical roles in regulating a variety of biological processes that include metabolism, stress response, cellular differentiation, and circadian rhythm, and also mediating adaptive responses to limited energy intake, such as fasting and diet restriction (Imai, 2010). For example, in skeletal muscle, both nutritional deprivation and exercise increase Nampt expression through the activation of AMP-activated protein kinase (AMPK), enhancing NAD+ biosynthesis and SIRT1 activity (Canto et al., 2010; Fulco et al., 2008). In pancreatic ß cells, both NAMPT-mediated NAD+ biosynthesis and SIRT1 regulate glucose-stimulated insulin secretion (GSIS) in response to glucose availability (Moynihan et al., 2005; Revollo et al., 2007). Additionally, in the liver and white adipose tissue (WAT), NAMPT and SIRT1 comprise a novel transcriptional-enzymatic feedback loop for the regulation of circadian rhythm, a powerful effecter for metabolism (Imai, 2010).
How nutritional and environmental perturbations affect the system dynamics of this NAMPT/NAD+/SIRT1-driven adaptive, systemic regulatory network, named the “NAD World” (Imai, 2010), still remains unclear. Here we show that HFD and aging compromise NAMPT-mediated NAD+ biosynthesis, contributing to the pathogenesis of T2D. Importantly, we also provide evidence that promoting NAD+ biosynthesis by using nicotinamide mononucleotide (NMN), a product of the NAMPT reaction and a key NAD+ intermediate, could be an effective intervention against diet- and age-induced T2D.