http://www.saltworkstechnologies.com
Technology
Saltworks Technologies is positioned to commercialize a
breakthrough desalination technology during a time of increasing
freshwater scarcity, rising energy prices, and mounting concerns
over carbon impacts.
Saltworks' patent pending technology employs an innovative
Thermo-Ionic™ energy conversion system that uses up to 80 per cent
less electrical/mechanical energy relative to leading desalination
technologies. The energy reduction is achieved by harnessing low
temperature heat and atmospheric dryness to overcome the
desalination energy barrier. Saltwater is evaporated to produce a
concentrated solution. This solution, which has concentration
gradient energy, is fed into Saltworks' proprietary desalting
device to desalinate either seawater or brackish water. Some
electrical energy is used to circulate fluids at a low pressure,
yet the bulk of the energy input is obtained through the
evaporation of saltwater.
Perfomance of this novel process improves in arid regions, which
happen to be the very regions that require freshwater. The
technology also requires less pre-treatment and chemicals than
traditional processes.
Applications for Saltworks' technology include producing drinking
water for communities and municipalities, irrigation water for
agriculture, and process water for industry. It is especially
well-suited for situations with low temperature thermal energy
(30-40 degrees Celsius) such as simple solar thermal or waste
heat.
The technology has been proof-tested by the National Research
Council of Canada and BC Hydro's Powertech Labs. An outfitted
1,000 litre a-day seawater pilot plant complete with chemical free
pre-treatment will soon be fully operational at a harbour location
in Vancouver, British Columbia.
http://rt.com/Sci_Tech/2009-12-17/ions-trick-desalinate-water.html
12/22/09
Ions
Trick
to Desalinate Water
Currently there are two major methods for seawater desalination,
both of them requiring a lot of energy. One uses the evaporation
and condensation cycle, the other one is based on reverse osmosis
filtering. Canadian company
Saltworks
Technologies says its technology
will consume four times less energy
per liter of fresh water produced, reports
Technology Review. Their
approach is based on manipulating the salt ions in water to trick
them out of the stream. The most energy-consuming part is the
preparation of water with increased salinity. From the normal 3.5%
of the sea water, it is boosted to at least
18% by evaporation. The
prototype plant company operates by using sprayers and sunlight,
but an industrial-scale version is expected to utilize waste heat
from some facility. Then the concentrated solution is fed into a
processing unit, where
ordinary
seawater circulates through polystyrene tubes. The plastic is
chemically treated to let either positive sodium or negative
chloride ions to pass. The lower salinity of water in the tubes
draws in corresponding ions. Then the two enriched streams are
connected to the third and final one, and draw salt ions out of
it. The result is desalinated water, which can be treated
with UF for disinfection and delivered to consumers.
http://www.technologyreview.com/energy/24237/?a=f
December 17, 2009
Sun-Assisted
Desalination
by
Tyler Hamilton
Energy-saving process uses free
heat to desalinate seawater.
A Canadian startup has built a pilot desalination plant in
Vancouver that uses a quarter of the energy of conventional plants
to remove salt from seawater. The process relies on concentration
gradients, and the natural tendency of sodium and chloride
ions--the key components of salt--to flow from higher to lower
salinity concentrations. If the system can be scaled up it could
offer a cheaper way to bring drinking water to the planet's most
parched regions while leaving behind a much lower carbon footprint
than other desalination methods.
"We've taken it from a benchtop prototype to a fully functional
seawater pilot plant," says inventor Ben Sparrow, a mechanical
engineer who established Saltworks Technologies in 2008 to
commercialize the process. "The plant is currently running on real
seawater, and we're in the final stage of expanding it to a
capacity of 1,000 liters a day."
Today most desalination plants are based on one of two approaches.
One is distillation through an evaporation-condensation cycle, and
the other is membrane filtration through reverse osmosis. But both
options are energy-intensive and costly.
Saltworks takes a completely different approach based on the
principles of ionic exchange. The process begins with the creation
of a reservoir of seawater that is evaporated until its salt
concentration rises from 3.5 percent to 18 percent or higher.
The evaporation is done in one of two ways: either the seawater is
sprayed into a shallow pond exposed to sunlight and dry ambient
air, or seawater is kept in a large tower that's exposed to waste
heat from a neighboring industrial facility. The second approach
is used in the commercial-scale plant. The concentrated water is
then pumped at low pressure into the company's desalting unit
along with three separated streams of regular seawater. At this
point the most energy-intensive part of the process is already
over.
Inside the desalting unit, which in the pilot plant is about the
size of a microwave oven, specially treated polystyrene bridges
connect two of the regular seawater streams to the highly
concentrated stream. Positive ions (largely sodium) and negative
ions (largely chloride) are drawn by diffusion through the
polystyrene, which has been chemically treated to manipulate
specific ions, from the concentrated steam into the weaker ones.
One bridge is treated to allow only positively charged ions to
pass, while the other bridge only allows negatively charged ions
to pass. But both allow other ions in salt water, including
magnesium, calcium, sulfate, and bromine ions, to pass through.
"The negatives all flow in one direction and the positives all
flow in another direction," Sparrow says.
The two regular streams--one now having a surplus of positive ions
and the other having a surplus of negative ions--are also
connected to the third saltwater stream, which is the target for
final purification. The two out-of-balance streams want to become
balanced again, so they essentially strip the third stream of all
positive and negative ions. The end result is de-ionized water
that only requires some basic chlorination or ultraviolet
treatment before being piped into homes and businesses.
Sparrow, who is also chief executive of Saltworks, says the
process uses low-pressure pumps to circulate the water, meaning
lightweight plastic pipes can be used instead of
corrosion-resistant steel. Saltworks cofounder and president
Joshua Zoshi says scaling up the system should be simple because
the plastics and ion-selective chemicals used are plentiful and
cheap. "Our next step is to engage with industry and work with
potential customers to get the technology out into the field,"
Zoshi says.
Much of the research and pilot-plant funding to date has come from
Canada's National Research Council, B.C. Hydro's Powertech Labs,
and Sustainable Development Technology Canada, a federal agency
that supports clean technology development through grants.
Rick Whittaker, chief technology officer at SDTC, says the company
has a reasonable chance of success because the science behind it
is sound and the approach is based largely on the creative
integration of existing technologies. "There's technical risk,"
says Whittaker. "But we're quite confident they can scale it up."
17
December,
2009
Ions trick to desalinate
water
A startup company is experimenting with technology, which
manipulates the ions in seawater to produce drinking water with
little energy consumption.
Currently there are two major methods for seawater desalination,
both of them requiring a lot of energy. One uses the evaporation
and condensation cycle, the other one is based on reverse osmosis
filtering.
Canadian company Saltworks Technologies says its technology will
consume four times less energy per liter of fresh water produced,
reports
Technology Review.
Their approach is based on manipulating the salt ions in water to
trick them out of the stream.
The most energy-consuming part is the preparation of water with
increased salinity. From the normal 3.5% of the sea water, it is
boosted to at least 18% by evaporation. The prototype plant
company operates by using sprayers and sunlight, but an
industrial-scale version is expected to utilize waste heat from
some facility.
Then the concentrated solution is fed into a processing unit,
where ordinary seawater circulates through polystyrene tubes. The
plastic is chemically treated to let either positive sodium or
negative chloride ions to pass. The lower salinity of water in the
tubes draws in corresponding ions.
Then the two enriched streams are connected to the third and final
one, and draw salt ions out of it. The result is desalinated
water, which can be treated with UF for disinfection and delivered
to consumers.
The solution is basically an inventive integration of existing
reliable technology, which gives the company high hopes for market
success.
US
Patent
Application # 20090314718
CA2649873
METHOD, APPARATUS AND PLANT FOR
DESALINATING SALTWATER USING CONCENTRATION DIFFERENCE ENERGY
Inventor: TANG JAMES [CA] ; ZOSHI JOSHUA
Applicant: SALTWORKS TECHNOLOGIES INC
EC: C02F1/469; C02F1/42; C02F1/469
2009-04-08
Abstract -- A method and
apparatus for desalinating saltwater using concentration
difference energy is disclosed. In order to desalinate saltwater
that is contained with in a product chamber, a drive cell is used
to generate a drive voltage. The product chamber has a
desalination voltage such that
when a sufficient voltage is applied to the product chamber,
anions and cations migrate out of the product chamber, thereby
desalinating the water. The sufficient voltage, which includes the
drive voltage and which is equal to or greater than the
desalination voltage, is applied to the product chamber,
consequently effecting desalination. Beneficially,
concentration difference energy
can be generated using a concentrated solution, which can be
generated using, for example, solar energy


















Description
BACKGROUND OF THE
INVENTION
[0002] 1. Field of the Invention
[0003] The present invention relates to a method, apparatus and
plant for desalinating saltwater. More particularly, the present
invention relates to a method, apparatus and plant for
desalinating saltwater by utilizing the energy difference that
exists between two solutions of different solute concentrations
that are separated by an ion exchange membrane.
[0004]
2. Background of the
Invention
[0005] Over one quarter of Earth's population does not have
adequate access to freshwater. Inadequate access to freshwater is
detrimental, as it can lead to disease and malnutrition, limit
agricultural development, and inhibit economic growth.
[0006] In contrast to freshwater, however, saltwater is readily
available. Saltwater in the form of seawater constitutes about 97%
of the water on Earth. Unless seawater is sufficiently
desalinated, though, it is not only undrinkable, but unsuitable
for agriculture. "Desalination" refers to the process of removing
anions and cations from saltwater. Seawater typically has a salt
concentration of about 3.5% by mass; that is, about 35 grams of
dissolved salt per liter of water. In contrast, drinkable water
typically has a salt concentration of, at most, about 0.04%.
[0007] Several desalination methods are currently known in the
art. One of the most popular methods at present is reverse osmosis
("RO"). RO involves mechanically forcing saltwater through
spirally wound, semi-permeable membranes at high pressure. The
membranes filter salt from the saltwater. Saltwater that is
filtered using RO requires extensive pre-treatment, which
increases RO's energy requirements. RO also suffers from
performance issues when the temperature of the saltwater is over
about 30.degree. C., which can be the case when the saltwater
source is water from a warm ocean or powerplant outlet, for
example.
[0008] Additional methods of desalination are multiple effect
distillation ("MED") or multi-stage flash ("MSF"). MED and MSF
desalinate saltwater by repeatedly evaporating and condensing the
saltwater over a series of multiple stages. The source of the
energy for MED and MSF processes is usually low pressure steam.
The primary drawback of MED and MSF processes is the large amount
of thermal energy these processes consume, which is typically an
order of magnitude higher than the electrical energy used by RO.
[0009] Another method of desalination is electrodialysis ("ED").
ED achieves desalination through a separation process whereby
dissolved salt ions are transferred from a feed stream to a
concentrate stream through ion exchange membranes under the
influence of an externally applied electric potential. This ion
transport is typically conducted using an ED stack, which is
constructed using an alternating arrangement of ion exchange
membranes, with feed and concentrate streams flowing between the
membranes. One problem with ED is that it consumes more energy
than RO for desalination of seawater, and that the source of such
energy is entirely in the form of an externally applied electric
potential. In addition to problems associated with energy
consumption, electrical hardware in the form of a direct current
power source or rectifiers to generate direct current from an
alternating current power source is required. A second problem
with ED is that often, as a result of the magnitude of the
externally applied electric potential, voltage gradients cause
salt ions to migrate not only through the ion exchange membranes
as intended but also through the manifolding used in the ED stack.
This results in circulating ionic current losses and reduces the
efficiency of ED.
[0010] Consequently, there is a need for a method and apparatus
for desalinating saltwater that improves on the prior art.
SUMMARY OF THE INVENTION
[0011] According to a first aspect of the invention, there is
provided an apparatus for desalinating saltwater. The apparatus
includes a plurality of drive cells for generating a drive
voltage, each drive cell having a diluent chamber for containing a
diluent of a first ionic concentration, a concentrate chamber for
containing a concentrate of a second ionic concentration that is
greater than the first ionic concentration, one of a cation or
anion exchange membrane forming a shared boundary between and in
ionic communication with the diluent and concentrate chambers, and
the other of the cation or anion exchange membrane forming a
shared boundary between and in ionic communication with each drive
cell and an adjacent drive cell. The apparatus also includes a
product chamber anion exchange membrane, a product chamber cation
exchange membrane, and a product chamber for containing the
saltwater to be desalinated. The product chamber is bounded on one
side by and is in ionic communication with the product chamber
anion exchange membrane and is bounded on another side by and is
in ionic communication with the product chamber cation exchange
membrane. The product chamber is in ionic communication with the
plurality of drive cells via the product chamber anion or cation
exchange membrane. The apparatus also includes a manifolding
assembly having diluent, concentrate and product manifolding
configured to convey the diluent to and away from the diluent
chamber, the concentrate to and away from the concentrate chamber,
and the saltwater to be desalinated to and desalinated saltwater
away from the product chamber, respectively.
[0012] The apparatus may further include a diluent chamber gasket,
a concentrate chamber gasket and a product chamber gasket
circumscribing each of the diluent chamber, the concentrate
chamber and the product chamber, respectively. Each of the diluent
chamber, concentrate chamber and product chamber gaskets can
contain therein a spacer for maintaining separation of cation and
anion exchange membranes.
[0013] The diluent, concentrate and product manifolding may
respectively include a diluent supply conduit and a diluent exit
conduit, a concentrate supply conduit and a concentrate exit
conduit and a product feed supply conduit and a product exit
conduit, each of which extend through the diluent, concentrate and
product gaskets. The diluent manifolding can have an inlet notch
in the diluent chamber gasket fluidly coupling the diluent supply
conduit to the diluent chamber and an outlet notch in the diluent
chamber gasket fluidly coupling the diluent exit conduit to the
diluent chamber. Similarly, the concentrate manifolding can have
an inlet notch in the concentrate chamber gasket fluidly coupling
the concentrate supply conduit to the concentrate chamber and an
outlet notch in the concentrate chamber gasket fluidly coupling
the concentrate exit conduit to the concentrate chamber; and the
product manifolding can have an inlet notch in the product chamber
gasket fluidly coupling the product feed supply conduit to the
product chamber and an outlet notch in the product chamber gasket
fluidly coupling the product exit conduit to the product chamber.
[0014] The apparatus for desalinating saltwater can also have an
anion discharge chamber and a cation discharge chamber, which are
in ionic communication with the product chamber through the
product chamber anion exchange membrane and the product chamber
cation exchange membrane, respectively.
[0015] The apparatus can also include anode and cathode
electrolyte chambers for containing an electrolyte; anode and
cathode stack end ion exchange membranes, the anode and cathode
electrolyte chambers in ionic communication with the plurality of
drive cells and product chamber through the anode and cathode
stack end ion exchange membranes, respectively; and an anode and a
cathode. The anode electrolyte chamber can be bounded on one side
by and be in ionic communication with the anode stack end ion
exchange membrane and can be bounded on another side by and be in
electrical communication with the anode. Similarly, the cathode
electrolyte chamber can be bounded on one side by and be in ionic
communication with the cathode stack end ion exchange membrane and
be bounded on another side by and be in electrical communication
with the cathode.
[0016] The plurality of drive cells, product chamber, anion
discharge chamber and cation discharge chamber can be arranged in
the shape of a ring. Alternatively, the plurality of drive cells,
product chamber, anion discharge chamber and cation discharge
chamber can be wound in the shape of a spiral.
[0017] Additionally, there may be provided one or both of a
voltage source or an electrical load electrically coupled between
the anode and cathode.
[0018] The anode and the cathode may each have a substrate having
a coating thereon. The substrate can be composed of a material
selected from the group consisting of titanium, niobium, tantalum,
iridium, palladium, steel, stainless steel, nickel and graphite,
and the coating can be composed of a material selected from the
group consisting of platinum, ruthenium, iridium, and an alloy
comprising platinum, ruthenium and iridium.
[0019] The apparatus may also include a electrolyte chamber fluid
conduit fluidly coupling the anode electrolyte chamber to the
cathode electrolyte chamber; and a pump in fluid communication
with the electrolyte chamber fluid conduit configured to pump the
electrolyte from one of the anode and cathode electrolyte chambers
to the other of the electrolyte chambers such that electrochemical
reaction by-products formed in one of the anode and cathode
electrolyte chambers can be used as a reactant in the other of the
electrolyte chambers.
[0020] The anode and cathode may be gas diffusion electrodes in
gaseous communication with each other such that gas produced at
one of the anode or cathode can be circulated to the other of the
anode or cathode.
[0021] The apparatus for desalinating saltwater may also include
first and second electrolyte chambers for containing an
electrolyte; first and second ion exchange membranes, the first
and second electrolyte chambers in ionic communication with the
plurality of drive cells and the product chamber through the first
and second ion exchange membranes, respectively; and porous first
and second end plates. The first electrolyte chamber can be
bounded on one side by and be in ionic communication with the
porous first end plate and can be bounded on another side by and
be in ionic communication with the first ion exchange membrane.
Similarly, the second electrolyte chamber can be bounded on one
side by and be in ionic communication with the porous second end
plate and can be bounded on another side by and be in ionic
communication with the second ion exchange membrane. The apparatus
can be sealed such that when the apparatus is submerged within a
conductive bath, ionic current will flow in the conductive bath
between the first and second electrolyte chambers through the
porous first and second end plates, respectively.
[0022] The apparatus can also include first and second electrolyte
chambers for containing electrolyte; first and second ion exchange
membranes, the first and second electrolyte chambers in ionic
communication with the plurality of drive cells and the product
chamber through the first and second ion exchange membranes,
respectively; first and second end plates, the first electrolyte
chamber bounded on one side by and in ionic communication with the
first end plate and bounded on another side by the first ion
exchange membrane, the second electrolyte chamber bounded on one
side by and in ionic communication with the porous second end
plate and bounded on another side by the second ion exchange
membrane; an electrolyte chamber fluid conduit fluidly coupling
the first electrolyte chamber to the second electrolyte chamber;
and a pump in fluid communication with the electrolyte chamber
fluid conduit configured to pump the electrolyte from one of the
first and second electrolyte chambers to the other of the
electrolyte chambers.
[0023] According to a further aspect of the invention, there is
provided an apparatus for desalinating saltwater capable of
operating in forward polarity and reverse polarity. The apparatus
includes a stack configured to receive a diluent of a first ionic
concentration, a concentrate of a second ionic concentration
greater than the first ionic concentration, and saltwater to be
desalinated. The stack has a plurality of drive cells, each drive
cell comprising a diluent/concentrate chamber, a
concentrate/diluent chamber, one of a cation or anion exchange
membrane forming a shared boundary between and in ionic
communication with the diluent/concentrate and concentrate/diluent
chambers, and the other of the cation or anion exchange membrane
forming a shared boundary between and in ionic communication with
each drive cell and an adjacent drive cell; a product/diluent
chamber anion exchange membrane and a product/diluent chamber
cation exchange membrane; a product/diluent chamber bounded on one
side by and in ionic communication with the product/diluent
chamber anion exchange membrane and bounded on another side by and
in ionic communication with the product/diluent chamber cation
exchange membrane, the product/diluent chamber in ionic
communication with the drive cell via the product/diluent chamber
anion or cation exchange membranes; a diluent/product chamber
anion exchange membrane and a diluent/product chamber cation
exchange membrane; a diluent/product chamber bounded on one side
by and in ionic communication with the diluent/product chamber
anion exchange membrane and bounded on another side by and in
ionic communication with the diluent/product chamber cation
exchange membrane, the diluent/product chamber in ionic
communication with the drive cell via the diluent/product chamber
anion or cation exchange membranes; and a manifolding assembly
comprising diluent/concentrate, concentrate/diluent,
product/diluent, and diluent/product manifolding respectively
configured to convey diluent to and away from the
diluent/concentrate and diluent/product chambers, concentrate to
and away from the concentrate/diluent chamber, and the saltwater
to be desalinated to and desalinated saltwater away from the
product/diluent chamber when the apparatus is operating in forward
polarity, and respectively configured to convey diluent to and
away from the concentrate/diluent and product/diluent chambers,
concentrate to and away from the diluent/concentrate chamber, and
the saltwater to be desalinated to and the desalinated saltwater
away from the diluent/product chamber when the apparatus is
operating in reverse polarity.
[0024] The apparatus can also include a diluent/concentrate
chamber gasket, a concentrate/diluent chamber gasket, a
product/diluent chamber gasket, and a diluent/product chamber
gasket circumscribing each of the diluent/concentrate chamber, the
concentrate/diluent chamber, the product/diluent chamber, and the
diluent/product chamber, respectively. Each of the
diluent/concentrate chamber, concentrate/diluent chamber and
product/diluent chamber gaskets can contain therein a spacer for
maintaining separation of cation and anion exchange membranes.
[0025] The diluent/concentrate, concentrate/diluent,
product/diluent, and diluent/product manifolding may respectively
include a diluent/concentrate supply conduit and a
diluent/concentrate exit conduit, a concentrate/diluent supply
conduit and a concentrate/diluent exit conduit, a product/diluent
supply conduit and a product/diluent exit conduit, and a
diluent/product supply conduit and a diluent/product exit conduit,
each of which extend through the diluent/concentrate,
concentrate/diluent, product/diluent, and diluent/product gaskets.
The diluent/concentrate manifolding can have an inlet notch in the
diluent/concentrate chamber gasket fluidly coupling the
diluent/concentrate supply conduit to the diluent/concentrate
chamber and an outlet notch in the diluent/concentrate chamber
gasket fluidly coupling the diluent/concentrate exit conduit to
the diluent/concentrate chamber. Similarly, the
concentrate/diluent manifolding can have an inlet notch in the
concentrate/diluent chamber gasket fluidly coupling the
concentrate/diluent supply conduit to the concentrate/diluent
chamber and an outlet notch in the concentrate/diluent chamber
gasket fluidly coupling the concentrate/diluent exit conduit to
the concentrate/diluent chamber; the product/diluent manifolding
further comprising an inlet notch in the product/diluent chamber
gasket fluidly coupling the product/diluent supply conduit to the
product/diluent chamber and an outlet notch in the product/diluent
chamber gasket fluidly coupling the product/diluent exit conduit
to the product/diluent chamber; and the diluent/product
manifolding further comprising an inlet notch in the
diluent/product chamber gasket fluidly coupling the
diluent/product supply conduit to the diluent/product chamber and
an outlet notch in the diluent/product chamber gasket fluidly
coupling the diluent/product exit conduit to the diluent/product
chamber.
[0026] The apparatus can also include first and second electrolyte
chambers for containing an electrolyte; first and second stack end
ion exchange membranes, the first and second electrolyte chambers
in ionic communication with the diluent/concentrate,
concentrate/diluent, product/diluent, and diluent/product chambers
through the first and second ion exchange membranes, respectively;
and first and second electrodes. The first electrolyte chamber can
be bounded on one side by and be in ionic communication with the
first stack end ion exchange membrane and can be bounded on
another side by and be in electrical communication with the first
electrode. The second electrolyte chamber can be bounded on one
side by and be in ionic communication with the second stack end
ion exchange membrane and can be bounded on another side by and be
in electrical communication with the second electrode.
[0027] The first and second electrodes can each have a substrate
having a coating thereon. The substrate can be composed of a
material selected from the group of titanium, niobium, tantalum,
iridium, palladium, steel, stainless steel, nickel and graphite,
and the coating can be composed of a material selected from the
group of platinum, ruthenium, iridium, and an alloy comprising
platinum, ruthenium and iridium.
[0028] According to a further aspect of the invention, there is
provided a plant for desalinating saltwater. The plant can include
any of the aforedescribed apparatuses for desalinating saltwater;
a first reconcentrator configured to remove water from the diluent
exiting the apparatus to generate the concentrate; and a
concentrate reservoir, in fluid communication with both the first
reconcentrator and the concentrate chamber, for holding the
concentrate.
[0029] The plant can also have a saltwater reservoir, in fluid
communication with the product chamber, for holding the saltwater
to be desalinated; a diluent reservoir, in fluid communication
with the drive cell, for holding the diluent; and a product
reservoir, in fluid communication with the product chamber, for
storing desalinated saltwater.
[0030] Additionally, the plant may have a pre-treatment center
fluidly coupled to the saltwater reservoir for treating the
saltwater to be desalinated prior to the saltwater entering the
saltwater reservoir.
[0031] The plant can also include a second reconcentrator in fluid
communication with the concentrate reservoir and the apparatus,
the second reconcentrator configured to remove water from the
concentrate exiting the apparatus.
[0032] The pre-treatment center can be fluidly coupled to the
diluent reservoir for treating the diluent prior to the diluent
entering the diluent reservoir.
[0033] One or both of the first and second reconcentrators can be
selected from the group consisting of an evaporative pond, an
evaporative spray pond, a natural draft evaporative tower, and a
forced draft evaporative tower. Additionally, the plant may also
include a heat exchanger, fluidly coupled to one or both of the
first and second reconcentrators, for transferring heat from a
heat source to one or both of the first and second
reconcentrators.
[0034] According to a further aspect of the invention, there is
provided a method for desalinating saltwater. The method includes
employing a plurality of drive cells to generate a drive voltage;
and applying a sufficient voltage across a product chamber
containing the saltwater to be desalinated and in ionic
communication with the plurality of drive cells, the product
chamber bounded by and in ionic communication with a product
chamber anion exchange membrane on one side and bounded by and in
ionic communication with a product chamber cation exchange
membrane on another side and having a desalination voltage such
that when a voltage is applied to the product chamber in excess of
the desalination voltage cations and anions migrate from the
saltwater through the product chamber cation and anion exchange
membranes, respectively, the sufficient voltage comprising the
drive voltage and being greater than or equal to the desalination
voltage.
[0035] Employing a plurality of drive cells to generate a drive
voltage can include flowing diluent of a first ionic concentration
through diluent chambers in the plurality of drive cells; and
flowing concentrate of a second ionic concentration through
concentrate chambers in the plurality of drive cells, the second
ionic concentration greater than the first ionic concentration,
one of a cation or anion exchange membrane forming a shared
boundary between and in ionic communication with the diluent and
concentrate chambers such that ions flow from the concentrate to
the diluent, and the other of the cation or anion exchange
membrane forming a shared boundary between and in ionic
communication with adjacent pairs of drive cells.
[0036] The method can further include flowing solution
having an ionic concentration greater than or equal to that of the
saltwater to be desalinated through anion and cation discharge
chambers, the anion and cation discharge chambers in ionic
communication with the product chamber via the product chamber
anion exchange membrane and the product chamber cation exchange
membrane, respectively.
[0037] The diluent can be the solution flowing through the anion
and cation discharge chambers. Additionally, the diluent and the
saltwater to be desalinated can be the same. The diluent and the
concentrate can also both be saltwater.
[0038] The drive voltage can be equal to or greater than the
desalination voltage. In such a case, desalination can be effected
without the application of any external voltage.
[0039] The method can further include flowing an electrolyte
through anode and cathode electrolyte chambers, the anode
electrolyte chamber bounded on a first side by and in ionic
communication with an anode stack end ion exchange membrane and
bounded on another side by and in electrical communication with an
anode, and the cathode electrolyte chamber bounded on a first side
by and in ionic communication with a cathode stack end ion
exchange membrane and bounded on another side by and in electrical
communication with a cathode, the anode and cathode electrolyte
chambers ionically communicative with the product chamber via the
anode and cathode stack end ion exchange membranes, respectively,
and the anode and cathode electrically communicative with each
other such that electrons flow from the anode to the cathode.
[0040] The diluent and the concentrate can flow through the
diluent and concentrate chambers, respectively, in countercurrent
directions.
[0041] The method can further include flowing the saltwater to be
desalinated through an initial desalination stage in series ionic
communication with the drive cell, the initial desalination stage
comprising an initial stage product chamber bounded on one side by
and in ionic communication with an initial stage product chamber
anion exchange membrane and bounded on another side by and in
ionic communication with an initial stage product chamber cation
exchange membrane, the initial stage product chamber having a
desalination voltage such that when a voltage is applied to the
initial stage product chamber in excess of the desalination
voltage cations and anions migrate from the saltwater through the
initial stage product chamber cation and anion exchange membranes,
respectively; flowing the saltwater to be desalinated through a
subsequent desalination stage, the subsequent desalination stage
in series ionic communication with the drive cell, the subsequent
desalination stage comprising a subsequent desalination stage
product chamber bounded on a first side by and in ionic
communication with a subsequent stage product chamber anion
exchange membrane and bounded on another side by and in ionic
communication with a subsequent stage product chamber cation
exchange membrane, the subsequent stage product chamber having a
desalination voltage such that when a voltage is applied to the
subsequent stage product chamber in excess of the desalination
voltage cations and anions migrate from the saltwater through the
subsequent stage product chamber cation and anion exchange
membranes, respectively, the saltwater to be desalinated flowing
through the initial stage and subsequent stage product chambers;
and applying the sufficient voltage across the initial and
subsequent stage product chambers, the sufficient voltage
comprising the drive voltage and being greater than or equal to
the sum of the desalination voltages of the initial and subsequent
stage product chambers.
[0042] One or both of the initial stage and subsequent stage
product chambers can include a plurality of product chambers, and
flowing the saltwater to be desalinated through the initial and
subsequent desalination stages can include flowing the saltwater
to be desalinated in parallel through the plurality of product
chambers of any given stage.
[0043] An external voltage across the anode and the cathode can
also be applied by using a voltage source electrically coupled
between the anode and cathode. An electrical load electrically
coupled between the anode and cathode can be powered when the
drive voltage is greater than the desalination voltage.
[0044] The electrolyte may be have a sodium, calcium, magnesium or
potassium cation and the anode and cathode ion exchange membranes
can both be cation exchange membranes. Alternatively, the
electrolyte may have a chlorine, sulphate or bromine anion and the
anode and cathode ion exchange membranes can both be anion
exchange membranes.
[0045] The electrolyte may be selected from the group consisting
of Na.sub.2SO.sub.4, NaCl, NaOH, HCl, Na.sub.3Fe(CN).sub.6,
Na.sub.2S.sub.4O.sub.6, Na.sub.2S.sub.2O.sub.3,
Na.sub.4Fe(CN).sub.6, K.sub.3Fe(CN).sub.6, K.sub.4Fe(CN).sub.6,
Na.sub.2S.sub.2O.sub.3, NH.sub.4Cl, NH.sub.4,
Na.sub.2Cr.sub.2O.sub.7, and CrCl.sub.3.
[0046] The method can further include pumping the electrolyte from
one of the anode and cathode electrolyte chambers to the other of
the anode and cathode electrolyte chambers such that
electrochemical reaction by-products formed in one of the anode
and cathode electrolyte chambers can be used as a reactant in the
other of the electrolyte chambers.
[0047] Additionally, the method can further include flowing
concentrate through first and second electrolyte chambers, the
first electrolyte chamber bounded on one side by and in ionic
communication with a first ion exchange membrane and bounded on
another side by and in ionic communication with a porous first end
plate and the second electrolyte chamber bounded on a first side
by and in ionic communication with a second ion exchange membrane
and bounded on a second side by and in ionic communication with a
porous second end plate, the first and second electrolyte chambers
ionically communicative with the product chamber via the first and
second ion exchange membranes, respectively; and submerging the
porous first and second end plates within a conductive bath such
that ionic current flows between the conductive bath and the
porous first and second end plates.
[0048] One advantage of the present invention is that by relying
on a drive voltage generated by the difference in concentrations
between diluent and concentrate to effect desalination, it is
possible to use solar energy or another form of readily accessible
low temperature energy (such as waste heat from a power plant) to
generate the concentrated solution that is used to generate the
drive voltage. Thus, energy that would otherwise be wasted can be
used to generate the concentrate and to effect desalination,
allowing for less electrically intensive desalination of saltwater
relative to prior art methods of and means for desalination. This
is especially beneficial as arid climates are those with abundant
available solar energy and are also those that are likely to
require desalination.
[0049] A further advantage of the present invention is that as
drive cells are used to generate the drive voltage, an external
power supply is not required, which can reduce the capital costs
associated with practicing the method and operating the apparatus
and plant of the present invention.
[0050] In contrast to ED, a further advantage of the present
invention is that voltage gradients do not built up to the same
extent as with an ED stack, and consequently less ion migration
occurs through stack manifolds as can occur in an ED stack. This
reduces circulated ionic current losses in the present invention
relative to ED.
[0051] In contrast to RO, a further advantage of the method of the
present invention is that its performance is not substantially
negatively affected when desalinating warm saltwater greater than
about 30.degree. C. and requires less pre-treatment as water is
not forced through a semi-permeable membrane that could foul.
[0052] Advantages of the apparatus of the present invention
include its ease of manufacturing, durability and robustness.
[0053] An additional advantage of the apparatus of the present
invention includes the ability to desalinate saltwater by
operating the apparatus at a lower pressure than comparable RO
systems, which lowers the cost of the components used to
manufacture the apparatus of the present invention relative to the
RO systems, which incorporate expensive allowed and stainless
steels and other high pressure components.
BRIEF DESCRIPTION OF THE DRAWINGS
[0054]
FIG. 1 is a
schematic view of a dialytic stack for desalinating saltwater
according to a first embodiment wherein the stack operates in
"forward polarity";
[0055]
FIG. 2 is a
schematic view of a dialytic stack for desalinating saltwater
according to a second embodiment wherein the dialytic stack
operates in "reverse polarity";
[0056]
FIG. 3 is a
schematic view of a dialytic stack for desalinating saltwater
according to a third embodiment wherein the dialytic stack employs
electrolyte recirculation using gas diffusion electrodes;
[0057]
FIG. 4 is a
schematic view of a dialytic stack for desalinating saltwater
according to a fourth embodiment wherein the dialytic stack is
configured to be submerged within a conductive bath;
[0058]
FIG. 5 is a
schematic view of a ring-shaped dialytic arrangement of cells for
desalinating saltwater;
[0059]
FIG. 6 is a
schematic view of a spiral-shaped dialytic arrangement of cells
for desalinating saltwater;
[0060]
FIGS. 7(a) and (c)
are sectional and exploded views, respectively, of a unipolar
dialytic stack in which assembly of various fluid chambers within
the dialytic stack and manifolding are illustrated. FIG. 7(b) is a
front elevation view of a gasket that circumscribes the various
fluid chambers contained within the dialytic stack;
[0061]
FIGS. 8(a)-(c) are
schematic, exploded and perspective views, respectively, of a
bipolar dialytic stack operable in both forward and reverse
polarities in which assembly of the fluid chambers, manifolding
and valves are illustrated;
[0062]
FIGS. 9(a) and (b)
are schematic views of a dialytic stack for desalinating saltwater
according to a further embodiment wherein the dialytic stack has
multiple desalination stages;
[0063]
FIG. 10 is a
schematic view of a plant that can be used to desalinate saltwater
continuously;
[0064]
FIG. 11 is a
schematic view of a plant that can be used to desalinate discrete
batches of saltwater;
[0065]
FIG. 12 is a
schematic view of an evaporative spray pond;
[0066]
FIG. 13 is a
schematic view of a natural draft evaporative tower; and
[0067]
FIG. 14 is a
schematic view of a forced draft evaporative tower.
DETAILED DESCRIPTION OF EXEMPLARY
EMBODIMENTS
[0068] Two ionic solutions that differ only in the concentration
of the solute dissolved therein have different amounts of chemical
energy. This difference in chemical energy is hereinafter referred
to as "concentration difference energy". For example, when equal
volumes of solutions of saltwater and freshwater are placed in
adjacent chambers and are separated from each other solely by a
membrane that is water permeable, but not ion permeable, the
concentration difference energy causes water from the freshwater
container to flow into and develop pressure in the saltwater
container.
[0069] Similarly, when equal volumes of solutions of saltwater and
freshwater are placed in adjacent chambers and are separated from
each other solely by a membrane that is ion permeable, but not
water permeable, the concentration difference energy results in a
voltage difference between the two chambers. A membrane that is
ion permeable, but not water permeable, is hereinafter referred to
as an "ion exchange membrane". Monopolar ion exchange membranes
include "cation exchange membranes" and "anion exchange
membranes". Cation and anion exchange membranes are those
membranes that allow only cations (positively charged ions) and
anions (negatively charged ions) to pass through, respectively.
Exemplary cation exchange membranes include Neosepta CMX, CM-1;
Ralex CMH-PES; Fumasep FKE, FKD; and Selemion CMV membranes.
Exemplary anion exchange membranes include Neosepta AM-1, AFN,
AMX; Ralex AMH-PES; Fumasep FAD; and Selemion DVS, APS membranes.
[0070] In order to desalinate saltwater, at least some of the
cations (primarily Na.sup.+) and anions (primarily Cl.sup.-) in
the saltwater need to be removed from the saltwater. Removing
these ions requires energy.
[0071] The embodiments described herein utilize concentration
difference energy to aid in removal of cations and anions from
saltwater, thereby desalinating the saltwater.
[0072] Referring now to FIG. 1, there is depicted a schematic view
of a dialytic stack 101 for desalinating saltwater. The dialytic
stack 101 is composed of a series of alternating concentrate and
diluent chambers 110 and 112, respectively. Flowing through each
of the diluent chambers 112 is a solution of a first ionic
concentration ("diluent") and flowing through each of the
concentrate chambers 110 is a solution of a second ionic
concentration ("concentrate"), with the second ionic concentration
being greater than the first ionic concentration. Diluent may
enter and exit the diluent chambers 112 via diluent supply and
exit conduits 104 and 132, respectively. Concentrate may enter and
exit the concentrate chambers 110 via concentrate supply and exit
conduits 102 and 130, respectively. Adjacent diluent and
concentrate chambers 112, 110 are separated from each other by one
of the cation and anion exchange membranes 120 and 122,
respectively. Located in the embodiment of FIG. 1 is a product
chamber 118 through which flows the saltwater to be desalinated
("product feed"). Product feed that has passed through the product
chamber 118 and has had at least some anions and cations removed
therefrom is hereinafter referred to as "product". The product
feed may enter the product chamber 118 via a product feed supply
conduit 106, and the resulting product exits the product chamber
118 via a product exit conduit 134. The product feed and the
diluent may both be seawater having a salt concentration of about
3.5% by mass, while the concentrate may be hyper-concentrated
seawater having a salt concentration of about 15%-28% by mass.
Alternatively, the diluent may be slightly concentrated saltwater
having a salt concentration of about 3.5% to about 6% by mass. The
salt concentration of the concentrate must be higher than the salt
concentration of the diluent and can be as high as the solubility
limit in water of whichever salts are present in the concentrate.
The manner in which this hyper-concentrated seawater can be
produced is discussed in more detail with respect to FIGS. 10-14,
below.
[0073] In the embodiment depicted in FIG. 1, each pair of chambers
140, 142, 144, 146, 148 (each a "drive cell") is composed of one
of the diluent chambers 112 and one of the concentrate chambers
110, the diluent and concentrate chambers 112, 110 separated from
each other by and in ionic communication with one of the anion
exchange membranes 122. As discussed above, the concentration
difference energy that exists between the concentrate and diluent
results in the drive cell generating a voltage ("drive voltage").
In an embodiment wherein the concentrate is about 18% aqueous
sodium chloride by mass and the diluent is about 3.5% aqueous
sodium chloride by mass, the theoretical drive voltage for each
drive cell is about 0.04 Volts.
[0074] In FIG. 1, the anion exchange membrane 122 forms a shared
boundary between and is in ionic communication with the diluent
and concentrate chambers 112, 110 of any given drive cell, and the
cation exchange membrane 120 forms a shared boundary between and
is in ionic communication with any given drive cell and an
adjacent drive cell (see, e.g. the drive cell 148 having the anion
exchange membrane 122 between its diluent and concentrate chambers
112, 110 and having the cation exchange membrane 120 between the
concentrate chamber 110 of the drive cell 148 and the diluent
chamber 112 of the adjacent drive cell 146). A cation or anion
exchange membrane 120 or 122 that contacts the fluid contained
within a chamber when the chamber is filled with fluid is said to
"bound" that chamber and, consequently, also be in ionic
communication with that chamber.
[0075] Each of the drive cells is separated from an adjacent drive
cell by one of the cation exchange membranes 120. As the ion
exchange membranes 120, 122 allow ions to flow from the
concentrate to the diluent chambers, the drive cells 140, 142,
144, 146, 148 are in ionic communication with each other. The
drive voltage generated by a group of drive cells in ionic
communication with each other is equal to the sum of the voltages
produced by each of the drive cells in the series. Consequently,
the total drive voltage produced by the drive cells 140, 142, 144,
146, 148 is about 0.20 Volts.
[0076] The dialytic stack 101 also contains a desalination cell
150, which is composed of one of the diluent chambers 112 and a
product chamber 118. The product chamber 118 is bounded on one
side by and is in ionic communication with one of the anion
exchange membranes 122 ("product chamber anion exchange membrane"
154) and is bounded on another side by and is in ionic
communication with one of the cation exchange membranes 120
("product chamber cation exchange membrane" 152). The diluent
chamber 112 that is separated from the product chamber 118 by and
is in ionic communication with the product chamber anion exchange
membrane 154 is hereinafter referred to as the "anion discharge
chamber" 162. The diluent chamber 112 that is separated from the
product chamber 118 by and is in ionic communication with the
product chamber cation exchange membrane 152 is hereinafter
referred to as the "cation discharge chamber" 160. The product
chamber 118 is in ionic communication with the drive cells via the
product chamber cation exchange membrane 152 on one side and via
the product chamber anion exchange membrane 154 on the other.
Flowing through the product chamber 118 is the saltwater to be
desalinated. Typically, the salt concentration of the product feed
as it enters the dialytic stack 101 is less than or equal to the
concentrations of the solutions in the chambers adjacent to the
product chamber 118.
[0077] In order to desalinate the product feed, a certain voltage
("desalination voltage") has to be applied across the product
chamber 118. In the illustrated embodiment, in order to desalinate
the product feed to a resulting product concentration of about
0.04% salt by mass such that anions and cations in the product
feed are driven from the product chamber 118 into adjacent diluent
chambers 112 containing diluent of about 3.5% salt by mass, the
desalination voltage is about 0.088 Volts.
[0078] When a sufficient voltage greater than the desalination
voltage is applied across the product chamber 118, anions migrate
towards one electrode, an anode 126, and cations migrate towards
another electrode, a cathode 124. Anions migrate from the product
chamber 118, through the product chamber anion exchange membrane
154, and into the anion discharge chamber 162. Similarly, cations
migrate from the product chamber 118, through the product chamber
cation exchange membrane 152, and into the cation discharge
chamber 160. In this way, saltwater can be desalinated by
employing the drive cell to generate the drive voltage, and by
applying the sufficient voltage, which includes the drive voltage,
across the product chamber. As described above, flowing through
the anion and cation discharge chambers can be a solution having
an ionic concentration greater than or equal to that of the
saltwater to be desalinated, such as the diluent.
[0079] The movement of ions through the dialytic stack 101 that
occurs when the sufficient voltage applied to the product chamber
118 is greater than or exceeds the desalination voltage represents
movement of ions through an ionic circuit. Various methods of
completing this ionic circuit are possible.
[0080] In FIG. 1, the ionic circuit is completed
electrochemically. Electrochemical completion of the ionic circuit
is achieved by disposing anode and cathode electrolyte chambers
114 and 116 between the stack of diluent and concentrate chambers
112, 110 and the anode 126 and cathode 124, respectively. A
suitable electrolyte flows into and out of the electrolyte
conduits via electrolyte supply conduits 108 and electrolyte exit
conduits 136, respectively. In the embodiment depicted in FIG. 1,
the electrolyte flows through the electrolyte chambers 114, 116 in
parallel; i.e., the electrolyte that flows through one of the
chambers 114, 116 is not used by the other chamber 114, 116.
However, in alternative embodiments (not shown), the electrolyte
may flow through the electrolyte chambers 114, 116 in series;
i.e., the electrolyte may flow into one of the electrolyte
chambers 114, 116 and, upon exiting this electrolyte chamber, be
directed into the other of the electrolyte chambers 114, 116.
Oxidation reactions (at the anode 126) and reduction reactions (at
the cathode 124) convert the ionic current into electric current
and complete the ionic circuit. The anode 126 and the cathode are
electrically communicative 124 via an electrical conduit 156,
thereby resulting in electrons flowing from the anode 126 to the
cathode 124.
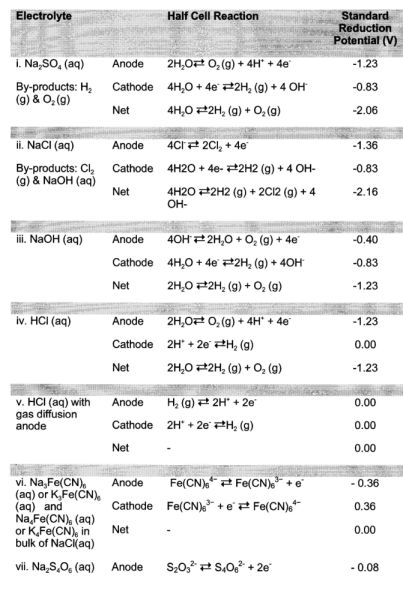
[0081] Table 1, below, lists exemplary electrolytes that can be
used in anode and cathode electrolyte chambers 114, 116, as well
as the associated electrochemical reactions and reduction
potentials that occur at the anode and cathode 126, 124:
TABLE-US-00001 TABLE 1 Exemplary Electrolytes Standard Reduction
Electrolyte Half Cell Reaction Potential (V) i. Na.sub.2SO.sub.4
(aq) Anode 2H.sub.2O O.sub.2 (g) + 4H.sup.+ + 4e.sup.- -1.23
By-products: H.sub.2 Cathode 4H.sub.2O + 4e.sup.- 2H.sub.2 (g) + 4
OH.sup.- -0.83 (g) & O.sub.2 (g) Net 4H.sub.2O 2H.sub.2 (g) +
O.sub.2 (g) -2.06 ii. NaCl (aq) Anode 4Cl.sup.- 2Cl.sub.2 +
4e.sup.- -1.36 By-products: Cl.sub.2 Cathode 4H2O + 4e- 2H2 (g) +
4 OH- -0.83 (g) & NaOH (aq) Net 4H2O 2H2 (g) + 2Cl2 (g) + 4OH-
-2.16 iii. NaOH (aq) Anode 4OH.sup.- 2H.sub.2O + O.sub.2 (g) +
4e.sup.- -0.40 Cathode 4H.sub.2O + 4e.sup.- 2H.sub.2 (g) +
4OH.sup.- -0.83 Net 2H.sub.2O 2H.sub.2 (g) + O.sub.2 (g) -1.23 iv.
HCl (aq) Anode 2H.sub.2O O.sub.2 (g) + 4H.sup.+ + 4e.sup.- -1.23
Cathode 2H.sup.+ + 2e.sup.- H.sub.2 (g) 0.00 Net 2H.sub.2O
2H.sub.2 (g) + O.sub.2 (g) -1.23 v. HCl (aq) with Anode H.sub.2
(g) 2H.sup.+ + 2e.sup.- 0.00 gas diffusion Cathode 2H.sup.+ +
2e.sup.- H.sub.2 (g) 0.00 anode Net -- 0.00 vi.
Na.sub.3Fe(CN).sub.6 Anode Fe(CN).sub.6.sup.4- Fe(CN).sub.6.sup.3-
+ e.sup.- -0.36 (aq) or K.sub.3Fe(CN).sub.6 Cathode
Fe(CN).sub.6.sup.3- + e.sup.- Fe(CN).sub.6.sup.4- 0.36 (aq) and
Net -- 0.00 Na.sub.4Fe(CN).sub.6 (aq) or K.sub.4Fe(CN).sub.6 in
bulk of NaCl(aq) vii. Na.sub.2S.sub.4O.sub.6 (aq) Anode
S.sub.2O.sub.3.sup.2- S.sub.4O.sub.6.sup.2- + 2e.sup.- -0.08 and
Na.sub.2S.sub.2O.sub.3 (aq) Cathode S.sub.4O.sub.6.sup.2- +
2e.sup.- S.sub.2O.sub.3.sup.2- 0.08 Net -- 0.00
[0082] Exemplary anode and cathode materials include substrate
metals such as titanium, niobium, tantalum, iridium, palladium,
stainless steel, steel, nickel and graphite; the substrate metals
may be optionally coated with platinum, ruthenium, iridium, or a
mixed metal oxide combination of any two or more of platinum,
ruthenium, and iridium.
[0083] Notably, the choice of which type of ion exchange membrane
("stack end membrane 158") is used to separate the diluent and
concentrate chambers 112, 110 from the electrolyte chambers 114,
116 is important. The stack end membrane 158 nearest to the anode
126 is hereinafter referred to as the "anode stack end ion
exchange membrane" and the stack end membrane 158 nearest to the
cathode 124 is hereinafter referred to as the "cathode stack end
ion exchange membrane". The anode electrolyte chamber 114 is
bounded on one side by and is in ionic communication with the
anode stack end ion exchange membrane, and the cathode electrolyte
chamber 116 is also bounded on one side by and is in ionic
communication with the cathode stack end ion exchange membrane. As
electrochemical reactions occur at the anode 126 and the cathode
124, the anode electrolyte chamber 114 is bounded on another side
by and is in electrical communication with the anode 126, and
similarly the cathode electrolyte chamber 116 is bounded on
another side by and is in electrical communication with the
cathode 124. In an embodiment that uses Na.sub.2SO.sub.4 as an
electrolyte, for example, cation exchange membranes 122 are used
as stack end membranes 158. This is because Na.sub.2SO.sub.4 is
composed of Na.sup.+ cations and SO.sub.4.sup.2- anions. By using
cation exchange membranes 122, only the Na.sup.+ cations can
travel between the electrolyte chambers 114, 116 into the adjacent
diluent and concentrate chambers 112, 110. As the diluent and
concentrate are both saltwater, none of the electrolyte, diluent,
or concentrate becomes polluted with new types of ions. Similarly,
if HCl were used as an electrolyte, anion exchange membranes would
typically be used to separate the electrolyte chambers 114, 116
and the diluent and concentrate chambers 112, 110.
[0084] In an alternative embodiment (not shown), the diluent and
the concentrate can flow through the dialytic stack 101 in
opposite, or countercurrent, directions. Doing so can help to
maintain a more even concentration difference between the diluent
and concentrate chambers 112, 110.
[0085] Referring now to FIG. 2, there is depicted a second
embodiment of a dialytic stack 201 that is configured to have a
polarity opposite that of the first embodiment of the dialytic
stack 101 depicted in FIG. 1. The dialytic stack 201 of FIG. 2 is
similar to the dialytic stack 101 of FIG. 1, with the exceptions
being that the product chamber 118 and anion and cation discharge
chambers 162, 160 have been shifted one chamber to the right, the
chamber that formerly served as the anion discharge chamber 162
has been replaced with a concentrate chamber 110, and the
remaining diluent and concentrate chambers 112, 110 have been
swapped. The result is a change of polarity of the drive voltage
and, consequently, a change in direction of ion migration.
Although FIG. 2 shows the product chamber 118 and anion and cation
discharge chambers 162, 160 having been shifted only one chamber
to the right relative to the embodiment depicted in FIG. 1, these
chambers could have been shifted any odd number of chambers to the
right or left.
[0086] Useful by-products may be created from the electrochemical
reactions occurring at the anode and cathode 126, 124 depending on
the electrolytes used in the dialytic stacks 101, 201 depicted in
FIGS. 1 and 2. For example, when the electrolyte used is aqueous
NaCl, Cl.sup.- anions are oxidized to Cl.sub.2 gas (see Table 1,
row ii, above). Cl.sub.2 gas can, for example, be used to treat
water via chlorination. Alternatively, if aqueous HCl were used as
an electrolyte, H.sub.2 gas would be produced at the cathode 124
(see Table 1, row v, above) and could be subsequently used to
produce power in fuel cells, for example. Other potential useful
by-products from the oxidation-reduction reactions occurring in
the electrolyte chambers 114, 116 include oxygen gas when using an
electrolyte of Na.sub.2SO.sub.4, NaOH or HCl; and sodium hydroxide
when using NaCl as an electrolyte.
[0087] FIG. 3 depicts a third embodiment of a dialytic stack 301
that pumps the electrolyte from one of the anode and cathode
electrolyte chambers 114, 116 to the other of the anode and
cathode electrolyte chambers 114, 116 such that electrochemical
reaction by-products formed in one of the anode and cathode
electrolyte chambers 114, 116 can be used as a reactant in the
other of the electrolyte chambers 114, 116. The dialytic stack 301
uses gas diffusion electrodes for the anode 126 and cathode 124
and circulates via pumping the electrochemical reaction
by-products from one of the anode 126 or cathode 124 to the other
of the anode 126 or cathode 124 for use as a reactant in an
electrochemical reaction and oxidation or reduction back to its
original form. This theoretically reduces the net voltage required
for the electrochemical reactions to zero. For example, in the
dialytic stack 301 of FIG. 3, an aqueous mixture of HCl and NaCl
can be used as the electrolyte in the electrolyte chambers 114,
116, and the stack end membranes 158 are anion exchange membranes
122. At the cathode 124, hydrogen ions are reduced to hydrogen
gas, which is forced to flow to the anode 126 where the hydrogen
gas is again oxidized to hydrogen ions. The hydrogen ions can then
be recirculated back to the cathode 124 via a pump (not shown)
where they are again reduced to hydrogen gas. The gas diffusion
electrodes that are used can be made using the same substrate and
coating materials as described above, and are configured to
provide sufficient resident time for the gas to be oxidized or
reduced at the anode 126 or cathode 124, respectively. Notably,
the by-products of the electrochemical reactions need not be
gaseous. For example, an aqueous mixture of Na.sub.3Fe(CN).sub.6
and Na.sub.4Fe(CN).sub.6 can be used as an electrolyte, which
results in Fe(CN).sub.6.sup.4- being oxidized to
Fe(CN).sub.6.sup.3- at the anode 126, which can then be circulated
to the cathode 124 for reduction back to Fe(CN).sub.6.sup.4-.
[0088] In the embodiment depicted in FIG. 3, then, the five drive
cells 140, 142, 144, 146, 148 generate a total of about 0.20
Volts. Assuming that Na.sub.3Fe(CN).sub.6 and Na.sub.4Fe(CN).sub.6
are used as electrolytes, the net electrode reduction potential
that needs to be overcome for the oxidation-reduction reactions to
occur is 0 Volts (see Table 2, row vi). The desalination voltage
of the desalination cell 150 is about 0.088 Volts. For a dialytic
stack that uses Neosepta AFN and CM-1 ion exchange membranes, has
a product chamber 118 that is 0.02 cm thick and has diluent and
concentrate chambers 112, 110 that are each 0.05 cm thick, the
five drive cells 140, 142, 144, 146, 148 generate a net drive
voltage of 0.20 Volts. The product chamber 118 has a desalination
voltage of 0.088 Volts and the net electrode reduction potential
is 0 Volts; consequently, the resulting stack open circuit voltage
is 0.20 Volts-0.088 Volts=0.112 Volts. In this embodiment, the ion
exchange membranes and the diluent, concentrate and product feed
contribute stack resistive losses of 43.OMEGA./cm.sup.2, which
results in an ionic current of 2.6 mA/cm.sup.2. Sufficient drive
voltage exists to desalinate the product feed in the product
chamber 118. The cations and anions in the product feed are driven
out of the product chamber 118, through the product chamber cation
and anion exchange membranes 152, 154, respectively, and into the
anion and cation discharge chambers 162, 160. The product solution
that exits the dialytic stack 101 has a salt concentration of
about 0.04% salt by mass and is drinkable. If an external voltage
is needed to effect desalination, it can be supplied by a voltage
source 128, which is electrically coupled to the electrical
conduit 156.
[0089] Instead of utilizing the voltage source 128 to supply any
additional voltage, additional drive cells may be added to the
dialytic stack 101 until a sufficient cumulative drive voltage is
achieved to effect desalination. If enough drive cells are added
such that voltage in excess of that required for desalination is
produced, the dialytic stack 101 may also act as a power source.
In such a case, an electrical load can be powered by electrically
coupling it between the anode 126 and cathode 124.
[0090] Referring now to FIG. 4, there is depicted another
embodiment of a dialytic stack 401 wherein the ionic circuit is
completed via fluid circulation by submerging the dialytic stack
401 in a conductive bath 404 that is contained within a storage
vessel 406. The bath 404 may be, for example, composed of
concentrate. Beneficially, and in contrast to completing the ionic
circuit electrochemically as is done in the embodiments
illustrated in FIGS. 1 to 3, completing the ionic circuit via
fluid circulation does not require an anode, cathode or power
supplies. Additionally, no external power must be supplied to
overcome the standard reduction potential of the electrochemical
reactions that take place when the ionic circuit is completed
electrochemically, thereby reducing the drive voltage that is
required to desalinate the product.
[0091] The dialytic stack 401 of FIG. 4 does not have an anode or
a cathode. Instead, the diluent chambers 112 and concentrate
chambers 110 of the dialytic stack 401 are sandwiched between
porous first and second end plates 408, which can be made of
non-conductive material such as polypropylene. Immediately
adjacent to the end plates 408 are concentrate chambers 110, which
act as first and second electrolyte chambers for containing an
electrolyte which, in this particular embodiment, is concentrate.
The first electrolyte chamber is bounded on one side by and is in
ionic communication with the porous first end plate and is bounded
on another side and is in ionic communication with a first ion
exchange membrane (the leftmost cation exchange membrane 120 in
FIG. 4); the second electrolyte chamber is bounded on one side by
and is in ionic communication with the porous second end plate and
is bounded on another side by and is in ionic communication with a
second ion exchange membrane (the rightmost anion exchange
membrane 122 in FIG. 4). These first and second electrolyte
chambers ionically communicate with the remainder of the diluent
and concentrate chambers 112, 110 via first and second ion
exchange membranes, respectively (the leftmost cation exchange
membrane 120 and rightmost anion exchange membrane 122 in FIG. 4).
In the dialytic stack 401 as illustrated in FIG. 4, anions migrate
from right to left and cations migrate from left to right.
Consequently, cations are drawn into the dialytic stack from the
conductive bath 404 near the porous end plate 408 on the left of
the dialytic stack 401 and anions are drawn into the dialytic
stack 401 from the conductive bath 404 near the porous end plate
408 on the right side of the dialytic stack 401. The deeper and
wider the conductive bath 404 and the higher its concentration,
the lower the resistance encountered by the migrating ions. In
practice, all of the diluent and concentrate chambers 112, 110 in
the dialytic stack 401 are sealed such that no fluid transfer
occurs between the bath 404 and the chambers 112, 110.
[0092] In an alternative embodiment (not shown), instead of
submerging the dialytic stack 401 within the conductive bath 404,
the first and second electrolyte chambers are filled with
concentrate. These concentrate chambers are fluidly coupled to
each other via an electrolyte chamber fluid conduit. In this
alternative embodiment, the end plates 408 are not porous. Forced
circulation can be provided via pumping to circulate concentrate
from one of the concentrate chambers to the other, thereby
completing the ionic circuit.
[0093] Referring now to FIG. 5, there is shown a dialytic
arrangement of cells in a ring configuration ("dialytic ring"
501). The dialytic ring 501 in FIG. 5 includes a concentrate
supply manifold 510 and a diluent supply manifold 512, which
receive concentrate and diluent from concentrate and diluent
supply conduits 104 and 102, respectively. Diluent and concentrate
are then conveyed to diluent and concentrate chambers 112, 110
which are fluidly coupled to the diluent and concentrate supply
manifolds 512, 510 and which, as in the aforedescribed
embodiments, are separated from each other by a series of
alternating cation and anion exchange membranes 120, 122. Product
feed is delivered directly to product chamber 118 from the product
feed supply conduit 106. Advantageously, cations and anions
migrate in opposite directions around the dialytic ring 501,
thereby achieving desalination of a given volume of product feed.
Compared to a dialytic stack 401 having the same number and
average thickness of chambers 110, 112, 118, the distance
migrating anions and cations have to travel in the dialytic ring
501 is less than the distance they have to travel in the dialytic
stack 401, and therefore the ionic resistance of the dialytic ring
501 is less than the ionic resistances of the dialytic stack 401.
Consequently, if the dialytic ring 501 and the dialytic stack 101,
201, 301 have the same number of chambers 110, 112, 118 of the
same average thickness, resistive losses will be lower in the
dialytic ring 501 than in the dialytic stacks 101, 201, 301. In
addition, electrodes are not required in the dialytic ring 501.
[0094] The dialytic ring 501 of FIG. 5 has seven drive cells and
one desalination cell 150. As with the embodiment of the dialytic
stack wherein the ionic circuit is completed using fluid
circulation, no energy is needed to drive any electrochemical
reactions. Consequently, assuming a concentrate concentration of
about 18% and a diluent concentration of about 3.5%, the seven
drive cells provide a cumulative drive voltage of 0.28 Volts,
which is well in excess of the voltage needed to desalinate the
product feed to about 0.04% salt by mass (approximately 0.088
Volts plus the voltage drop due to parasitic resistive losses).
Following desalination, the product exits the dialytic ring 501
via a product exit manifold 508, the diluent exits the dialytic
ring 501 via a diluent exit manifold 504, and the concentrate
exits the dialytic ring 501 via a concentrate exit manifold 506.
[0095] Referring now to FIG. 6, there is depicted a dialytic
arrangement of cells in a spiral configuration ("dialytic spiral
601"). As in previous embodiments, the dialytic spiral 601 is
composed of an alternating arrangement of cation and anion
exchange membranes 120, 122. Product feed, diluent and concentrate
can be supplied to the gaps between the alternating ion exchange
membranes 120, 122 through supply conduits 102, 104, 106. The
dialytic spiral 601 can be created by, for example, arranging the
ion exchange membranes 120, 122 along with chamber spacers and
gaskets flat on a surface and then rolling them as depicted in
FIG. 6. Beneficially, rolling the membranes 120, 122 aids in high
volume manufacturing; and reduces exposure of sealing surfaces,
which reduces the likelihood that the spiral 601 will leak.
Production techniques are similar to those used in spirally wound
reverse osmosis modules, such as the DOW.TM. 210 EDI module.
[0096] Referring now to FIGS. 7(a) and (c), there are depicted
sectional and exploded views of a unipolar dialytic stack 701 that
can be used to desalinate saltwater. By "unipolar", it is meant
that the direction of ionic movement in the dialytic stack 701 is
not reversible during operation. FIG. 7(a) is a sectional view of
the dialytic stack 701 having an alternating arrangement of
diluent and concentrate chambers 112, 110. The anion exchange
membrane 122 forms a shared boundary and is in ionic communication
with adjacent diluent and concentrate chambers 112, 110 of any
given drive cell; the cation exchange membrane 120 forms a shared
boundary between and is in ionic communication with any given
drive cell and an adjacent drive cell. The product chamber 118 is
disposed within the dialytic stack 701 and is bounded on one side
by and is in ionic communication with the product chamber anion
exchange membrane 154, and is bounded on another side by and is in
ionic communication with the product chamber cation exchange
membrane 152. The anode and cathode 126, 124 and anode and cathode
electrolyte chambers 116, 114 are disposed on either end of the
dialytic stack 701. Between the anode electrolyte chamber 116 and
the drive cells and product chamber 118 are the stack end
membranes 158 in the form of the anode and cathode stack end ion
exchange membranes. The anode electrolyte chamber 116 is bounded
on one side by and is in ionic communication with the anode stack
end ion exchange membrane and is bounded on another side by and is
in electrical communication with the anode 126. The anode
electrolyte chamber 116 is ionic communication with the drive
cells and the product chamber 118 via the anode stack end ion
exchange membrane. Similarly, the cathode electrolyte chamber 114
is bounded on one side by and is in ionic communication with the
cathode stack end ion exchange membrane and is bounded on another
side by and is in electrical communication with the cathode 124.
The cathode electrolyte chamber is in ionic communication with the
drive cells and product chamber 118 via the cathode stack end ion
exchange membrane. The anode and cathode, ion exchange membranes
and diluent, concentrate and product chambers are sandwiched
between two end plates 714.
[0097] Referring now also to FIG. 7(b), there is depicted a front
elevation view of a gasket 708 having a spacer 710 that is used as
part of a manifolding assembly that includes diluent manifolding
configured to convey diluent to and away from the diluent chambers
112, concentrate manifolding to convey concentrate to and away
from the concentrate chambers 110, and product manifolding to
deliver the saltwater to be desalinated to and desalinated
saltwater away from the product chamber 118. It is advantageous
for the chambers to be as thin as practically possible to limit
ionic resistance while also promoting fluid flow without an
excessive pressure drop. In FIG. 7(b), the spacer 710 is a mesh
spacer that maintains separation of adjacent ion exchange
membranes when the dialytic stack 701 is in operation by
preventing adjacent ion exchange membranes from contacting each
other as a result of fluid flow through the chambers 110, 112,
118. The spacer 710 can be similar to Industrial Netting's XN-3234
or ON-6200. The gasket 708 can be formed using materials such as
ethylene propylene diene M-class rubber (EPDM), silicon, nitrile,
santoprene, viton, neoprene, PTFE (Teflon), natural rubber, and
PVC. As is evident from FIG. 7(c), the gaskets 708 and ion
exchange membranes are layered in an alternating series to form
the various diluent, concentrate, product, and electrolyte
chambers. Each gasket 708 circumscribes a volume that acts as one
of the diluent, concentrate or product chambers, and each such
chamber is bounded on one side by and in ionic communication with
one ion exchange membrane and bounded on another side by and in
ionic communication with another ion exchange membrane.
[0098] Each gasket 708 has punched through its perimeter a series
of holes that make up part of the diluent, concentrate or product
manifolding. In FIG. 7(b), one side of the gasket 708 has the
diluent supply conduits 104 extending therethrough and the
opposing side of the gasket 708 has the diluent exit conduits 132
extending therethrough. Similarly, the product feed supply
conduits 106 and the product exit conduits 134 extend through
opposing sides of the gasket 708. The gasket 708 has a series of
inlet notches 738(a) extending through one side of the gasket 708,
with each inlet notch 738(a) being fluidly coupled to the
concentrate supply conduit 102, and on an opposing side has a
series of outlet notches 738(b) extending therethrough, with each
outlet notch 738(b) being fluidly coupled to the concentrate exit
conduit 130. The gasket 708 depicted in FIG. 7(b) is thus
configured to be a concentrate chamber 110. Only the concentrate
supply and exit conduits 102, 130 are fluidly coupled to the
concentrate chamber 110 via the inlet and outlet notches 738(a),
(b); consequently, any product (or product feed) and diluent
passing through the product feed supply conduits 106, product exit
conduits 134, diluent supply conduits 104 and diluent exit
conduits 132 are sealed from the concentrate chamber 110, while
concentrate will flow from the concentrate supply conduits 102,
through the inlet notches 738(a), into and through the concentrate
chamber 110, and then out through the outlet notches 738(b) on the
opposite side of the gasket 708 and into the concentrate exit
conduits 130. Similarly, for the gasket that circumscribes the
product chamber 118, only notches that fluidly couple the product
feed supply and exit conduits 106, 134 to the product chamber 118
are present, and for the gaskets that circumscribe the diluent
chambers 112, only notches that fluidly couple the diluent supply
and exit conduits 104, 132 to the diluent chamber 112 are present.
The gaskets 708 when pressed together to form the dialytic stack
701 form a fluid tight seal, thus securely containing the contents
of the diluent, concentrate and product chambers. Additionally,
the concentrate supply and exit conduits 102, 130, diluent supply
and exit conduits 104, 132 and product feed supply and product
exit conduits 106, 134 from various gaskets 708 align together
when the gaskets 708 are pressed to form the dialytic stack 701,
thus forming the concentrate, diluent, and product manifolding,
respectively. Concentrate, diluent and product feed can
consequently flow through the concentrate, diluent and product
manifolding and be delivered to the concentration, diluent and
product chambers 110, 112, 118.
[0099] In the depicted embodiment, electrolyte is pumped into and
out of electrolyte chambers 114, 116 via conduits 108, 136,
respectively.
[0100] Referring now to FIG. 8, there are depicted schematic (FIG.
8(a)), exploded (FIG. 8(b)) and perspective (FIG. 8(c)) views of a
bipolar dialytic stack 801, in which assembly of the chambers with
manifolding and valves is illustrated. By "bipolar", it is meant
that the dialytic stack 801 is operable in forward and reverse
polarities. In forward polarity, a schematic view of the dialytic
stack 801 is the dialytic stack 101 of FIG. 1. In reverse
polarity, a schematic view of the dialytic stack 801 is the
dialytic stack 201 of FIG. 2. Visible in FIG. 8(a) are sixteen
two-way valves, inlet valves 844(a)-(h) and outlet valves
844(i)-(p) that can be configured for both forward polarity and
reverse polarity operation. Table 2, below, specifies how these
two-way valves 844(a)-(p) are configured in these modes:
TABLE-US-00002 TABLE 2 Valve Settings in Forward Polarity and
Reverse Polarity Operation Forward Polarity Reverse Polarity Valve
Operation Operation 844(a) Opened Closed 844(b) Closed Opened
844(c) Closed Opened 844(d) Opened Closed 844(e) Closed Opened
844(f) Opened Closed 844(g) Opened Closed 844(h) Closed Opened
844(i) Opened Closed 844(j) Closed Opened 844(k) Closed Opened
844(l) Opened Closed 844(m) Closed Opened 844(n) Opened Closed
844(o) Closed Opened 844(p) Opened Closed
[0101] Referring now to FIG. 8(b) and as with the embodiments of
the dialytic stack depicted in FIGS. 1 and 2, the dialytic stack
801 is composed of an alternating series of cation and anion
exchange membranes 120, 122. Between the cation and anion exchange
membranes 120, 122 are a series of alternating diluent/concentrate
chambers 812 and concentrate/diluent chambers 810. The anion
exchange membrane 122 forms a shared boundary and is in ionic
communication with adjacent diluent/concentrate and
concentrate/diluent chambers 812, 810 of any given drive cell; the
cation exchange membrane 120 forms a shared boundary between and
is in ionic communication with any given drive cell and an
adjacent drive cell. Also located within the dialytic stack 801
are a product/diluent chamber 818 and diluent/product chamber 819,
which in the depicted embodiment are adjacent to each other. The
product/diluent chamber 818 is bounded on one side by and is in
ionic communication with a product/diluent chamber anion exchange
membrane 854 and bounded on another side by and is in ionic
communication with a product/diluent chamber cation exchange
membrane 852. Similarly, the diluent/product chamber is bounded on
one side by and is in ionic communication with a diluent/product
chamber anion exchange membrane 856 and bounded on another side by
and is in ionic communication with a diluent/product chamber
cation exchange membrane 852. In this particular embodiment, the
diluent/product chamber cation exchange membrane 852 and the
product/diluent chamber cation exchange membrane 852 are the same
ion exchange membrane because the product/diluent and
diluent/product chambers 818, 819 are adjacent to each other,
although this is not the case when the chambers 818, 819 are not
adjacent.
[0102] As with the dialytic stack 701, the dialytic stack 801 has
a manifolding assembly that includes diluent/concentrate,
concentrate/diluent, product/diluent, and diluent/product
manifolding respectively configured to convey diluent to and away
from the diluent/concentrate chamber 812 and diluent/product
chamber 819, concentrate to and away from the concentrate/diluent
chamber 810, and the saltwater to be desalinated to and the
desalinated saltwater away from the product/diluent chamber 818
when the dialytic stack 801 is operating in forward polarity, and
respectively configured to convey diluent to and away from the
concentrate/diluent chamber 810 and product/diluent chamber 818,
concentrate to and away from the diluent/concentrate chamber 812,
and the saltwater to be desalinated to and the desalinated
saltwater away from the diluent/product chamber 819 when the
dialytic stack 801 is operating in reverse polarity.
[0103] As with the dialytic stack 701, the dialytic stack 801
includes gaskets (not labeled in FIG. 8) that each circumscribe a
volume that acts as one of the diluent/concentrate,
concentrate/diluent, diluent/product, or product/diluent chambers,
and each such chamber is bounded on one side by and is in ionic
communication with one ion exchange membrane and is bounded on
another side by and is in ionic communication with another ion
exchange membrane. Structurally, the manifolding assembly of the
dialytic stack 801 is identical to that of the dialytic stack 701
with the exception of the addition of an additional
diluent/product manifolding to another side of the gaskets used in
the dialytic stack 801. Structurally, the diluent/concentrate
manifolding, concentrate/diluent manifolding, and product/diluent
manifolding correspond to the diluent manifolding, concentrate
manifolding, and product manifolding of the dialytic stack 701,
respectively. For example, inlet notches fluidly couple
diluent/concentrate supply conduits 804 in one of the gaskets of
the dialytic stack 801 to the diluent/concentrate chamber 812,
thereby allowing the solution flowing through the
diluent/concentrate conduit (either diluent or concentrate) to
enter the diluent/concentrate chamber 812. Outlet notches fluidly
couple the diluent/concentrate chamber 812 to diluent/concentrate
exit conduits 830, thereby allowing the solution in the
diluent/concentrate chamber 812 to exit the stack 801.
[0104] The first and second electrodes 824, 826 and first and
second electrolyte chambers 814, 816 are disposed on either end of
the dialytic stack 801. The electrodes 824, 826 are both operable
as either anodes or cathodes, depending on whether the dialytic
stack 801 is operating in forward or reverse polarity. The first
electrolyte chamber 814 is bounded on one side by and is in ionic
communication with a first stack end ion exchange membrane 858,
and is bounded on another side by and is in electrical
communication with the first electrode 824. Similarly, the second
electrolyte chamber 816 is bounded on one side by and is in ionic
communication with a second stack end ion exchange membrane 858,
and is bounded on another side by and is in electrical
communication with the second electrode 826. The electrodes, ion
exchange membranes and various chambers are sandwiched between two
end plates 714.
[0105] The electrodes 824, 826 can be composed of a substrate and
a coating applied thereon. The substrate can be, for example,
titanium, niobium, tantalum, iridium, or palladium. The coating
can be platinum, ruthenium, iridium, or a mixed metal oxide
combination of the three. Alternatively, the electrodes 824, 826
can be sacrificial and deteriorate over time. In such an
embodiment, the electrodes 824, 826 can be formed from an uncoated
substrate of stainless steel, steel, nickel, copper, or graphite.
[0106] Operation of the dialytic stack 801 in forward polarity
will now be described. Diluent, concentrate and product feed are
supplied through the arrows labeled "D", "C" and "P" in FIG. 7(a).
Diluent is pumped into the dialytic stack 801 through the valves
844(a), 844(g) and the diluent/concentrate supply conduits 804 and
diluent/product supply conduits 806. The diluent is pumped through
the diluent/concentrate and diluent/product manifolding and is
delivered to the diluent/concentrate chambers 812 and the
diluent/product chamber 819 in the dialytic stack 801. Diluent
exits the dialytic stack 801 through the diluent/concentrate exit
conduits 830 and diluent/product exit conduits 832 and the valves
844(i), 844(p). Concentrate is pumped into the dialytic stack 801
through the valve 844(d) and concentrate/diluent supply conduit
802. The concentrate is pumped through the concentrate/diluent
manifolding and is delivered to the concentrate/diluent chambers
810. The concentrate exits the dialytic stack 801 through
concentrate/diluent exit conduit 828 and the valve 844(l). Product
feed is pumped into the dialytic stack 801 through the valve
844(f) and product/diluent supply conduit 805. The product feed is
pumped through the product/diluent manifolding and is delivered to
the product/diluent chamber 818. The product exits the dialytic
stack 801 through product/diluent exit conduit 831 and the valve
844(n).
[0107] Following exiting the dialytic stack 801, diluent,
concentrate and product are diverted through three-way valves
846(a)-(c). Normally, the valves 846(a)-(c) are set such that the
diluent, concentrate and product are directed along the conduits
labeled "D", "C" and "P". The dialytic stack 801 can also be
operated in purge mode, in which case the diluent, concentrate and
product are all diverted to the "waste" conduit, labeled "W".
While transitioning from operation in forward polarity mode to
reverse polarity mode or vice versa, the dialytic stack 801 can be
temporarily operated in purge mode so as to flush away any
unwanted diluent, concentrate and product from the valves and
conduits.
[0108] When operating in reverse polarity, diluent is pumped into
the dialytic stack through the valves 844(c), 844(e) and the
concentrate/diluent supply conduit 802 and the product/diluent
supply conduit 805. The diluent is pumped through the
concentrate/diluent and product/diluent manifolding and delivered
into the concentrate/diluent chambers 812 and the product/diluent
chamber 818. The diluent exits the dialytic stack through the
concentrate/diluent exit conduit 828, the product/diluent exit
conduit 831 and the valves 844(k), 844(m). Concentrate is pumped
into the dialytic stack 801 through the valve 844(b) and through
the diluent/concentrate supply conduit 804. The concentrate is
pumped through the diluent/concentrate manifolding and delivered
to the diluent/concentrate chambers 812 and exits the dialytic
stack through the diluent/concentrate exit conduit 830 and the
valve 844(i). Product feed is pumped into the dialytic stack 801
through the valve 844(h) and the diluent/product supply conduit
806. The product feed is pumped through the diluent/product
manifolding and delivered to the diluent/product chamber 819 and
the product exits the dialytic stack through the diluent/product
exit conduit 832 and the valve 844(o). In both forward and reverse
polarities, electrolyte is pumped into and out of end chambers
114, 116 via conduits 108, 136, respectively.
[0109] In addition to pumping product feed, diluent and
concentrate into the dialytic stack through the valves 844(a)-(h)
and product, diluent and concentrate out of the dialytic stack
through the valves 844(i)-(p) ("forward flow" mode), the flow of
solutions within the dialytic stack can also be reversed such that
product feed, diluent and concentrate are pumped into the dialytic
stack through the valves 844(i)-(p) and product, diluent and
concentrate are pumped out of the dialytic stack through the
valves 844(a)-(h) ("reverse flow" mode). Reverse flow mode can be
used to flush the dialytic stack 801. Furthermore, the dialytic
stack 801 is able to operate in a mode wherein some of the
solutions enter the dialytic stack 801 through the valves
844(a)-(h) and other solutions enter the dialytic stack through
the valves 844(i)-(p) ("countercurrent flow" mode). For example,
when pumping the product feed and diluent into the dialytic stack
801 through the valves 844(a), (f) and (h), concentrate can be fed
into the dialytic stack 801 through valve 844(l). This allows the
diluent and concentrate to flow through the dialytic stack 801 in
countercurrent directions, which as mentioned above can help to
maintain a more even concentration difference between the diluent
and concentration chambers 812, 810.
[0110] One benefit of being able to run the dialytic stack in both
forward and reverse polarities is that periodic reversal of stack
polarity can be used to prevent scaling and fouling of the
membranes 120, 122 and electrodes 824, 826 and thereby extend the
life of the ion exchange membranes 120, 122 and the electrodes
824, 826.
[0111] Referring now to FIGS. 9(a) and (b), there is depicted a
multi-stage dialytic stack 901. The multi-stage stack 901 has four
desalination stages 1-4. Input to the first stage are concentrate,
diluent and product feed through the concentrate, diluent and
product feed supply conduits 102, 104, 106. Each stage in the
dialytic stack 901 purifies the product by a certain percentage
such that the product that exits via the product exit conduit 134
is desalinated. As is evident in FIG. 9(a), the product flows
through adjacent desalination stages in the depicted dialytic
stack 901 in opposing directions, although this is not required in
all embodiments.
[0112] Referring now to FIG. 9(b), there is depicted a detailed
view of a portion of desalination stages 3 and 4 of the dialytic
stack 901. The dialytic stack 901 includes a plurality of drive
cells, each of which is composed of a diluent chamber 112 and a
concentrate chamber 110 ionically communicative via an ion
exchange membrane. Desalination stage 3 also has a plurality of
initial stage product chambers 914, each of which is bounded on a
first side by and in ionic communication with an initial stage
product chamber anion exchange membrane and bounded on another
side by and in ionic communication with an initial stage product
chamber cation exchange membrane. The output of the initial stage
product chambers 914 is directed into a plurality of product
chambers in stage 4, subsequent stage product chambers 916, each
of which is bounded on a first side by and in ionic communication
with a subsequent stage product chamber anion exchange membrane
and bounded on another side by and in ionic communication with a
subsequent stage product chamber cation exchange membrane. The
plurality of product chambers that make up the initial stage
product chambers 914 and the plurality of product chambers that
make up the subsequent stage product chambers 916 allow product to
flow in parallel in any given stage. In any given stage, having
multiple product chambers purify saltwater in parallel allows the
total volume of product feed that can be handled by the dialytic
stack 901 to be increased relative to a dialytic stack 901 that
has only a single product chamber per stage. Additionally,
incorporating multiple desalination stages into a single stack
results in lower capital costs than having a separate stack for
each desalination stage. The initial stage and subsequent stage
product chambers 914, 916 each have a desalination voltage.
Consequently, in order to effect desalination in both the initial
stage and subsequent stage product chambers 914, 916
simultaneously, the sufficient voltage that is applied across the
product chambers 914, 916 must be greater than or equal to the sum
of the desalination voltages of the product chambers 914, 916.
This sufficient voltage includes the drive voltage generated by
the drive cells and any external voltage applied to the dialytic
stack 901.
[0113] Although the initial and subsequent desalination stages of
FIG. 9 are shown as being desalination stages 3 and 4, the terms
"initial desalination stage" and "subsequent desalination stage"
refer to any two desalination stages in a dialytic stack wherein
the saltwater to be desalinated travels through the initial
desalination stage prior to traveling through the subsequent
desalination stage; i.e., to any two stages in series
communication with each other.
[0114] Referring now to FIG. 10, there is depicted a plant 1001
that can be used to desalinate saltwater using a continuous flow
process. The plant 1001 includes a water source 1004, which can be
the ocean or a brackish water supply, for example. Saltwater from
the water source 1004 is directed to a pre-treatment system 1006
that treats the saltwater prior to desalination. The pre-treatment
system 1006 is designed to remove debris, suspended solids and
organic and inorganic matter that can foul, plug or damage the
equipment used in the plant 1001. From the pre-treatment system
1006, the treated saltwater is diverted to a saltwater reservoir
1010, which holds product feed that is to be desalinated, and to a
diluent reservoir 1026, which holds diluent. In the depicted
embodiment, the diluent and the product feed to be desalinated are
the same, although this is not a requirement for all embodiments.
Also present in the plant 1001 is a concentrate reservoir 1020,
for holding concentrate. Concentrate, diluent and product feed
from reservoirs 1020, 1026 and 1010 are pumped through a series of
three dialytic stacks 1002 in order to desalinate the product
feed. Other embodiments could use more or less than three dialytic
stacks with the desalination occurring in stages as the product
feed passes through the dialytic stacks. The plant 1001 utilizes
three dialytic stacks 1002, with product feed flowing through the
dialytic stacks 1002 in series and concentrate and diluent flowing
through the dialytic stacks 1002 in parallel. Following use in the
dialytic stacks 1002, used diluent can be returned to the diluent
reservoir 1026 via a valve 1040; back to the water source 1004 via
a second valve 1042; and to a first reconcentrator 1032 for
generating concentrate from the used diluent, as discussed in more
detail below. Used concentrate is returned to a second
reconcentrator 1034 for reconcentration prior to storage in the
concentrate reservoir 1020, also discussed in more detail below.
Product is stored in product reservoir 1018 for retrieval and use.
In an alternative embodiment (not shown), used concentrate could
be returned to the first reconcentrator 1032 and then to the
second reconcentrator 1034, beneficially maintaining a higher
concentration in the concentrate reservoir 1020. Also in
alternative embodiments (not shown), output from the first
reconcentrator 1032 could be conveyed directly into the
concentrate reservoir 1020, or used concentrate could be returned
directly to the concentrate reservoir 1020 instead of to the
second reconcentrator 1034.
[0115] Exemplary first and second reconcentrators 1032, 1034 are
depicted in FIGS. 12-14, discussed below. The first reconcentrator
1032 is used to increase the concentration of used diluent prior
to transferring the used diluent to the second reconcentrator
1034, which is used to further increase the concentration of the
used diluent prior to transferring it into the concentrate
reservoir 1020 and to increase the concentration of the solution
stored in the concentrate reservoir, if necessary. Both the first
and second reconcentrators 1032, 1034 utilize evaporation to the
atmosphere for increasing the concentration of saltwater until the
saltwater attains a salt concentration suitable for use as
concentrate. In one embodiment, solar energy can be directly
transferred to the reconcentrators 1032, 1034 (e.g. by having the
sun shine on a spray pond 1201, as depicted in FIG. 12) to
increase the concentration of saltwater. In alternative
embodiments (not depicted), the first and second reconcentrators
1032, 1034 can be fluidly coupled to a heat exchanger 1324
(depicted in FIGS. 13 and 14) which, in turn, obtains heat from an
external heat source such as a nearby power or process plant or a
solar thermal collector. Low grade thermal energy from a power
plant may be, for example, waste heat (such as from a power plant)
that may range from about 30 to 150 degrees Celsius. Exemplary
heat exchangers are shell and tube, plate, and phase change heat
exchangers. The reconcentrators 1032, 1034 may use a combination
of energy obtained via the heat exchanger 1324 or directly from
the sun to aid in evaporation.
[0116] In contrast to known means and methods for desalinating
saltwater, the plant 1001 is essentially able to store low grade
thermal energy, such as solar energy, in the form of concentrated
saltwater; this stored chemical energy is transformed to
desalinate the product feed in the dialytic stacks 1002. Notably,
areas that are dry and arid and consequently likely to require
desalination technology are also those areas that tend to have
less humid atmospheres, receive a great deal of solar radiation
and therefore have environments in which water readily evaporates.
Beneficially, areas in which the plant 1001 is likely to function
best are those areas in which the plant 1001 is needed most.
[0117] Referring now to FIG. 11, there is depicted a second
embodiment of a plant 1101 that can be used to desalinate
saltwater in a batch flow process. As with the first embodiment of
the plant 1001, saltwater is collected from the water source 1004,
receives pre-treatment in the pre-treatment system 1006, and is
then diverted to a combined saltwater and product reservoir 1110
and the diluent reservoir 1026. Although the plant 1101 uses only
one dialytic stack 1102, multiple dialytic stacks could be used as
is done in the first embodiment of the plant 1001. In contrast to
the first embodiment 1001 of the plant that pumps product feed in
series through three dialytic stacks 1002, though, the second
embodiment 1101 of the plant does not use the product feed
reservoir 1018 of the first plant embodiment 1001 to receive
desalinated product. Instead, desalinated product is returned back
to the combined saltwater and product reservoir 1110. In this way,
a discrete batch of saltwater to be desalinated can be transferred
from the water source 1004 and stored in the combined saltwater
and product reservoir 1110, which can then be pumped through the
dialytic stack 1002 until the water in the combined saltwater and
product reservoir 1110 has been sufficiently desalinated. As with
the first embodiment of the plant 1001, the reconcentrators 1032,
1034 increase the concentration of saltwater by evaporation to the
atmosphere until the saltwater is ready to be used as concentrate.
[0118] Referring now to FIGS. 12-14, there are depicted three
examples of reconcentrators. FIG. 12 depicts an evaporative spray
pond 1201; FIG. 13 depicts a natural draft evaporative tower 1301;
and FIG. 14 depicts a forced draft evaporative tower 1401.
[0119] The evaporative spray pond 1201 depicted in FIG. 12
includes a pond surface 1214 in which is a shallow layer of pond
catchment 1232. The pond catchment 1232 is fluidly coupled to a
nozzle header 1206, nozzle riser 1204, and spray nozzle 1202.
While only one spray nozzle 1202 is depicted in FIG. 12, the
nozzle header 1206 may be coupled to a plurality of spray nozzles
1202. Concentrated seawater can be pumped from the catchment 1232
to the concentrate reservoir 1020 via fluid conduit 1236;
similarly, fluid from the concentrate reservoir 1020 can be pumped
to the nozzle header 1206 and sprayed through the spray nozzle
1202 via fluid conduit 1238. Some of the water in the spray
emanating from the spray nozzle 1202 will evaporate as the spray
falls towards the catchment 1232, thereby increasing the salt
concentration of the saltwater in the catchment 1232. A louvred
fence 1212 may be used to prevent water droplets from diffusing
away from the pond 1212.
[0120] Labeled A, B, C and D in FIG. 12 are four different ways in
which the pond surface 1214 may be constructed. Surface A is
constructed of a layer of heat capacitive material 1218, such as
sand, on which is a layer of a dark, highly conductive material
1216 such as black steel. Surface B is similar to surface A except
that a layer of insulation 1222 is laid under the heat capacitive
material 1218 to better retain heat. Surface C is constructed of a
layer of the heat capacitive material 1218 on which is a layer of
a dark, sealing membrane surface 1226 such as PVC, polypropylene
or EPDM. Surface D is similar to surface C except that a layer of
the insulation 1222 is under the layer of heat capacitive material
1218.
[0121] In an alternative embodiment (not shown), the nozzle 1202,
nozzle riser 1204 nozzle header 1206 and louvered fence 1212 can
be removed from the evaporative spray pond 1212. The result is an
evaporative pond, which can also be used as a reconcentrator.
[0122] In a further alternative embodiment (not depicted), the
heat exchanger 1324 may be fluidly coupled to the nozzle header
1206 to provide additional energy that can be used in the
evaporation process. The more heat is supplied via the heat
exchanger 1324, the smaller the area of the pond catchment 1232
needs to be. The heat exchanger 1324 can, for example, supply 60%
of the energy used in the evaporation process, with the remaining
40% coming from energy absorbed by the surface area of the pond
catchment 1232.
[0123] The natural draft evaporative tower 1301 is composed of a
tower base 1314 inside of which lies a shallow layer of tower
catchment 1316 and on which is supported a tower housing 1302. As
with the spray pond 1201, the tower catchment 1316 can be pumped
to the concentrate reservoir 1020 via the fluid conduit 1236, and
fluid from the concentrate reservoir 1020 can be pumped to a
dispersive nozzle 1304 via the fluid conduit 1238. The fluid first
passes through the heat exchanger 1324 that is coupled to an
external heat source as described above, which provides low grade
heat to the fluid to replace heat lost during evaporation. Spray
falling from the nozzle 1304 evaporates as it falls towards the
catchment 1316, aided by an influx of air through air intakes
1310, thereby increasing the salt concentration of the catchment
1316. The spray also hits fill material 1312, which provides
increased surface area for the saltwater to flow over and
increases mass transfer between the saltwater and the air, thereby
aiding in evaporation.
[0124] The forced draft evaporative tower 1401 is similar to the
natural draft evaporative tower 1301 as it is composed of a tower
base 1418 inside of which lies a shallow layer of tower catchment
1420 and on which is supported a tower housing 1402. The tower
catchment 1420 can be pumped to the concentrate reservoir 1020 via
the fluid conduit 1236, and fluid from the concentrate reservoir
1020 can be pumped to a dispersive nozzle 1304 via the fluid
conduit 1238. The fluid first passes through the heat exchanger
1324 that is coupled to an external heat source as described
above, which provides low grade heat to the fluid to replace heat
lost during evaporation. Spray falling from the nozzle 1304
evaporates as it falls towards the catchment 1420, aided by an
influx of air through air intakes 1310 and by the draft provided
by a fan 1412, thereby increasing the salt concentration of the
catchment 1316. The spray also hits fill material 1312, which
provides increased surface area for the saltwater to flow over and
increases mass transfer between the saltwater and the air, thereby
aiding in evaporation. Demister 1414 helps to prevent spray from
damaging the fan 1412 and escaping from the top of the tower 1401.
[0125] Any of the reconcentrators depicted in FIGS. 12-14 can
reside on land or float at sea.
EXAMPLE
[0126] An example of a plant 1001 composed of a four stage
dialytic stack 901 coupled to a reconcentrator 1032 in the form of
an evaporative spray pond 1201 will now be discussed.
[0127] The dialytic stack has a total of 800 drive cells and 100
desalination cells. The cation exchange membrane 120 used is a
Neosepta AFN membrane. The anion exchange membrane 122 used is a
Neosepta CM-1 membrane. Each membrane 120, 122 has a surface area
of 1500 cm.sup.2 (each membrane 120, 122 has a height of 50 cm and
a depth of 30 cm) that interfaces with the product, diluent, or
concentrate. The thickness of each of the product, diluent and
concentrate chambers is 0.02 cm. The anode and cathode 124, 126
are made of platinised titanium. The gaskets between chambers are
made of polypropylene.
[0128] The plant 1001 generates 1 m.sup.3 of drinkable water per
day at 0.04% salt concentration per day. The product feed has a
salt concentration of 3.50% when it is input into the dialytic
stack 901. The diluent also has a salt concentration of 3.50% and
the concentrate has a salt concentration of 18.00% when they are
input into the dialytic stack 901. As they leave the dialytic
stack 901, the diluent and concentrate have salt concentrations of
4.90% and 16.90%, respectively. Diluent, concentrate and product
feed flow into the dialytic stack 901 at rates of 38.7
m.sup.3/day, 36.4 m.sup.3/day and 1.01 m.sup.3/day, respectively.
Diluent and concentrate flow out of the dialytic stack 901 at
38.92 m.sup.3/day and 36.16 m.sup.3/day, respectively.
[0129] The salt concentration of the water in the water source
1004 is 3.50%. Water is drawn from the water source 1004 at a rate
of 39.75 m.sup.3/day. Of this water, 1.01 m.sup.3/day is sent to
the saltwater reservoir 1010, while 38.74 m.sup.3/day is sent to
the diluent reservoir 1026. A portion of the used diluent is sent
from the dialytic stacks 1002 to the concentrate reservoir 1020 at
a rate of 10.9 m.sup.3/day. The remaining portion of the used
diluent is discharged directly to the saltwater reservoir 1010 at
a rate of 28.0 m.sup.3/day and is not sent to the diluent
reservoir 1026.
[0130] The evaporative spray pond 1201 has a pond surface 1214
area of 75 m.sup.2. The pond 1201 uses three nozzles 1202. The
percent of water that evaporates per nozzle spray cycle is 4.0%.
The spray pond 1201 accepts fluid from the dialytic stacks 1002 at
a concentration of 16.90%, and returns fluid to the concentrate
reservoir 1020 at a concentration of 18.00%.
[0131] While illustrative embodiments of the invention have been
described, it will be appreciated that various changes can be made
therein without departing from the scope and spirit of the
invention. The invention is therefore to be considered limited
solely by the scope of the appended claims.




















