
rexresearch.com
Process and apparatus for electrolytic
extraction of magnesium hydroxide from seawater, brines or
concentrated solutions uses a two-zone cell at high pH with
membrane separation
DE102004039593
Extraction of magnesium hydroxide involves (A) introducing a salt
solution or brine into both the cathode (1) and anode (2) zones of
a 2-chamber electrolytic cell, the zones being separated by a
membrane (3); (B) applying a direct current between cathode (4)
and anode (5) of a strength giving a high pH in the salt
electrolyte in the cathode zone (1); and (C) loosening the
accreted material (9) and removing it from cathode zone (1). An
independent claim is also included for an apparatus for the
extraction comprising (1) a membrane (3)-separated electrolytic
cell with one or more anodes (5) and cathodes (4) near the
membrane (3); (2) an inlet (6) for introducing the solution into
cathode zone (1) and an outlet (7) for removing used process
electrolyte and the Mg(OH)2; (3) an opening (8) in the anode zone
(2) introducing and removing the solution; (4) a device removing
the Mg(OH)2 (9) from cathode (4); and (5) a conveyor (11)
transporting the Mg(OH)2 from the cathode zone (1).DE102004039593
DESCRIPTION
The present invention relates to a method and an apparatus for the electrolytic extraction of magnesium hydroxide, especially from seawater, concentrated seawater, brines or concentrated salt solutions.
Magnesium hydroxide is required in substantial quantities for industrial purposes, particularly in the production of paper and heat-resistant materials. It is used for the refining of sugar and in the production of uranium. In the medical field, it is an important antacids (antacid) and laxative. Magnesium hydroxide or brucite is a raw material for the production of magnesium and magnesium alloys, which are increasingly used, in particular in vehicle construction.
Seawater is a large reservoir of magnesium hydroxide. It is known that magnesium hydroxide can be precipitated from seawater by the alkalinity of the water by additions of dolomitic limestone or slaked lime is increased. However, no attempts have been made to obtain magnesium hydroxide from seawater, concentrated seawater, brines or other mineral solutions directly using electrolytic process based on an industrial mass production.
After oxygen, hydrogen, chlorine and sodium magnesium is the fifth most abundant element in seawater. A cubic mile of seawater contains 6,125,000 t magnesium. (Life Nature Library, The Sea, The Matchless Phenomenon of the Sea, Leonard Engel, and the editors of Time-Life Books New York, 1972, p 11). Thus 660-3000 cubic meters of sea water must be processed, depending on the efficiency of the process used to obtain a ton of magnesium from magnesium hydroxide compound.
From US Pat. No. 4,246,075 the Mineralakkretion (mineral deposit) of structures with large surfaces, structural components and elements is known. By providing a direct current between electrodes is connected in an electrolyte such as seawater, calcium carbonate, magnesium hydroxide and hydrogen is generated at the cathode, while oxygen and chlorine generated at the anode. The electrochemical accretion of minerals is used to produce structures with large surface elements and building components, consisting of a hard and resistant material. To prepare such surfaces, structural components and elements of hard and resistant material, is a prefabricated form, consisting of electrically conductive material as a cathode in a volume of electrolyte such. B. seawater submerged. One or more anodes are immersed in the vicinity of the structure and a direct current is switched for a time period sufficient to Akkretisierung a solid cover of the material of the form / structure, between the electrodes.
US Pat. No. 4,246,075 shows the use of Mineralakkretionstechnologie to build an OTEC (Ocean Thermal Energy Conversion) facility. The drawings show self-cleaning cathode in the Mg (OH) 2 chamber. The description in column 6, lines 51-55 refers to 8 and indicates that the system in addition to the conversion of thermal energy into electricity and chlorine, hydrogen, ammonium, Mg (OH) 2 (brucite) as a byproduct of the electrolysis process, which for the production can be used by buoyancy of cold seawater in the deep water tubes, manufacture. Means the application of heat, the brucite directly on the mineral periclase (MgO) can be reduced. This could produce potentially competitive cost investment refractory magnesium. This application proposals have so far not brought on an industrial scale for use.
The US Patent Nos. 4,440,605 and no. 4,461,684 describe the application of similar Mineralakkretionstechnologie for repairing concrete reinforced structures and protection against biodegradation.
The patent J 61177.385-A describes the production of magnesium hydroxide electrodeposited from seawater with high salinity. By applying a high current density and a low temperature magnesium hydroxide can be produced with a low calcium carbonate content. 2 shows a conveyor belt, which obviously also functions as a cathode, deposited on the magnesium hydroxide, and is then transported out of the cell.
The patent SU 1,193,177-A describes a method of extraction of magnesium hydroxide by the electrolysis of water enriched with minerals, eg. B. underground water, as a starting material in a membrane / diaphragm cell, the starting material is brought into the anode and cathode compartments of the cell. 10 V and at a pH value in the cathode compartment of Figs 10.5;, taking the electrolysis with a current density of 0.4 to 0.8 A / dm <2>, a current of 8 Figs 11 is performed. It is claimed that these conditions result in an extraction of 100-percentage magnesium hydroxide without impurities. The patent indicates, however, that this process has only been carried out in a glass jar, wherein the anode and cathode compartments by means of a Schott crucible (crucible) were separated with a capacity of 50 mg. However, it is neither a process nor a device for the production of magnesium hydroxide disclosed.
K. Menzel & quot; Electrochemical deposition of mineral substances from seawater "in & quot; Natural Constructions, messages of the SFB 230, Issue 1-From the subprojects / Collaborative Research Centre 230" University of Stuttgart / University of Tübingen, 1988 describes the coating of electrodes made from wire cloth & quot; self-repairing "mineral coatings in seawater by means of electricity. The mechanisms of precipitation and electrochemical conditions to obtain growing, solid layers are discussed and examined experimentally.
In the experiments, a chloride-free anode electrolyte (KOH solution) was used in order to avoid the production of chlorine gas at the anode. The anode compartment from the cathode compartment through a porous glass filter (1 micrometers pore diameter), or by a ceramic diaphragm separated. This description refers to the addition of magnesium hydroxide and calcium carbonate in cathode for producing components and not to the industrial production of Mahnesiumhydroxyd as raw material for further processing.
In summary, currently is not a suitable method, nor a device for the electrolytic extraction of magnesium hydroxide, especially from seawater, concentrated seawater, brines or concentrated salt solutions or aqueous solutions of chloride free salts (hereinafter, the term & quot; salt solutions "Pooled) available, the is economically feasible on an industrial scale.
The invention aims to remedy this. The invention has for its object to provide a process for the electrolytic extraction of magnesium hydroxide, especially from salt solutions, which can be used economically on an industrial scale. According to the invention, this object is achieved by a method which comprises the following method steps: & Ndash; Introducing saline into a cathode compartment of a two-chamber electrolysis cell & ndash; Introducing saline solution into the anode compartment of the cell, the anode compartment separated from the cathode compartment by at least one membrane, Figs; Applying a direct current between anode and cathode with a current intensity which is adapted to generate a high pH value in the electrolyte salt solution in the cathode compartment, Figs; Detachment of akkretisierten material and transporting it from the cathode compartment. & Ndash; Introducing saline into a cathode compartment of a two-chamber electrolysis cell & ndash; Introducing saline solution into the anode compartment of the cell, the anode compartment separated from the cathode compartment by at least one membrane, Figs; Applying a direct current between anode and cathode with a current intensity which is adapted to generate a high pH value in the electrolyte salt solution in the cathode compartment, Figs; Detachment of akkretisierten material and transporting it from the cathode compartment.
& Ndash; Introducing saline into a cathode compartment of a two-chamber electrolysis cell & ndash; Introducing saline solution into the anode compartment of the cell, the anode compartment separated from the cathode compartment by at least one membrane, Figs; Applying a direct current between anode and cathode with a current intensity which is adapted to generate a high pH value in the electrolyte salt solution in the cathode compartment, Figs; Detachment of akkretisierten material and transporting it from the cathode compartment. &
Ndash; Introducing saline into a cathode compartment of a two-chamber electrolysis cell & ndash; Introducing saline solution into the anode compartment of the cell, the anode compartment separated from the cathode compartment by at least one membrane, Figs; Applying a direct current between anode and cathode with a current intensity which is adapted to generate a high pH value in the electrolyte salt solution in the cathode compartment, Figs; Detachment of akkretisierten material and transporting it from the cathode compartment.
The invention provides a process for the electrolytic extraction of magnesium hydroxide, in particular of salt solutions is created, which is economically feasible on an industrial scale.
The electrolysis causes an increase of the catholyte pH, to magnesium hydroxide is precipitated. The pH may be raised so far that magnesium hydroxide is precipitated with a high degree of purity. The pH of the catholyte is determined by the applied current density between the electrodes. The akkretisierte material can be subsequently detached from the cathode.
The term & quot; Membrane "are hereinafter all the means for the separation of anolyte and catholyte, in particular ion-exchange membranes or ion-selective membranes as well as glass or ceramic filter subsumed.
Preferably the akkretisiete material is removed from the cathode by scraping, scraping, brushing or the use of vibration. In this way, the economy of the process is increased.
In the invention, the cathode is moved away from the membrane, while magnesium hydroxide accumulates at the cathode. In this way a small distance between the diaphragm and the effective cathode surface with increasing accretion is ensured.
Preference is given from the cathode, this is moved away from the membrane at a greater distance to remove the akkretisierten material. This is an in-process removal of the material allows. In addition, a possible damage of the diaphragm is counteracted.
In embodiment of the invention the hydrogen gas which is produced in the cathode compartment and the oxygen and chlorine gas produced in the anode compartment is collected. In this way, the economy of the process is further increased. When the anode electrolyte from a freshwater solution is with chlorine-free salt, only oxygen is produced at the anode. This may be advantageous with respect to the mixture of chlorine gas and oxygen, which is generated by brine electrolyte at the anode. The chlorine-free salt is preferably potassium hydroxide, but it may also be another salt, such as sodium hydroxide. If the anolyte consists of salt water, more salts may be added in order to increase the electrical conductivity.
Another embodiment of the invention, the heat of the discharged electrolyte process is used via a heat exchanger for heating of fresh saline electrolyte for supplying the cathode compartment. Thereby, the efficiency of the process is increased. the fresh saline electrolyte for supplying the cathode compartment are fed by means of ion exchange hydroxyl ions of the discharged electrolyte process is advantageous.
process key variables such as temperature, pH, current density, voltage, salinity, flow rate of the catholyte or deposition rate of magnesium hydroxide are preferably detected by sensors and used as input variables for process control. This is a continuous process optimization possible. The detected variables are processed by a computer which adjusts the relevant parameters in order to maintain predetermined conditions of the procedure. An integrated sensors microprocessor effector device automatically controls the deposition process of the magnesium hydroxide, for example in terms of optimization of energy recovery or purity of the magnesium hydroxide and creates a documentation of the process.
The invention is also based on the object, a device for the electrolytic extraction of magnesium hydroxide to provide particular salt solutions that can be used economically on an industrial scale. According to the invention this object is achieved by an apparatus substantially comprising & Ndash; an electrolytic cell comprising at least one anode compartment and a cathode compartment which are separated by at least one membrane, Figs; one or more anodes and one or more cathodes in the respective compartments, arranged in the vicinity of at least an associated membrane, Figs; at least one inlet for the supply of saline solution into the cathode compartment & ndash; at least one outlet for removing spent electrolyte and process magnesium hydroxide from the cathode compartment, & ndash; at least one opening for introducing saline solution into the anode compartment and / or derive, Figs; at least one device for the removal of magnesium hydroxide from the cathode, Figs; Conveying means for removal of magnesium hydroxide from the cathode compartment...
With the invention an apparatus for the electrolytic extraction of magnesium hydroxide, in particular of salt solutions is created, which is economically feasible on an industrial scale. The discharged from the drain magnesium hydroxide may be in solid, flaked or finely suspended, milky form. Preferably, a separate opening for the end of the anolyte is provided.
In embodiment of the invention is the device to replace the akkretisierten magnesium hydroxide from the cathode, a wiper or scraper. In another embodiment of the apparatus for the relief of akkretisierten magnesium hydroxide is from the cathode, a mechanical vibration generator. In both cases, an economical replacement of the magnesium hydroxide is guaranteed.
In the invention, the cathode is relatively movable in translation to the associated membrane at intervals of 2 to 150 mm. Thereby, the distance between the cathode and the membrane can be regulated. This moving mechanism causes the cathode during the precipitation of magnesium hydroxide, which progressively builds up on the cathode surface, is removed from the membrane to the extent that a constant distance between the membrane and the surface of the magnesium hydroxide is provided. Furthermore, the cathode may be at greater distances (eg. B.> 80 mm) are brought from the membrane in order to remove magnesium hydroxide from the cathode and to prevent the massing of loose Magnesiumhydroxydpartikeln.
Advantageously, the anode is moved translationally relative to the associated membrane at a distance of 1 to 30 mm. This controllability of the process flow is further increased.
In a further embodiment of the invention the cathode is continuously relatively movable to the associated membrane according to the growth of the akkretisierten material. This is a constant distance between the charged with akkretisiertem material cathode and the membrane obtained, whereby a continuous process flow is guaranteed.
In development of the invention controllable baffles for influencing the flow behavior of the electrolyte are provided in the cathode compartment. These cause an even and uniform flow through the electrolyte in the cathode compartment.
In a further embodiment of the invention, at least a transducer and / or vibrator in the anode compartment and / or on the anode is arranged. In this way, the permanent formation of oxygen bubbles and chlorine gas bubbles on the anode surface is prevented. Preferred in the cathode compartment, at least one transducer to emit sound waves into the electrolyte is angeord net.
In an embodiment of the invention, the anode side facing the cathode in a corrugated or serrated surface. By this comparable with the surface of a pattern of iron filings, the electrolytically active surface area is increased. Furthermore, an increased adhesion of the magnesium hydroxide is thereby effected on the cathode surface.
In an advantageous embodiment of the invention, the cathode consists of a series of tubular units with halbkreisartigem cross section whose flat part is electrically conductive and whose curved part consists of a thermally insulating material, wherein the units are arranged such that the electrically conductive part of the membrane facing. The arrangement of the profiles is preferably vertical parallel, alternatively, a horizontal or diagonal arrangement is possible.
Preferably, at least one tubular unit comprises a controllable heating element and a thermally conductive fluid. This allows the temperature of the electrolyte to be increased at the cathode surface, whereby the Ausfällrate of the magnesium hydroxide is increased. The temperature of the electrolyte is preferably between 20 ° C and 98 ° C.
In the invention, a heat exchanger is provided which is arranged between inlet process electrolyte outlet and Speisesalzlösung-. This can be heated by leaking warm process solution by heat exchange from the device fresh incoming brine so cold salt water can be effectively used as a raw material without further costs. If, however, warm saline, for example. Sea water or heated concentrated seawater from desalination plants used as raw material, the heating of the cathode surface and the electrolyte can be omitted.
In a preferred embodiment of the invention, sensors are provided for measuring process parameters in the electrode compartments. In this way, the monitoring and manipulation of process parameters is made possible to optimize the production of magnesium hydroxide in an economic and qualitative point of view. Some operationally variable quantities are, for example: temperature of the electrolyte, pH value of the input and process electrolyte composition of the electrolyte, flow rate, spacing of the electrodes, transmission rates of hydroxyl ions from leaking process electrolyte in fresh incoming brine, frequencies and power of sonochemical transducer in the cathode and the anode compartment, the anode frequencies vibrators, temperature on the cathode surface, electric current density and voltage between the electrodes, chemical-physical properties of the precipitated magnesium hydroxide and its composition, Ausfällungsraten of magnesium hydroxide. These variables can be detected by sensors in and on the device and processed by a programmed microprocessor, which performs optimal operational conditions according to targets by adjusting the appropriate variable sizes by means of suitable effectors.
The consumption of electricity in the precipitation of magnesium hydroxide from brine solutions by the process and apparatus of the present invention can be reduced by the smallest possible spacing is established between the electrodes and the electrode materials have a high electrical conductivity losses through the electrical resistance of the brine and to keep the electrode material as small as possible. Thus, for example, the cathode preferably of copper or silver, alternatively electrically highly conductive substrate materials with gold, silver, or copper plating can be used as the cathode. To economically advantageous to produce magnesium hydroxide from brines, the use of the lowest possible electric power voltages and currents is required. Several devices or Electrolytic cells may be combined into an electrolysis system.
Other developments and refinements of the invention are indicated in the remaining dependent claims. An embodiment of the invention is shown in the drawings and will be described below in detail. Show it:
1 -- a cross-section through a device according to the invention;
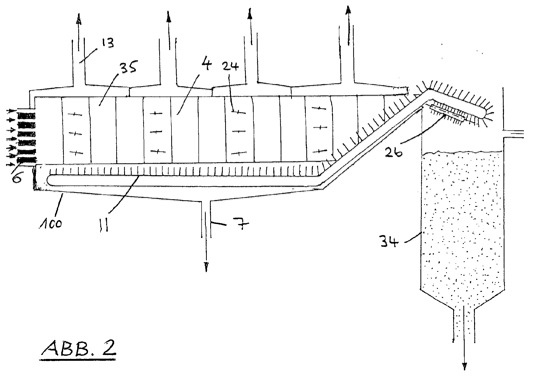
2 -- a longitudinal section through the device of Figure 1;
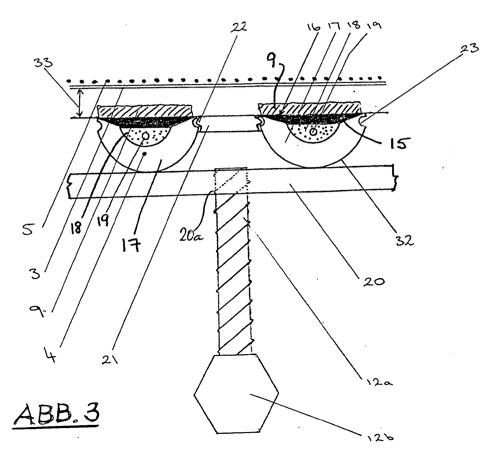
3 -- the detailed view of 'A' of the apparatus of Figure 1 with parts of the anode, the membrane and the cathode with precipitated brucite;
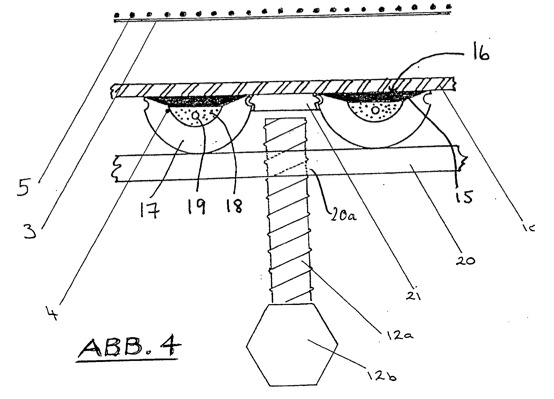
4 -- the detailed view of 'A' 3 with altered arrangement. It shows the cathode in the inactive position and the active detachment device for brucite;
5 -- the detailed view of a fin arrangement on a frame as part of a cathode compartment;
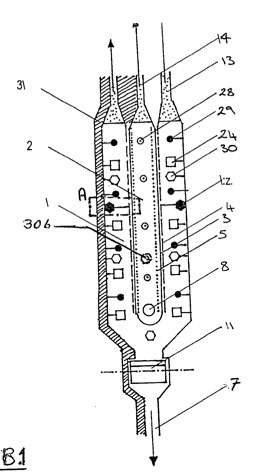

The selected as the exemplary embodiment consists Before direction from a process pan (100) is arranged longitudinally in an anode compartment (2) and a cathode compartment (1) in front, behind and below the anode compartment (2) is provided. The process pan (100) has an inlet (6) for supplying the brine. The cathode compartment is formed substantially in the form of a tuning fork and surrounds the substantially rod-shaped anode compartment (2) (see FIG. 1 ). Cathode compartment (1) and anode compartment (2) separated by one or more ion-permeable membranes (3). The cathode (4) or Anodes (5) of the cathode or Anode compartment (1 or 2) with a & ndash; & Not shown ndash; regulated DC source.
The cathode compartment (1) has, below its curvature to an outlet (7) to the end of the process solution. The legs of the cathode compartment (1) are formed by tubular profiles (32) with a semicircular cross section, formed from a flat cathode part (15) of electrically highly conductive material such as silver and copper, or a highly conductive support material with a coating, for example of gold, silver , copper, platinum, and a rounded heat-insulating profile (17). The electrically conductive, flat-shaped portion of each so designed cathode unit (4) has a corrugated surface (16), comparable to a metal file. Between the flat cathode part (15) and the rounded profile (17) is a cavity (18) formed, which is filled with a thermally conductive but electrically non-conductive fluid. In the cavity (18) an electric heating element (19) is provided centrally. Between the juxtaposed cathode units (4) are spacers (21) are provided which ensure a uniform, stable distance of the tubes (32) to each other.
On the spacers (21) guide rails (22) in the grooves (23) of the profiles (17) of two adjacent cathode units (4) are formed, engage. The spacers (21) are arranged at regular intervals vertically along the cathode units (4), wherein intermediate spaces are formed, so that the electrolyte can circulate freely. The panels (35) thus formed are modular components of the cathode compartment (1). In the exemplary embodiment, each of the panels (35) twenty cathode units (4) to (ten pairs of the cathode unit shown in Figure 3).
The heat insulating profile (17) of said cathode units (4) are fastened to a rigid frame (20), which in turn is connected to a displacement device (12). About the displacement device (12), the cathode units (4) can be moved continuously to the membrane (3) to or from it. The displacement device (12) comprises in the application example, a threaded rod (12a) into a corresponding internal thread (20a) of the frame (20) engages, and a motor (12b) through which the threaded rod can be driven in rotation in both directions. The displacement device (12) causes the spacing (33) between cathode (4) and one on the outer surface (16) growing mineral layer (9) and the membrane (3) during the accretion remains constant at all times or that Figs from a -- Not shown -- Computer determined optimum distances are adjusted. Further, on the displacement device (12), the distance (33) between cathode (4) and diaphragm (3) are sufficiently large set for the stripping of the mineral layers.
The distance (33) in Figure 3 is not drawn to scale for reasons of clarity.
The cathode (4) are opposite the diaphragm (3) arranged in the smallest possible and adjustable distance therefrom. The smallest possible distance describes the smallest possible distance between the cathode (4) and diaphragm (3), which ensures an unobstructed and continuous flow of electrolyte between these, also under the influence of Konvektionströmungen or other forces acting on the electrolyte in the cathode compartment (1 ) act. In this case, sufficient space for the growing accretion (9) on the diaphragm (3) facing towards the cathode surface (1) in the cathode compartment must at all times be present (see FIG. 3 ). Further, a scraper (10) between cathode (4) and diaphragm (5) must be moved in order to remove the akkretisierte material (9).
The scraper (10) consists essentially of a guided wire or a taut plastic cord, which is arranged horizontally. Optionally, also a provided with bristles profile are used. The scraper (10), driven by one or more motors (12b), to the full height of the cathode compartment (1) vertically up and come down. When the cathode (4) are provided for stripping, removes the vertical movement of the scraper (10) over the surface (16) of the cathode (4) slides, the akkretisierte material (9) from the cathodes (4) and the material drops onto a conveyor belt (11) which is arranged beneath the curvature in the lower part of the cathode compartment (1) and into a container (34) at the end of the conveyor belt (11) opens (see FIG. 2 ). Provided with a rotary brush device (26) is arranged on the container side under the conveyor belt (11) and removes the residual mineral from the conveyor belt (11), which is collected in the container (34).
The conveyor belt is preferably porous, so that the process used electrolyte can flow via the outlet (7), while the mineral (16) on the tape (11) remains to be transported in the container (34). In order to separate the mineral material which is in the leaked from the cathode department process electrolyte listed, after leaving the cathode compartment through the drain (7), various filters of known type can be arranged here.
The scraper (10) has a smooth surface, so that during the stripping the Akkretionsmaterials (9) from the cathode surface (16) remains a small part of the mineral in the cavities of the knurled surface. This is advantageous because thus nucleation of precipitating magnesium hydroxide and the subsequent film formation can be facilitated.
Due to the displacement device (12), the cathode (4) progressively from the membrane (3) are moved during the accretion and a typical distance of 1-120 mm at any time between the membrane (3) and the cathode (4), or, while accretion, are held between the outer surface of akkretisierten material (9) and the membrane (3). Furthermore, by the displacement device (12), the cathode (4) to be further separated from the membrane (3) so that the scraper (10) between cathode (4) and diaphragm (3) can be moved to the akkretisierte material (9) to remove.
Transducer (29) are provided on the wall of the cathode compartment (1). This is to sonochemical modules which act by application of sound waves in the frequency range 4-18000 Hz in the electrolyte on the Akkretionsmatrix, whereby the production rate of magnesium hydroxide is improved.
Furthermore, a number of motors driven by fins or lamellae (24, 25) are arranged within the cathode compartment (1). These control the flow of the electrolyte, starting at the inlet (6) until the end (7) such that a complete contact of fresh electrolyte (saline) takes place with the cathode. In Figure 2, which normally horizontally disposed deflection blades (24), the adjustable cause a uniform flow of the electrolyte through the cathode compartment, is shown.
Fig. 5 shows a series of adjustable guide vanes (25) generally vertically in comparison with the horizontally arranged fins (24), connected to the rigid frame (20) between the cathode (4) to the upwardly flowing electrolyte gaps between the spacers (21 ) to conduct so that the electrolyte sweeps the cathode surfaces (16). The combination of the various fins (24) causes a spiral flow of the electrolyte in the cathode compartment, which allows the best possible use of the electrolyte.
In the anode compartment (2), an inlet (8) is provided for supplying the anolyte to the curvature of the cathode compartment (1) facing side. The anode (5) is preferably made of a highly conductive material such as platinum, a platinum-coated carrier material or, for example, mixed metal oxides on a titanium carrier (eg. B. from Raney titanium), preferably shaped so that the anode surface is maximized. The anodes (5) opposite the membrane (3) disposed in the smallest possible and adjustable distance therefrom. & Quot; The smallest possible distance "means in the case of anodes (5) like as in the cathode (4), so that an unobstructed and continuous flow of the electrolyte between the anodes (5) and membrane (3), also under the influence of Konvektionströmungen or other forces which act on the electrolyte in the anode compartment, is ensured. The typical distance is in the range of 1-50 mm.
In the anode compartment (2) transducer (28) are further adapted to remove by emission of acoustic frequencies oxygen and chlorine gas bubbles, which are electrically insulating and affect Akkretionsvorgang negative. Thus, the effectiveness of the accretion process is increased. In addition, in the anode compartment (2) and & ndash; not shown & ndash; be arranged vibrators.
Both in the cathode compartment (1) and in the anode compartment (2) are sensors (30, 30b) is provided, the one, for example, parameters such as pH, salinity, temperature, flow rates and the like, important for an economical accretion process parameters on sharing computer that the conditions for an optimized accretion determined according to information received, for example, by fine-tuning the heating of the cathode (4), flow rate, current and voltage between the electrodes (4, 5) and other parameters to optimize Mineralakkretion required of magnesium hydroxide and comply with the targets.
The membrane (3), disposed between the electrodes (4, 5) consists of a material having ion-specific selectivity of known design and separates the anolyte from the catholyte as well as the resulting gases in the compartments. It is, for example, an ion exchange membrane. Alternatively can be used with a pore diameter of about 1 micrometer, for example, a diaphragm of the known type or a ceramic or glass material.
The procedure of the present invention will be illustrated with reference to the selected embodiment hereinafter: A water / potassium hydroxide solution (5-60 weight percent KOH) is passed through the inlet (8) into the anode compartment (2) and filtered Salzlö is sung in the adjacent cathode compartment (1) through the inlet (6) of the process pan left (2). A DC power source is connected to the electrodes (4, 5) so that an electric current with an output voltage of 1.1 to 6 V and an initial current density of 0.8 to 38 A / m <2> effective cathode surface flows. The current density is adjusted so that the pH of the electrolyte at the cathode (4) is at least 9.7 (at normal sea water). Electrolytes, vary the composition of the normal sea water, for example, concentrated seawater from desalination plants or sols, various pH values require to produce magnesium hydroxide with a high degree of purity.
This deviates from the norm for normal seawater electrolyte pH values can be determined experimentally by the built in device sensors in conjunction with a microprocessor and control mechanisms and effectively used in the production process.
Advantageously, the gap that separates the electrodes as small as practically possible, based on production conditions, maintained in order to efficiently produce magnesium hydroxide. The anode (5) is typically 1- 30 mm from the membrane (3) is removed, the arrangement of the cathode (4) is of another kind, since it can be variably adjusted relative to the diaphragm (3).
During the accretion process the distance between the membrane (3) and the diaphragm (3) facing the mineral layer (9) on the cathode (4) is automatically kept constant by means of a controlled motor (12b) (1 -50 mm), while the mineral layer ( 9) grows. This causes electrolyte at any time can circulate between the membrane (3) and the mineral layer (9) and thus raw materials are transported to the Mineralakkretion in close proximity to the growing mineral layer (9). Another advantage of automatic represents the minimization of electrical resistance losses in the electrolyte.
After a certain amount of magnesium hydroxide in the cathode (4) is formed, the mineral is processed in the following manner continued: The cathodes are removed by a displacement device (12) of the diaphragm side, so that the vertical movement of Mineralabstreifers (10) effectively the can solve Akkretionsprodukt of the cathode surfaces (16) on the porous conveyor belt (11) is lowered and is supported by the same from the cathode compartment (1) into the container (34). The conveyor belt (11) remaining stocks of one or more brush-like devices (26) is removed (2).
The stripping action of the mineral layers may be performed while the apparatus contains an electrolyte and is electrolytically active, or if the device contains no electrolyte.
When the apparatus electrolyte contains, a continuous flow of electrolyte in the cathode compartment (1) is maintained while electrolyte and stripped material (9) by means of the porous conveyor belt (11) and / or by the outlet (7) leaving the cathode compartment (1) , The out of the drain (7) and overflowed in the tank (34) collected salt water / magnesium hydroxide mixture is filtered to acquire the magnesium hydroxide. The saltwater is a & ndash; & Not shown ndash; Hydroxylaustauscher supplied which is supplied with fresh salt water, which is provided as the electrolyte for processing in the apparatus. Also, the thermal energy used salt water through a & ndash; also not shown & ndash; Heat exchanger on the fresh salt water electrolyte are transmitted.
In alternative modes of operation of the apparatus can advantageously be on the operation of the conveyor belt (11) can be dispensed. For this purpose, the conveyor belt is removed from the apparatus. The salt water / Magnesiumhydroxydgemisch is then optionally discharged for further use in heating or Hydroxylaustauschern through the outlet (7) for filtering and.
Besides the production of magnesium hydroxide from brines and hydrogen, oxygen, and in the case of an alternative use of the device chlorine gases are produced as byproducts of the electrolysis of water or salt water. These gases pass through openings (13, 14) of the cathode or Anode chamber (1, 2) and can be collected with known devices. Oxygen and chlorine bubbles formed at the anode (5), by the action of vibrators and transducers (28) in the anode compartment (2) or on the anode (5) are mounted, removed from the anode surface.
The device is thermally insulated to minimize heat losses. 1 shows the isolation (31) only at the head of the unit, as this is provided as one in a succession of similar units. For all ranked devices arranged a thermal insulation is provided.
In the embodiment, the following operating conditions are: voltage between the electrodes 1.1 -6.0 V; Current density at the cathode 0.8 Figs; 48.0 A / m <2> effective cathode surface; Temperature of the electrolyte 3 & ndash; 98 ° C.
The pH of the catholyte is within the range of 8.3 Figs; 14.5; preferably from 8.7 to 13.0; more preferably from 9.3 to 12.8; and in particular between 9.5. and 12.6.
The critical pH for precipitation of magnesium hydroxide in seawater is 9.7.
A specific combination of the values given above is required to obtain the desired composition of the precipitated material. It should be noted that a relatively low current density at the cathode (4) leads to the Akkretionsprodukt brucite in its hard crystalline form, while is precipitated with use of higher current density in the brucite soft soap-like shape.